Materials Compiled
Structure of matter
Types of Atomic and Molecular Bonds
Primary atomic bonds
Ionic bonds - Ceramics
B/w + and - ions
Electron transfer
Materials are hard, brittle, electrically, and thermally insulating
Covalent bonds - Polymers and ceramics
Sharing electrons → Directional
Materials are hard, brittle, electrically and thermally insulating
Metallic bonds - Metals
Sea of donated valence electrons
Materials and thermal and electrically conductive, and can readily undergo plastic deformation
Secondary atomic and molecular bonds - weaker than primary bonds
Polar forces (ex/ H-bonds) - Polymers
Covalently bonded atoms have dipole
Van der Waals forces
Properties from Bonding
Ceramics (ionic and covalent)
Large bond energy
Large Tm
Large E (elastic modulus)
Small CTE (coefficient of thermal expension)
Metals (metallic)
Variable bond energy
Variable Tm
Variable E
Moderate CTE
Polymers (covalent and secondary)
Directional properties with secondary bonding dominates
Small Tm
Small E
Large CTE
Structural arrangement of atoms in solids
Crystal Structure
Regular arrangement of atoms and molecules in space for minimal internal energy
Organized in lattice forms (FCC, BCC, HCP)
Polycrystalline structures: aggregates of many single crystals organized in different orientations → Grain boundaries
Noncrystalline Structure
Amorphous materials
Random arrangement of atoms (polymers and some ceramics - porcelain)
Tg vs Tm
Tg = Glass transition temperature (noncrystalline materials)
At Tg, materal is “softer” but not fully a liquid
Tm = Crystalline melting/fusion temperature
Surface energy and wetting
Surface energy is higher than bulk → B/c there are “dangling'“ bonds
A material with a lower surface energy can wet a surface with a higher energy
Elevate surface energy via polishing to make it easier to wet
Wetting in Dentistry
Key for adhesion
Key to take accurate dental impressions
Physical properties of dental materials
Thermal properties
Thermal conductivity: How easily heat transfers through a material
Metals > Non-metals
Large metallic restorations need insulating cement to protect pulp
How insulating the base material is (Lining efficiency) = Thickness / sqrt(thermal diffusivity)
Thermal diffusivity: Rate at which temperature of body changes as heat passes through
Depends on thermal conductivity, density, and specific heat
Inversely proportional to density
Specific heat: quantity of heat to raise temperature of a unit mass of a material by 1 degree C
Thermal expansion: Change in length per unit of the original length when the temperature is raised by 1 degree C
Must know original length
\alpha=\frac{\Delta L}{L\cdot\Delta T}
CTE of dental materials
Clinical consequences: Expansion and contraction of material, leakage around restorations, shrinkage of wax patterns, differential shrinkage of PFM crowns
Biological consequences: Microleakage along tooth/restoration interface, Corrosion products from amalgam reduce microleakage
Rheological properties
Rheology: Study of flow of materials
Viscosity: Resistance to flow of a material under an applied stress
Resistance is due to internal friction
Shear stress = F/A
Shear strain rate = V/d
Viscosity = Shear Stress / Shear Strain
Rheological Behavior of Fluids
Newtonian: Viscosity is constant with respect to stress
Pseudoplastic (shear thinning): Viscosity decreases after initial stress
Dilatant (shear thickening): Viscosity increases with stress
Plastic: Some initial stress needed, then linear (like newtonian)
Optical properties
In dentistry, interaction of light with restorative material must mimic the interaction with teeth
Light and Color in Dentistry
Visible light wavelength: 400-700 nm
Wavelength of max. visual perception: 550 nm (green)
Spectral Distribution of Light
Different light sources have different wavelength distributions
Color measurement system
Munsell system
Hue (wavelength)
Circumference
Chroma (intensity)
Radius
Value (light/dark)
Bottom → Top
CTE
L*, a*, b* systems
L = value
a* = red/green
b* = blue/yellow
Color Perception
Metamerism: Change in color matching of two objects under different light sources (have different wavelength distribution)
Translucency/Opacity: Amount of incident light transmitted by an object that scatters part of the light
Gloss: Proportion of specualr reflection to diffused reflection of light
Gloss = specular / diffused
Fluorescence: 300-400 nm absorbed → 400-450 emitted (blueish)
Near enamel edges - hard to minic
Color Rendering Index
The degree to which a light source can render the color of an object compared to a reference source
CRI: Measurement of how well a light source displaces color compared to a natural light source (ex/ sunlight)
0 - 100
Shade Matching
Light sources (at least 2)
Background color (neural gray)
Tarnish and Corrosion
Tarnish: surface discoloration of a metal
Presence of saliva, bacteria, chemicals
Formation of thin films of oxides, sulfides, and chlorides
Corrsion: reaction of metallic material with environ,ent
Progressive and destructive
Degredation of material and possible by-products
Other properties
Electrical - Galvanism
Can be caused by… opposing metal restorations or be in 1 tooth
Mechanical Properties of Dental Materials
Stress and strain: What are they and why are they used instead of load and deformation
Stress and Strain
Stress: force per unit area (independent on sample size)
Types of stress: Tensile, shear, compressive
Strain: material deformation in response to stress (change in length per initial length)
Dimensionless
Types of strain: Tensile, shear, compressive
Stress-strain curve
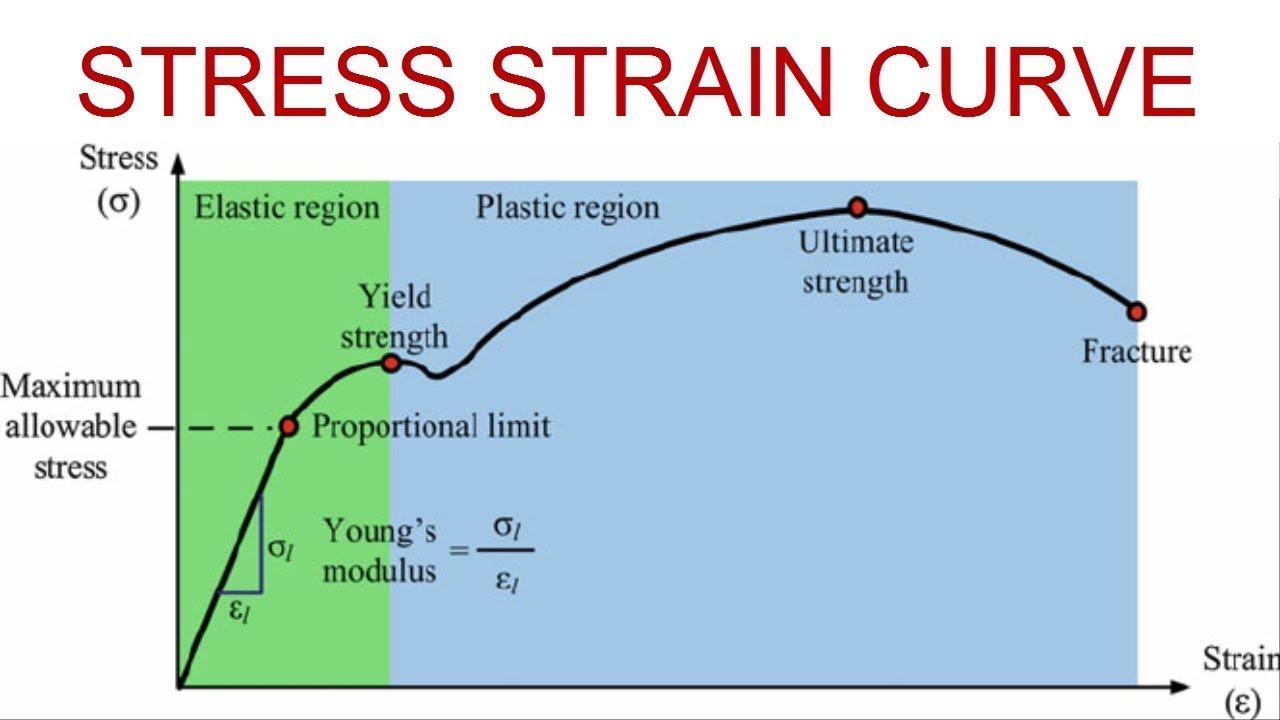
PL = Proportional limit
Value of stress at which the stress/strain curve deviates from the initial linear relation
Elastic limit: Stress corresponding to the 1st measurable permanent deformation
Somewhere between the PL and YS
Yield strength: strength corresponding to a designated amount of permanent strain 0.2%
Ultimate tensile strength: Maximum stress without fracture
Elastic and plastic behaviors: When loads are small, how much deformation occurs?
