Nucleic Acids and Proteins
CHAPTER 2A: The Functional Diversity of Proteins
Proteins are also known as polypeptides.
Proteome: all the proteins within a cell or a living organism at a given time.
Polypeptide: a long chain of amino acids.
Proteins one of the four types of biomacromolecules
Amino acids are the repeating units or else monomers that make up a polypeptide chain or protein.
Types of Proteins
Enzyme: an organic molecule/protein, that catalyses (speeds up) specific reaction.
Peptide Hormone: a signalling protein that is responsible for regulating physiiology or behaviour.
Antibody: a protein that is produced by plasma cells during the adaptive oimmune response that is specific to an antigen and combats pathogens in a variety of ways. It is also known as immunogobulin.
Haemoglobin: a type of protein that is responsible for carrying oxygen in red blood cells and is an example of a protein with a quaternary structure.
STRUCTURE OF AMINO ACIDS
Amino acids serve as the building blocks of proteins.
Amino acids are the repeating units or else monomers that make up a polypeptide chain or protein.
There are 20 types of different amino acids
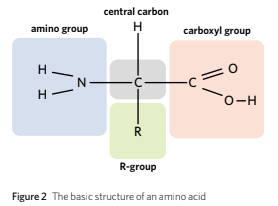
The chemical structure of amino acids is composed of a central carbon, a carboxyl group, an amino acid group, an R-group and a hydrogen atom.
Carboxyl Group
The central carbon is bonded to a hydrogen atom, a carboxyl group, amino group and an R-group. The R-group of an amino acids is reponsible and determines the type of the amino acid.
This explains the reason why amino acids are not only made up of carbons, hydrogen, oxyegn and nitrogen but they are also made up of other elements such as sulfur (S) depending on the R-group of the amino acids.
 Due to the proteins’ chemical properties, it determines the way it interacts with other/surrounding proteins. For example, a protein with a hydrophobic R-Group tend to form bonds with other prteins with hydrophoicR-Group than proteins with hydrophilic R-Group.
Due to the proteins’ chemical properties, it determines the way it interacts with other/surrounding proteins. For example, a protein with a hydrophobic R-Group tend to form bonds with other prteins with hydrophoicR-Group than proteins with hydrophilic R-Group.
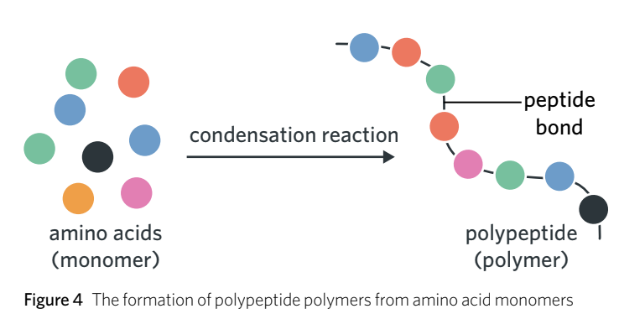
The joining/combining of two adjacent amino acids are held by polypeptide bonds which occurs in the ribosome through a condensation reaction.
PROTEIN STRUCTURE
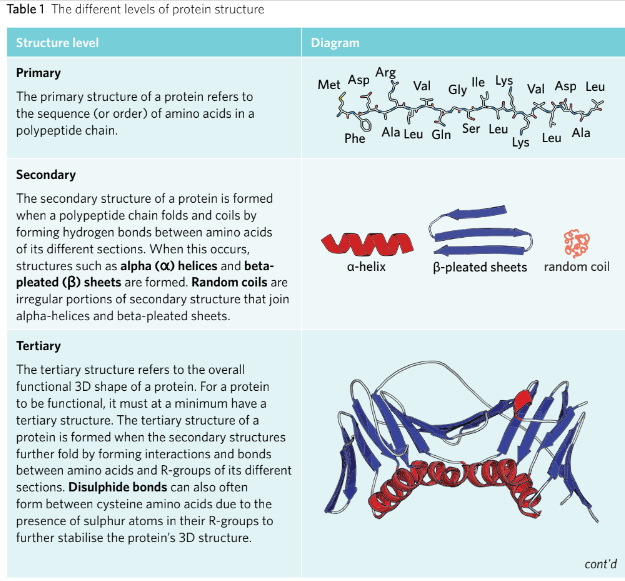
Proteins has for level of structure; primary, secondary, tertiary and quaternary.
A protein can only becomes FUNCTIONAL if it succeeds in tertiary or maybe even quaternary structure.
PRIMARY: The first level of protein structure and refers to the sequence(order) of the amino acids in a polypeptide chain.
SECONDADRY: The level of protein structure where the amino acid chains form either of alpha-helices, Beta-pleated sheets and random coil.
TERTIARY: The level of protein structure and refers to the functional 3D shape polypeptide chains.
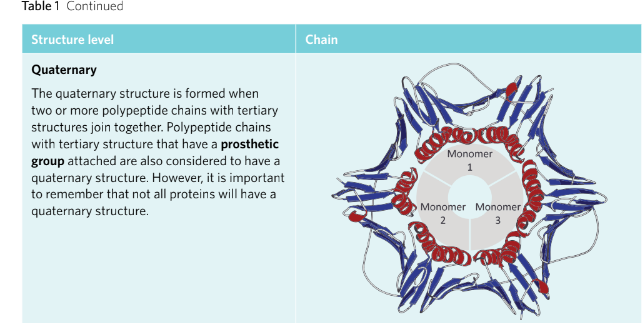
QUATERNARY: The level of protein structure where two or more polypeptide chains or other other non-protein molecules/groups are added to the structure to form a complete functional protein.
