Chapter 10 - Thermochemistry
10.1 Energy as a Reactant or Product
Kinetic Energy: energy of an object in motion
Potential Energy: energy of an object because of position or composition (chemical energy)
Internal Energy: sum of all kinetic and potential energies of a system
Thermodynamics: study of energy and its transformations
First Law of Thermodynamics: energy cannot be created or destroyed but it can change from one form to another
Universe = system + surroundings
Work: force over a distance (F*d)
Internal energy of a system decreases when heat flows out of a system or if work is done by the system on its surroundings. The internal energy of a system increases when heat flows into a system or if work is done on a system. ΔE = q+w, where q is heat and w is work.
State function: internal energy depends on the potential and kinetic energy of a system, not the means of which these energies changed; ΔE = Efinal - Einitial
Heat: energy in the process of being transferred from a higher temperature object to a lower temperature one
Thermal Energy: total internal energy of a system is related to its heat
Translational Kinetic Energy: motion of particles as they collide with each other; average kinetic energy is directly proportional to its absolute kelvin temperature
Non-translational Kinetic Energy: rotational kinetic energy and vibrational kinetic energy
10.2 Transferring Energy and Doing Work
Open System: matter and energy can be transferred between a system and its surroundings
Closed System: only energy can flow between system and surroundings
Isolated System: neither matter nor energy can flow between system and surroundings
Exothermic: transfer of energy from system to surroundings, usually detected by an increase in temperature ΔH < 0
Endothermic: transfer of energy from surroundings to system ΔH > 0
Pressure-Volume Work: work associated with the expansion or compression of a gas ΔE=−PΔV
10.3 Enthalpy and Enthalpy Changes
Enthalpy: measure of the total energy of a system H=E+PV
Enthalpy Change (ΔH): quantity of heat transferred in or out from a system during a chemical reaction or phase change ΔH = ΔE + Δ(PV)=qp
Enthalpy of Fusion: energy required to convert one mole of a substance at its melting point into the liquid state
Enthalpy of Vaporization: energy required to convert one mole of a liquid substance at its boiling point into the gaseous state
10.4 Heating Curves and Heat Capacity
specific heat capacity: quantity of thermal energy required to riase the temperature of one gram of a substance by 1 degree Celsius at a constant pressure
Q=mcΔT, where m is mass, c is specific heat capacity and ΔT is the change in temperature
molar heat capacity: quantity of energy required to raise the temperature of one mole of a substance by one degree Celsius at a constant pressure, q=ncΔT where c is the molar heat capacity
The relative lengths of line segments in a heat curve represent phase changes; longer lengths correspond with greater enthalpies of phase changes
10.5 Enthalpies of Reaction and Calorimetry
Calorimetry: changes in temperature with a known heat capacity, a calorimeter, are used to determine the energy released or absorbed by a process occurring inside the calorimeter
Enthalpy of Reaction: magnitude of energy absorbed or released during a chemical reaction; proportional to quantity of reactants consumed; difference in enthalpy between products and reactants
Thermochemical Equation: adding enthalpy change that accompanies mole ratio
Coffee Cup Calorimeter: q*(rxn)=-q(calorimeter)=C(calorimeter)*ΔT=(4.18)mΔT
Bomb Calorimeter: used to measure energy released during a combustion reaction; sealed vessel capable of withstanding high pressures that is submerged in water in a heavily insulated container, oxygen is introduced and mixture is ignited. the thermal energy generated by the reaction flows into the walls of the bomb and into the water of the calorimeter
The heat capacity of the calorimeter (calorimeter constant) also needs to be known because it absorbs some of the energy from the reaction q=ΔE
10.6 Hess’s Law and Standard Enthalpies of Reaction
Hess’s Law: change in enthalpy that accompanies a process that occurs in more than one step is the sum of the enthalpy changes that occur in each of those steps
we can combine chemical equations by describing reactions in a way that gives us an equation for the final reaction by multiplying coefficients and reversing reactions, which directly impact the enthalpy value
Standard Enthalpy of Reaction: enthalpy change that accompanies a reaction under standard conditions: 1 bar of pressure and solutions with a concentration of 1 M. No standard temperature, but 25 C is common.
Standard State: most stable physical state of a substance under standard conditions
10.7 Enthalpies of Reaction from Enthalpies of Formation and Bond Energies
Standard Enthalpy of Formation: relative enthalpy change for a formation reaction; pressure of 1 bar when one mole of substance is formed from constituent elements in their standard states - will be a value of 0.
Breaking bonds is endothermic and forming bonds is exothermic; exothermic reactions occur when more energy is released forming bonds than energy needed to break bonds; endothermic reactions occur when more energy is consumed in breaking bonds than released in forming bonds
Bond energy: enthalpy change that occurs when one mole of bonds in the gas phase is broken

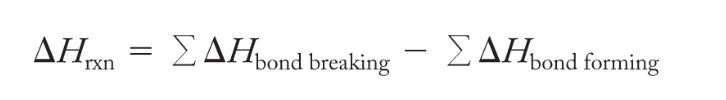 10.9 More Applications of Thermochemistry
10.9 More Applications of Thermochemistry
Fuel Values: energy produced during the combustion of 1 gram of a substance
as the number of carbon atoms per molecule increases, the hydrogen to carbon ratio decreases, making fuels less effective
Fuel Density: quantity of energy released per unit volume of a liquid fuel
 Knowt
Knowt