Elastic strain: Strain which disappears completely when the applied force is removed
Ex/ Pulling on a rubber band
Permanent (plastic strain): Strain which remained permanently after the applied force is removed
Ex/ Bending a metal rod
Elastic modulus and hardness: What are they and how are they determined
Elastic Modulus/Modulus of elasticity/Young's modulus
E = stress/strain (within the elastic range or the linear portion of the curve)
Determined by interatomic and intermolecular forces (the stronger the force, the more rigid the material)
Material dependent
Elastic modulus: resistance to elastic deformation
Important to consider elastic modulus of implants relative to bone.
If elastic modulus of implant much higher than bone, can lead to bone loss
Tooth: Enamel = 70-90 GPa; Dentin = 15-20 GPa
Stiffness and Modulus
Stiffness = force/deflection
Modulus = stress/strain
Higher stiffness = Higher modulus
Steeper slope in elastic region
Yield strength and tensile strength: What are they and how are they determined
Ultimate Strength
UTS: The stress corresponding to the maximum value of applied stress a material can withstand without rupturing
Peak of stress/strain curve
Fracture Strength
Fracture point: The value of stress at which a material ruptures into 2 or more portions
Yield Strength
Yield strength: strength corresponding to a designated amount of permanent strain 0.2%
Toughness, ductility, and resilience: What are they and how do we measure them?
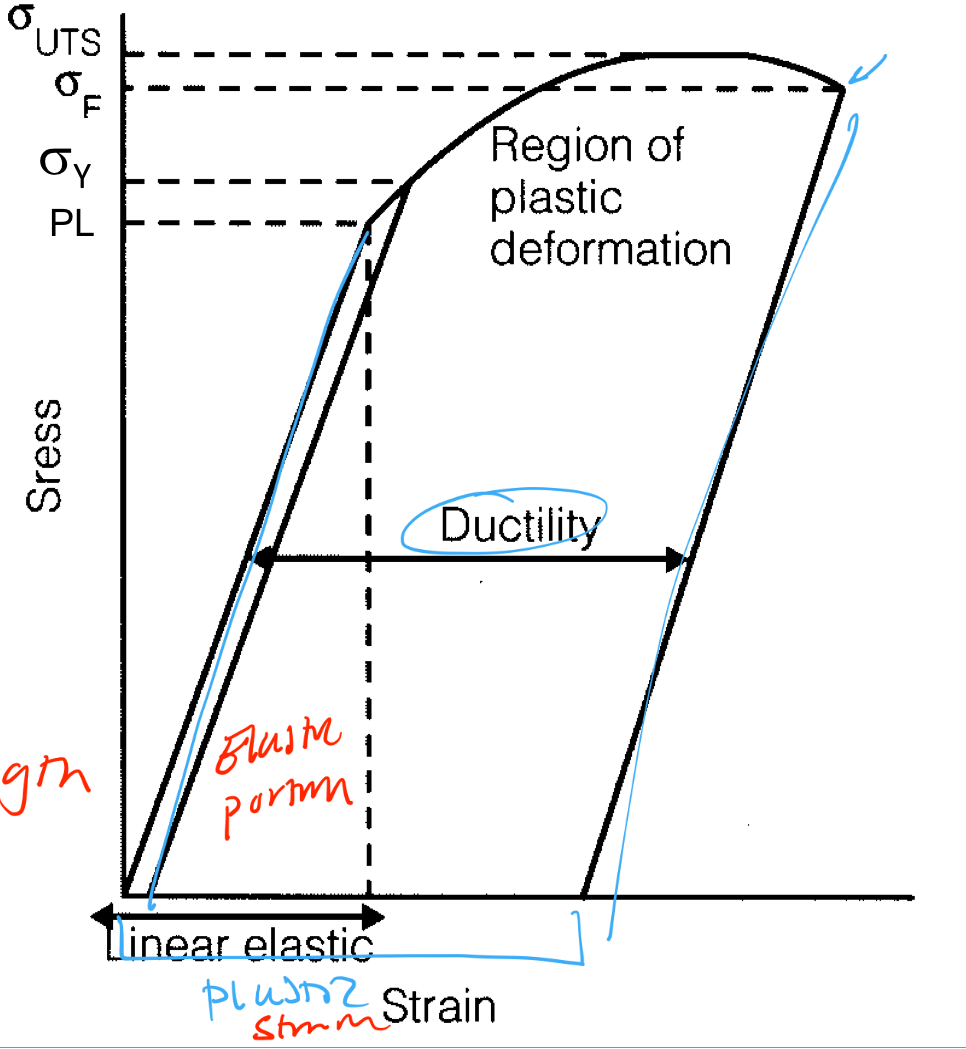
Ductility and Elongation
Ductility: The ability of a material to undergo permanent tensile deformation without fracturing
Elastic + plastic deformation
Plastic tensile strain at failure
The more ductile the material, the more strain it can withstand
Malleability
The ability of a material to undergo permanent compressive deformation without fracture
Brittleness
Material behavior characterized by fracture with little or no prior permanent deformation
Modulus of Resilience
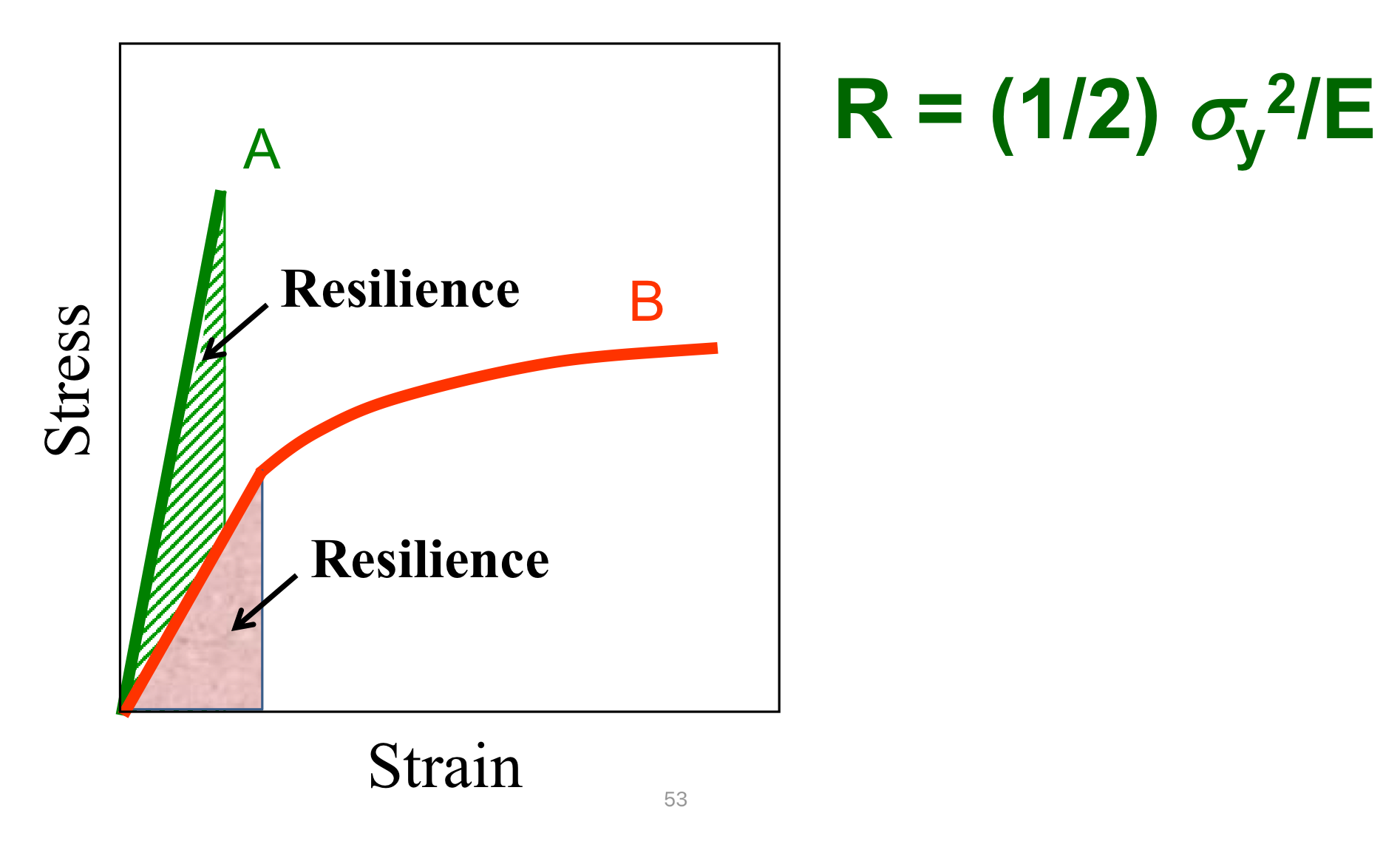