Random Coil: and irregular protein structure that is neither an a-helix or B-pleated sheets.
Disulphide Bond: a strong covalent bond between two sulphur atoms.
CHAPTER 2B: Nucleic Acid
Nucleic Acid: class of macromolecule which includes both DNA and RNA. All nucleic acids are polymers composed of nucleotide monomers.
The Two Types of Nucleic Acid:
- DNA - Deoxyribonucleic Acid
- RNA - Ribonucleic Acid
Large polymers composed from nucleotide monomers.
Responsible for storing genetic information and help produce proteins for survival.
Structure of Nucleotide
The monomer subunit of a nucleic acid
A Nucleotide is composed of a phosphate group, a five-carbon (pentose) sugar and a nitrogenous base.
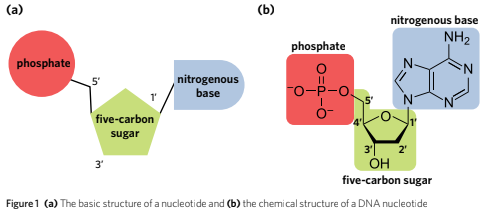 Nucleotides are bonded together through/by phosphodiester bonds which are a strong covalent bonds that are formed via a condensation reaction.
Nucleotides are bonded together through/by phosphodiester bonds which are a strong covalent bonds that are formed via a condensation reaction.
The DNA
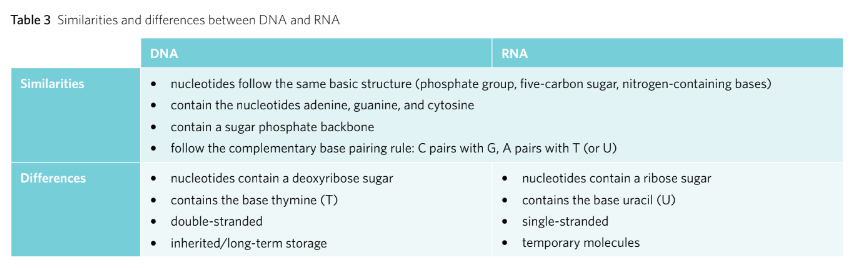
The DNA (Deoxyribonucleic Acid) is a double stranded nucleotides bonded together via complementary base pairing. Due to this the DNA’s shape turns into a double helix that runs in an anti-parallel to each other.
DNA is composed of two polynucleotide chains which run anti-parallel to each other. The two chains are joined through the complementary base pairing meaning that hydrogen bond is utilised to join pairs of nucleotides.
In the nuclear DNA the double helix structurew of DNAs coils around proteins called ‘histones’ to condense and form tightly packed chromosomes.
The RNA
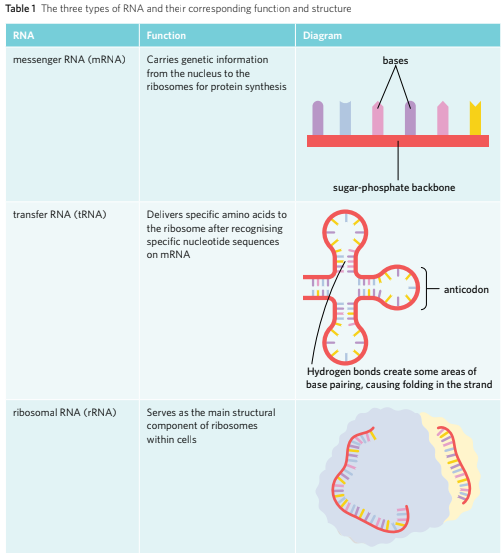
RNA serves many different roles and functions within the cell however its primary function is protein synthesis. There are many different RNAs such as the mRNA, tRNA and rRNA, each serving different and specific roles and functions in protein synthesis.
Whilst DNA is inherited from generation to generation, RNA however, is synthesised on demand.
mRNA: Messenger RNA are the RNA molecules that are produced during the transcription and carry the genetic code/information from the nucleus to the ribosome.
tRNA: Transfer RNA are the RNAs that are responsible for recognising specific codons on the mRNA starnd and adds the corresponding amino acid to the polypeptide chain during the protein synthesis.
rRNA: Ribosomal RNA are the RNAs that are the key components of ribosomes.
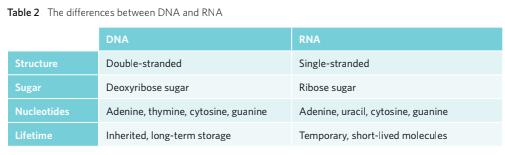 VCAA Examiner Tips: When comparing between the similarities and differences of RNA and DNA, one shouldn’t rely on the double and single stranded factor as there are viruses with RNA that are double stranded and viruses that contain single stranded DNA molecules.
VCAA Examiner Tips: When comparing between the similarities and differences of RNA and DNA, one shouldn’t rely on the double and single stranded factor as there are viruses with RNA that are double stranded and viruses that contain single stranded DNA molecules.
Chapter 2C: Genes
Transcription, RNA processing (post-transcirption modifications) and translation are the process of reading and interpreting genes within DNA for protein synthesis.
The Process:
During transcription and post-transcription modifications, DNA sequence of a gene is copied into RNA nucleotides in the form of mRNA. The mRNA is then decoded(translated) into specific amino acids in the translation to create a polypeptide chain.
NOTE: These process is only and only possible due to the existence of the genetic code.
The Genetic Code:
It is a series of rules
The set of rules by which the information is encoded in genetic material.