Resilience: The amount of recoverable energy stored in a material during elastic deformation (material does’t suffer damage)
Area under linear portion of stress/strain curve
The larger the area, the more resilient
Toughness
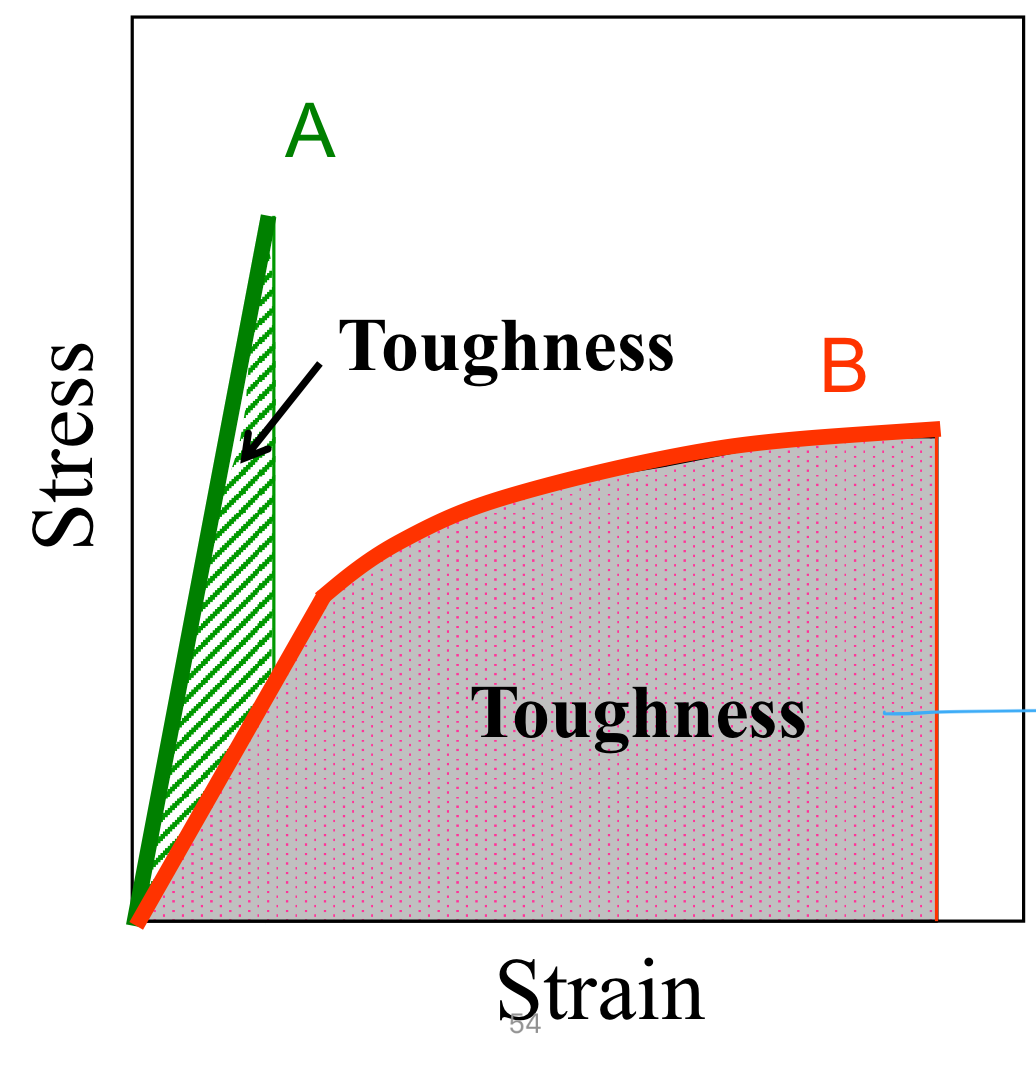
Toughness: Energy to break a unit volume of material
Area under the stress/strain curve
Include elastic and plastic regions
Hardness
Resistance of a material to plastic or permanent deformation
In metals, hardness = YS * 3
Determined by the “scratch test”
The harder material can scratch the softer material
Cyclic mechanical properties
Failure of materials due to cyclic loading and unloading
Fails occur at stresses lower than UTS
Growth of small cracks which become larger upon cycling until failure
Fatigue limit
Stress at which material can withstand an unlimited number of cycles
Influenced by how polished the surface is, temperature, and wetness
The more polished, the greater the fatigue resistance
Increase temperature decreases fatigue resistance
Wetness decreases fatigue resistance
Mechanical properties of enamel and dentin
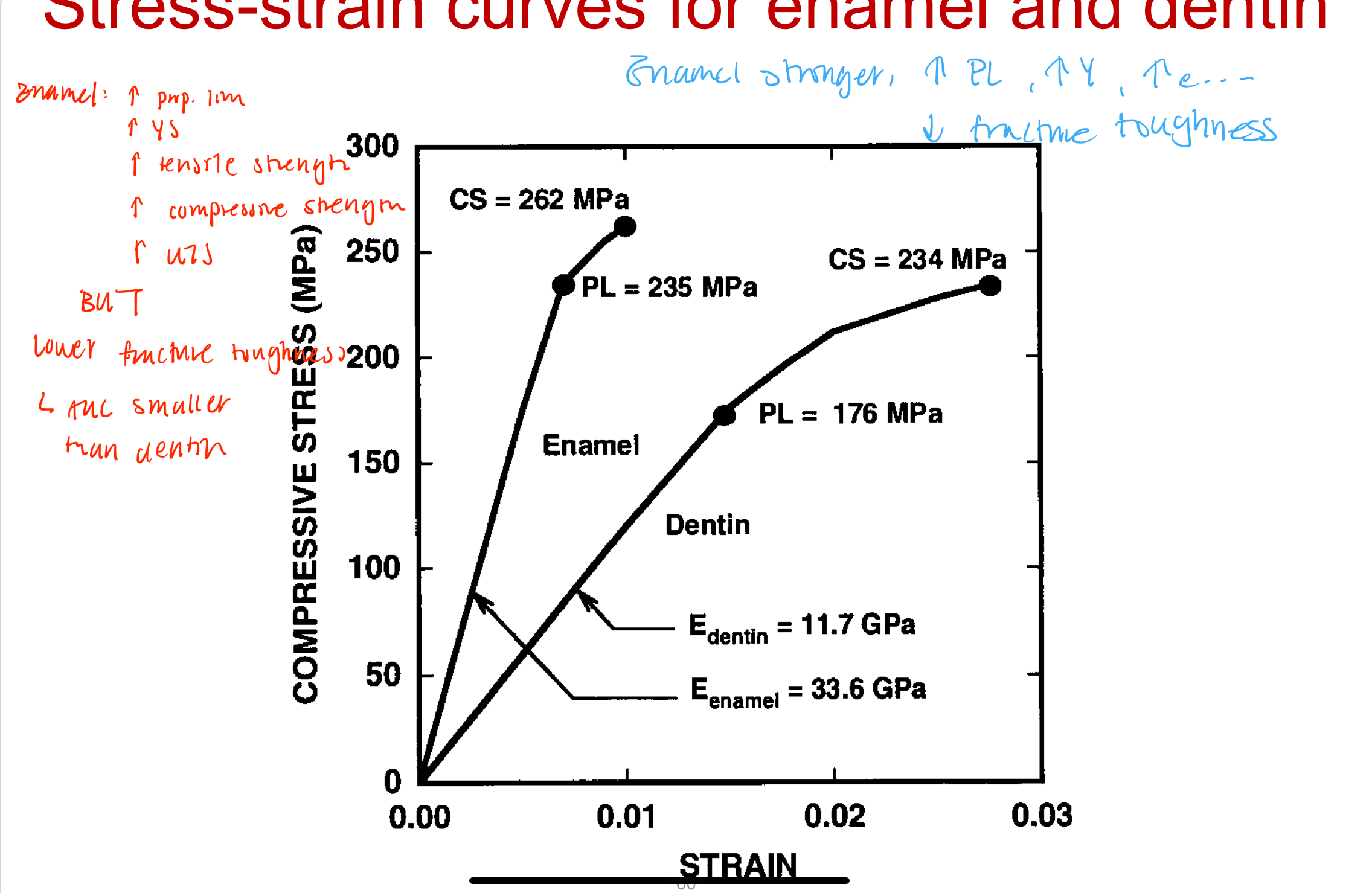
Enamel compared to dentin
Increase: PL, YS, Tensile strength, Compressive strength, UTS
Decrease: Fracture toughness (AUC smaller than dentin)
Composites
What is the basic composition of dental composites?
Dental Composites
A combination of materials in which each component retains its identity
Composed of…
Matrix (high molecular weight monomers, low molecular weight monomers, polymerization control additioves)
Fillers (particles, fibers, whiskers)
Interfacial coupling agents (organic silanes)
What are the roles of the matrix phases, filler particles, coupling agents, and polymerization control additives?
Matrix (Continuous Phase)
High molecular weight monomers: Increase strength and viscosity, decrease filler loading
Ex/ Bis-GMA, UDMA, Sirolane
Low molecular weight monomers: Decrease viscosity, increase filler loading and polymerization kinetics
Ex/ TEGMA
Polymerization Control Additives
Activators/initiator
Light activation = Camphorquinone
Chemical activation = Organic peroxide
Inhibitor
BHT and Hydroquinone
Fillers (inorganic - Dispersed Phase)
Dental composites primarily have noncrystalline (glassy) silicates
Ex/ borosilicate glass, quartz, zirconia
Can also add radio-opacity
Generally harder and stronger than the matrix
By adding filler to composites….
Decrease CTE
Increase hardness
Decrease shrinkage (fillers physical dimensions won’t change)
Decrease water absorption
Interfacial Coupling Agents - Organic Silanes
Pros…
Silanes are the most effective coupling agent and they work best with filler materials have a lot of Si-O bonds (ex/ silica)
Reduces viscosity and glass hydrophilicity → Chemical bonding from matrix to filler
Raise strength and wear resistance for composites
Cons
Silanation reaction may be reverse in water
Problems such as: multi-layers, bonding (increase vulnerability to break between interfaces)
Other Components
Inorganic pigments for shade development
What are the clinical indications of various composites
Polymerization Shrinkage
Increased filler → Decreased shrinkage
High stress due to high elastic modulus → Stiffer
How to resolve
Increase amount of filler
Add composite in 2mm increments
Use low shrinkage monomers
Working and Setting Time
Light cure: “On demand”
Reaction continues for 24hrs
Chemically cured: 3-5 mins
Depth of Cure
2 mm composite at a time
Mechanical Properties
Abrasion and wear
Toothbrush abrade and wear weak polymers first → rough surface
If inter-particle spacing becomes small, wear is greatly reduced (ex/ use of nanohybrids → highly polished surface)
Occlusal wear resistance
Hybrid composites wear rate similar to dental amalgam
Thermal Properties
Water Sorption
Solubility
Color and Color Stability
Biocompatibility
Translucency
Composites for special application
Multipurpose composites
Microfilled composites
Packable composites
Flowable composites
Laboratory composites
Core composites
Provisional composites
Metals and Alloys
What are pure metals?
Noble metals
Elements with good metallic surfaces that retain their luster in clean dry air
Indicate the inertness of the element
Resist oxidation, tarnish, and corrosion during heating, casting, and soldering
Platinum group (6 metals) - include platinum, palladium, iridium, ruthenium
Gold
The term precious metals indicates how expensive a metal is based on supply and demand
Gold content of a dental alloy
Karat/Carat (K): Parts of pure gold per 24
Fineness: Parts of pure gold per 1000 (used for gold solders)
Pennyweight
Solidification of pure metals
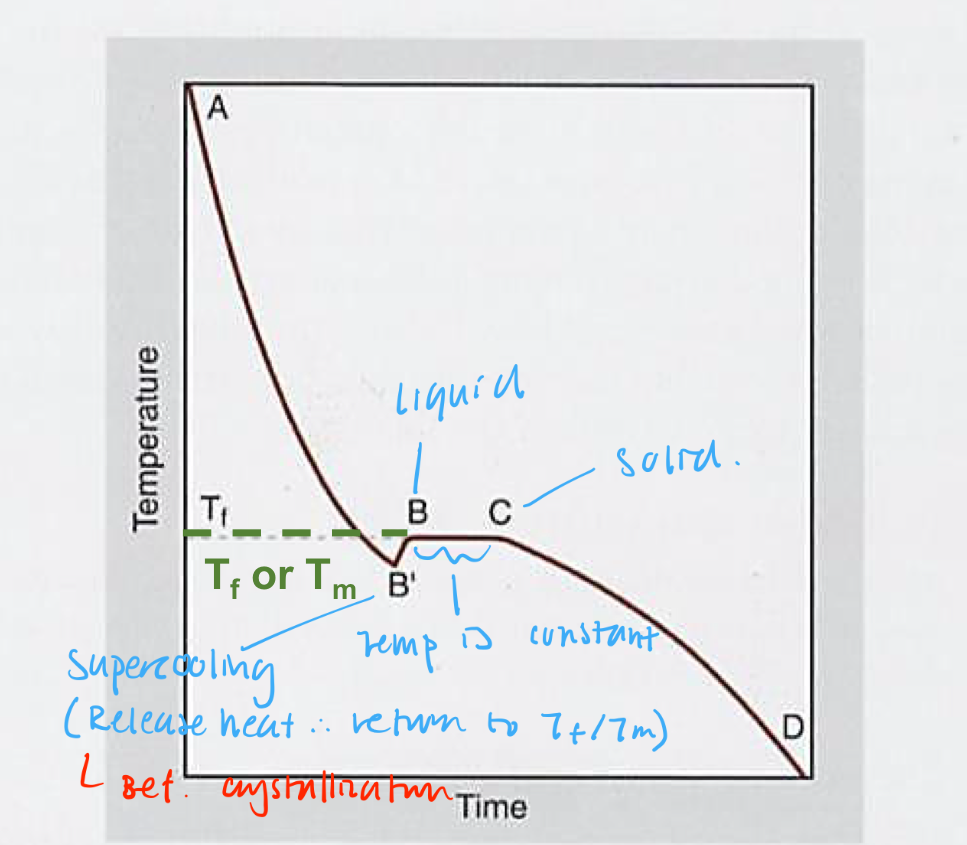
Have fixed Tm/Tf
Supercooling may occur before crystallization
Formation of grain structures
Stages
Atoms aggregate to form an embryo
Nuclei formation: embryo increases in size
Branch/dendrite formation
Grains become recognizable
Grains are formed
A metal has formed grain structures
Crystal Growth
Homogenous nucleation → Non-uniform grain
From nucleus without external agents
Heterogeneous nucleation → Uniform grain
Discreet particles are used to form nucleus
Grain Boundaries
Junction between grains or crystals
Have higher energy (due to unsatisfied bonds)
Increases strength of material → Cracks don’t propagate as well
Grain morphology and size
Equiaxed grains: Equal in size in all directions
Average size of grains in microstructure
Small grain size → Better physical and mechanical properties
Controlling grain sizes
Rapid cooling
Mold design
Vibration during solidification
High thermal differential between mold wall and alloy
Use of nucleating agents (grain refiner) - best way to do it
What are dental alloys?
Alloy
Mixture of 2 or more metals that are mutually soluble in the molten condition; or a mixture of a metal and a non-metal
Improves physical and mechanical properties
Categorizing Alloys
ADA specification #5: Gold-based alloys
Can have any composition so long they pass the tests for toxicity, tarnish, yield strength, and percent elongation
I → IV (soft → extra-hard)
ADA classification
High noble: >40 wt% Au and >60 wt% of the noble metal elements
Noble: > 25 wt% of the noble metal elements (Au, Pd, Pt)
(Predominantly) Base metal (PB): < 25 wt% pf the noble metal
Principle element: Listed in declining order of composition (highest to lowest)
Except certain elements that affect physical properties or represent potential biocompatibility concerns
Descriptive classification
Normal-fusing alloys (good for all-metal restorations)
Ex/ Silver-palladium
High-fusing alloys (mostly for PFM)
Ex/ Palladium-silver
Why are pure metals not useful for most dental applications
Alloys have better physical and mechanical properties than pure metals
Understand phase diagrams for dental alloys
Definitions/Characteristics
Phase: A physically distinct, homogenous, and mechanically separable portion of a system
Alloys solidify over a range of temperatures
Exist as solid and liquid
No one Tm
Solid solutions
Substitutional: Have similar properties therefore can replace to form alloy
Atomic size - variation within 15%
Valence - behaves the same with other elements
Chemical affinity
Lattice type (FCC, BCC, HCP)
Interstitial: Have different sizes
Can distort lattice and make dislocation movement difficult
Increase strength, hardness, and proportional limit
Decrease ductility and resistance to corrosion
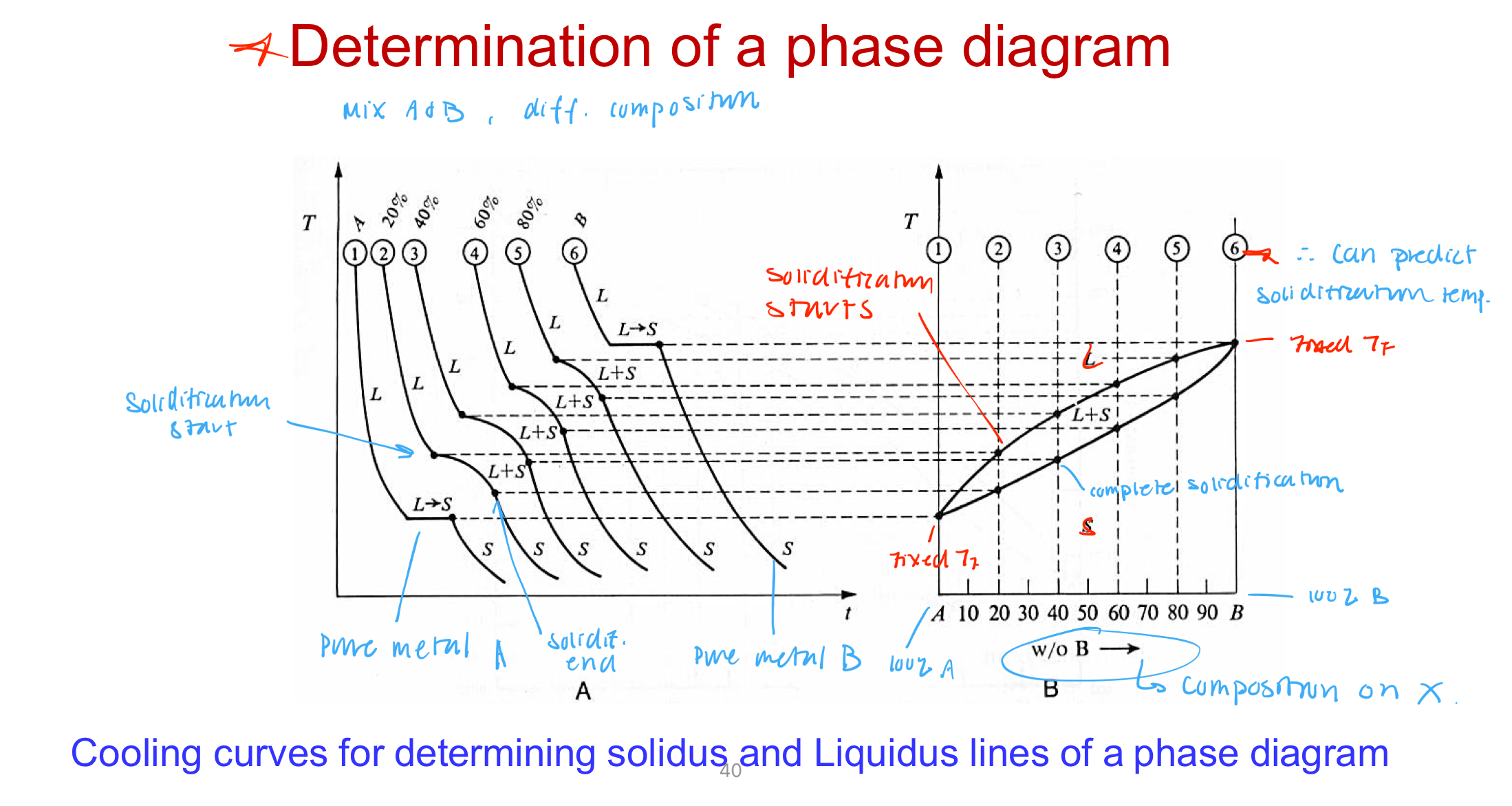
Liquidus curve: Above line, all liquid (solidification begins once you hit the curve)
Solidus curve: Below the line, all solid
Know the role of each element in dental alloy
Gold (Au)
Soft, (most) malleable and ductile
Relatively low strength
Tarnish resistant in air and water at any temp
Insoluble in sulfuric, nitric, or hydrochloric acids
Soluble in a combination of nitric and sulfuric acids (aqua-regia)
Physical properties:
E: low (weak bonds)
Tm: low (weak bonds)
CTE: large (weak bonds)
Platinum (Pt)
Tough, malleable, and ductile
Very high cost (usually replaced by Pd)
High corrosion resistance
Higher melting temp than porcelain
Physical properties:
E: higher than gold → Higher Tm and lower CTE
Palladium (Pd)
Not used in pure state
Replaced Pt in dental casting alloys
Cheaper than Pt
Helps prevent corrosion of silver in the oral environment
Physical propertiesL
E: higher than gold → Higher Tm and lower CTE
Silver (Ag) - NOT A NOBLE METAL
Malleable and ductile
Best known conductor of heat and electricity
Harder than gold
Unaltered in clean dry air
Severe tarnishing in the oral environment → Pits and porosities
Recognize the importance of some properties of the alloys
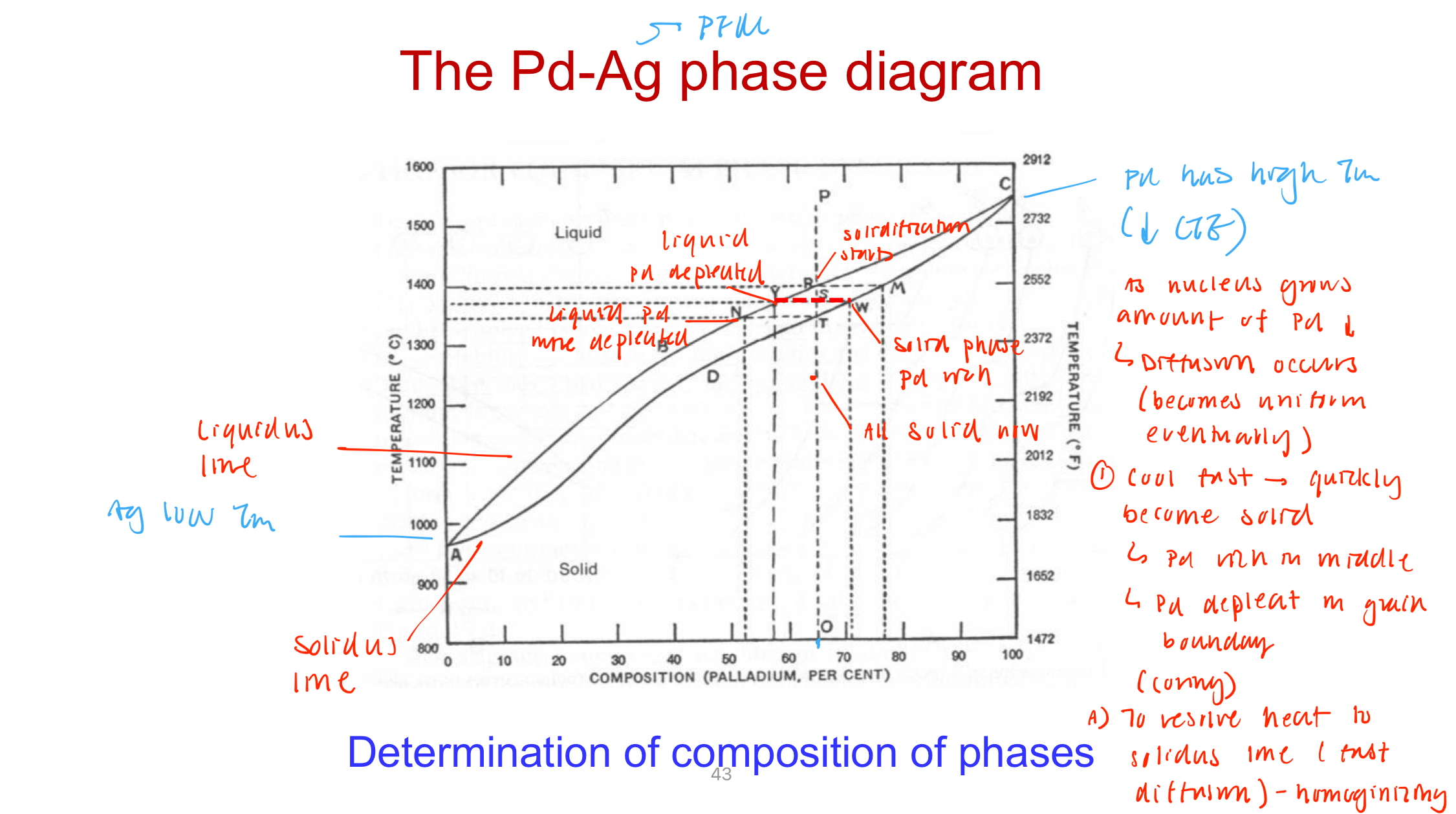
Coring: inhomogenous alloy composition because of non-equilibrium cooling rates
Happens during solidification (one element in higher concentration in the center) → can lead to corrosion
Homogenizing with heat treatment can undo the coring
Eutectic system
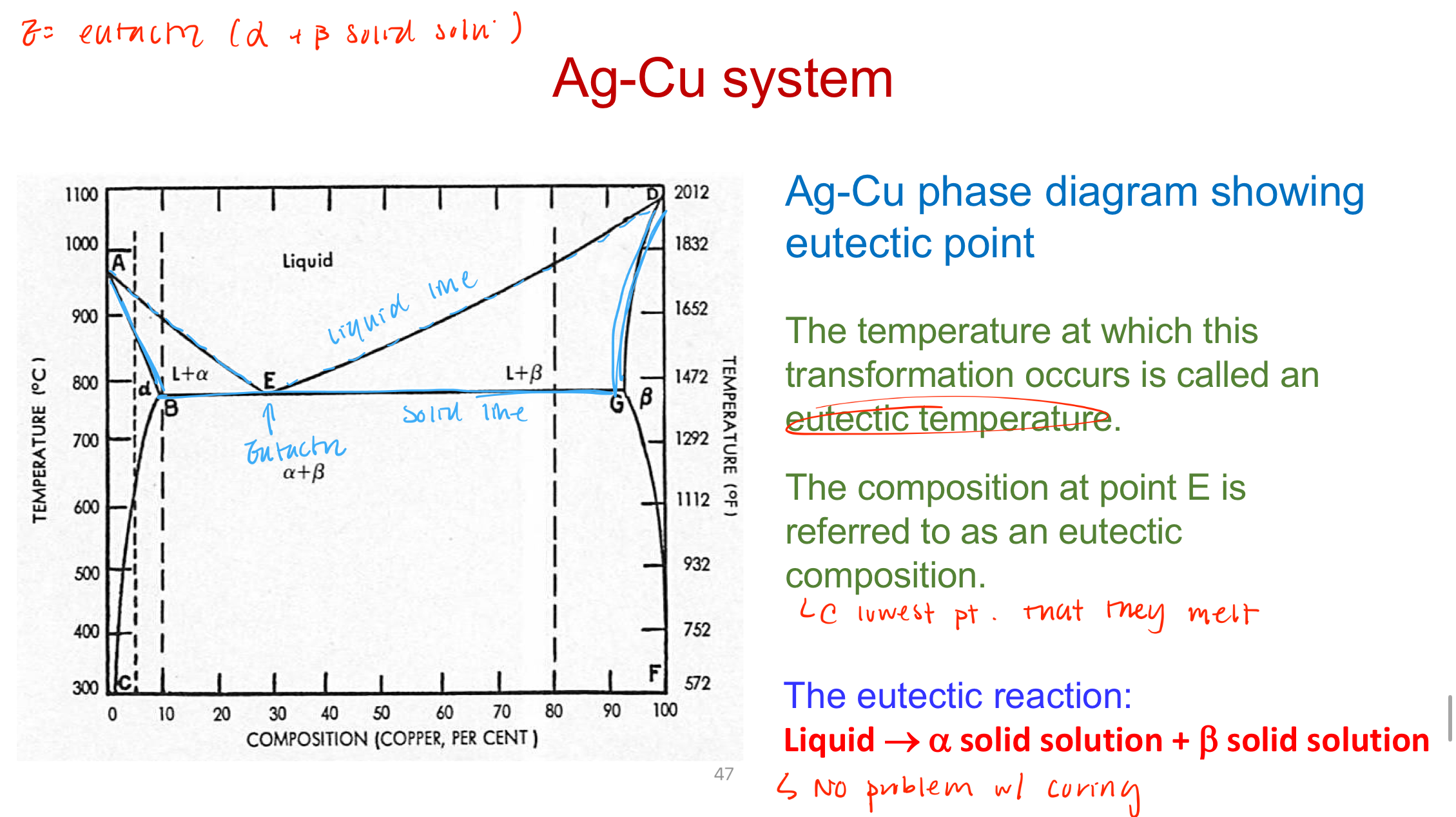
Liquid freezes and forms 2 different phases (alpha and beta) - point is known as the eutectic temperaure
Alloys in eutectic systems are…
Brittle, high hardness and strength, poor tarnish and corrosion resistance
Peritectic Alloys
Takes place between a previously precipitated phase and the liquid to produce a new solid
Liquid + Beta solid solution → alpha solid solution (susceptible to coring)
Alloys in pertectic systems are…
Susceptible to coring
Brittle
Boor corrosion resistance
Intermetallic compounds
An alloy with definite (fixed) proportions of 2 or more metals (elements in clearly defined atomic ratios)
Clearly defined stoichometry
Properties of Metals and Alloys
Cast metals and alloys = Not deformed
Wrought metals and alloys = Plastically deformed
Have changes in microstructure and physical properties
Ex/ direct filling gold, orthodontic wires, titanium dental implantss
Is deformation good or bad?
Mechanism of Deformation
Deformation occurs when bonds between atoms are ruptured
Stress needed = E/15
Large stresses required to produce slip in a perfect lattice
E/15 » YS (through experimentation) because all metals have impurities and defects
What is the reason for deformation?
What types of defects arise in solids?
Types of Imperfections
Point defects
Vacancy atoms: Distorts plane → Collapse
Interstitial atoms
Self-interstitial: Distorts plane → Extra atoms bulges lattice
Interstitial atoms: Typically smaller
Substitutional atoms
Line defects
Dislocation: “extra” half plane of atoms → Edge dislocation
Atomic arrangement next to a dislocation line is strained
Dislocation slips to proceed at stresses lower than predicted yield stress
Only need to break one bond at a time (propagates)
Movement of dislocation → slip → plastic deformation
Stops once reaches the edge of the crystal
Area defects
Grain boundaries: Impedes dislocation motion
Need more energy to overcome boundary
Volume defects
Cracks, pores, inclusions
How do defects affect material properties
Wrought
T/S: high
Ductility: low
Corrosion resistance: low
Cast
T/S: low
Ductility: high
Corrosion resistance: high
Are defects undesirable?
Not necessarily, can strengthen material
What are the methods for strengthening metals and alloys?
Strengthening Strategies
Decrease grain size: Add grain refiners
In metals… increase number of nucleation sites or pin grain boundaries by fine particles
Solid solution strengthening
Substitutional solid solution: Adds local stresses → Increase strength (stress spots making it more difficult to dislocate)
Interstitial solid solution: Interstitial strengthening can lock planes from shearing and pit the surface in compression
Precipitate strengthening
Hard precipitates are difficult to shear
Ex/ alpha titanium precip. into beta titanium → Locks up beta titanium while maintaining ductility
The closer the precipitates are, the more difficult it is to shear
Cold work: Physically plastic deforming (but becomes more brittle)
Dislocation becomes more difficult (trapped by the dislocation that happens during cold-working)
Increase YS and tensile strength
Decrease ductility (fractures more easily)
Annealing
Annealing can reduce dislocation density and increase grain size
Reverse effects of cold work
Heat metal to half its fusion temperature
Stages: recovery, recrystallization, grain growth
Application: Implants
Modern titanium implants are stronger than those from 30 years ago
Use CP4 Ti → strengthened via cold working (480 → 760)
Can return to 480 after annealing
Introduction to Dental Polymers
Uses in Dentistry
Impression materials
Denture base materials: PMMA/acrylic
Composite resin restorative materials: Bos-GMA, UDMA, Sirolane
What is a monomer?
Resins: monomer - nonmetallic materials synthesized from organic compounds that can be molded when soft and hardened for use
Plasticizers
Added to monomers to increase solubility and decrease brittleness of a polymer
Facilitates slipping of polymer chains along each other
Effects:
Reduce strength
Decrease hardness
Lower Tg
What is a polymer?
Long chain molecules consisting of many repeating units
Classification Based on Origin
Natural: Proteins and nucleic acids, polyisoprenes, polysaccharides
Synthetic: PMMA, Nylon, Teflon
Classification based on thermal behavior
Thermoplastic resins: Soften under heat and pressure and harden when cooled
No chemical reaction
Generally soluble in organic solvents
Ex/ PMMA, waxes
Thermoset resins: Harden by a chemical reaction and generally insoluble in organic solvents
Ex. Alginates, epoxy, Bis-BMA
Cross-linking
Formation of bridges between chains of polymers to form a 3D network
Effects:
Increase Tg
Increase strength
Decrease solubility
Decrease water-sorption
Physical Properties of Polymers
Degree of polymerization (DP): Average number of repeating monomeric units in a polymer molecule
Large polymer = Large DP = Increase strength
Degree of conversion: Fraction of DB converted to single bonds after polymerization
Increase degree of conversion = Increase strength
Strength Properties
Strong influence of temperature on strength
As temperature increases…
Physical properties decrease
Strength decrease
Ductility increase
Other influences: Composition, molecular weight, structure, residual monomer (conversion rate)
Biological Properties
Taste, smell, toxicity, soft tissue irritation influenced by…
Water uptake (will structurally change dimensions)
Solubility
Residual monomer (no less than 25%)
Bond to tooth structure
What is the difference between condensation polymerization and addition polymerization?
Polymerization: A series of chemical reactions by which a macromolecule is formed from a single monomer
Condensation Polymerization (step-growth)
Repeating units are joined by functional groups
Reaction is slow (hard to make large molecules)
Results in a by-product and is exothermic
Ex/ Mercaptan + lead dioxide → polysulfide rubber + lead oxide + water
Lead dioxide = catalyst (survives entire reaction)
Addition Polymerization
General steps: initiator + monomer → activated monomer → Propagation → Termination
Successive reactions between monomer molecules to form a polymer without formation of volatile by-products
Features: the presence of an unsaturated group, readily form giant molecules, chain growth can continue indefinitely, exothermic reaction
Ex/ polymerization of MMA → PMMA
Initiated by benzoyl peroxide
Activators and Inhibitors
Activators of addition polymerization: Heat, tertiary amine, light (UV, visible), Microwave energy
Inhibitors of polymerization: Impurities, hydroquinone (esp in composites), oxygen
Structure of Polymers
Linear
Branched
Copolymerization
Polymerization of 2 or more monomers to form a polymor
Dental Ceramics
Define ceramic in general terms and give examples of ceramics and their applications in dentistry
Ceramics: Solid material composed of inorganic nonmetallic compounds (ex/ pottery, clay, cements, glass)
Key characteristic - Brittle (stress concentrations at surface imperfections lead to crack initiation, propagation, and failure)
Dental ceramics
Gypsum products
Cement powders (ZnO, MgO)
Orthodontic bracket
Fillings,
Veneers
Crows and fixed pros.
Implants and abutments
Differentiate the composition and structure of crystalline and non-crystalline ceramics
Crystalline vs Non-Crystalline Ceramics
Crystalline (Ex/ Quartz, cristobalite, tridymite)
Non-Crystalline (Ex/ Amorphous SiO2 glass, fused silica)
Classes of Dental Ceramics
Silicate ceramics
Porcelain: Mimics optical properties of enamel and dentin
Feldspathic ceramics, leucite ceramics, fluor-apatite ceramics
Glass ceramics: Add crystallites to strengthen and toughen material
Leucite, lithia
Glass-Infiltrate (no longer used)
Oxide ceramics
Polycrystalline
Aluminum oxide (no longer used)
Zirconia
Non-Crystalline: Dental Porcelains
Feldspathic ceramics: Best mimics optical properties of enamel and dentin
Predominantly glassy material
Mainly feldspar, minimal clay and quartz components
Other porcelains: feldspathic porcelain, feldspar, aluminosilicate glass
Potash (with K2O) and soda (with Na2O) feldspar
Always use oxides in porcelains
By adding K2O and Na2O → Lower Tm and Increase CTE (glass modifier)
Characteristics…
Amorphous - non-crystalline
Highly translucent
Easy to make tooth shades
BRITTLE
Structure and properties of feldspathic ceramics
Low strength: Low resistance to crack initiation
Low toughness: Low resistance to crack propagation
Glass Ceramics
Leucite glass-ceramic
Add leucite to add strength and toughness
Dispersion strengthening
Lithia glass-ceramics
Lithium disilicate (has a higher crystalline content - “log” shaped)
By compressing, can force crystals to be perpendicular to applied stress
Stronger and tougher than leucite glass-ceramics but still relatively low
Best used for anterior bridge and single tooth restorations
Oxide Ceramics - Zirconia (Polycrystalline ceramic - glass free)
Much higher strength (harder to initiate crack)
Very strong, but highly opaque
Describe methods of strengthening ceramics for dental application
Leucite Reinforced Feldspar
Has a similar refractive index to porcelain
Increases strength
Faster acid etch rate → Increase mechanical interlocking with cement
Role of Components
Feldspar
SiO2 (glass network)
Oxides of potassium, sodium and calcium (glass network modifier)
Alumina
Increase strength and viscosity (glass network intermediate)
Leucite crystal formation
Metal oxides (opacity) and pigments
Fracture of Porcelain Crowns
Feldspathic ceramic crowns
Reinforcement with crystals can make it harder
Strengthening Porcelain Restorations
Strong core materials: zirconia, glass ceramics, and metals
Ex/ Porcelain veneered glass-ceramic, PFM
Strengthen via having a strong framework
List the advantages and disadvantages of ceramic material
Advantages
Natural appearance
High resistance to wear and distortion
Excellent biocompatibility (chemically inert)
Low or no corrosion
Considerably less expensive
Disadvantages
Brittle
Tensile stresses can cause crack propagation and fracture
High compressive strength (10x the tensile strength)
No dislocation motion (slip) - Ionic bonding → too much electrostatic repulsion
Hard, difficult to polish
Wear opposing teeth (harder than enamel)
Produce clicking sound on contact (especially zirconia)
Difficult to bond to denture base material