Chapter13:Acid and Bases
Arrhenius concept - acids produce hydrogen ions in aqueous solution, while bases produce hydroxide ions \n Brønsted–Lowry model - an acid is a proton (H+) donor, and a base is a proton acceptor
conjugate base - everything that remains of the acid molecule after a proton is lost \n conjugate acid - formed when the proton is transferred to the base \n conjugate acid–base pair - two substances related to each other by the donating and accepting of a single proton
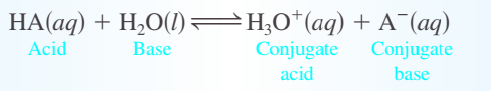
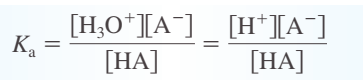 Ka - acid dissociation constant
Ka - acid dissociation constant
Brønsted–Lowry model is not limited to aqueous solutions but also reactions in gas phase
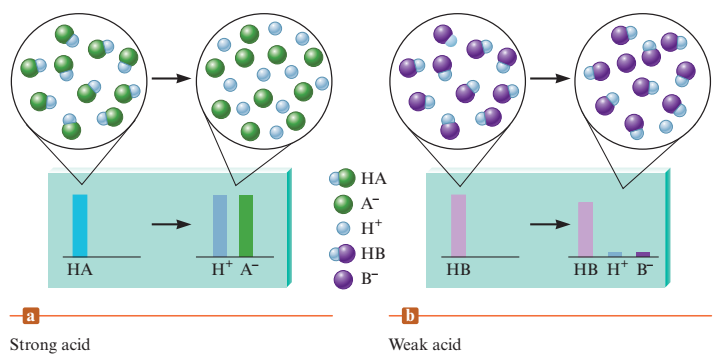
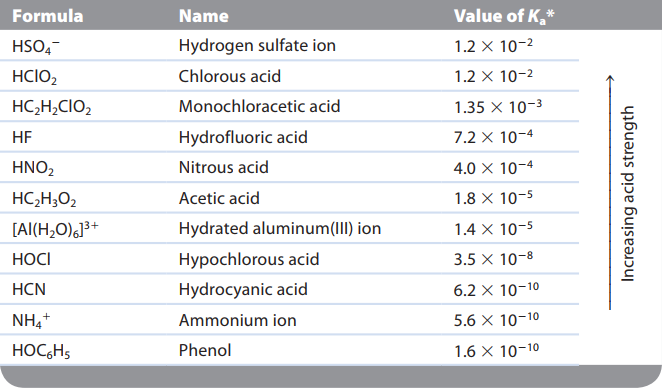
Acid strength - equilibrium position of its dissociation(ionization) reaction
strong acid - equilibrium lies far to the right
weak acid - equilibrium lies far to the right \n ”strong acid yields a weak conjugate base”
diprotic acid - acid having two acidic protons
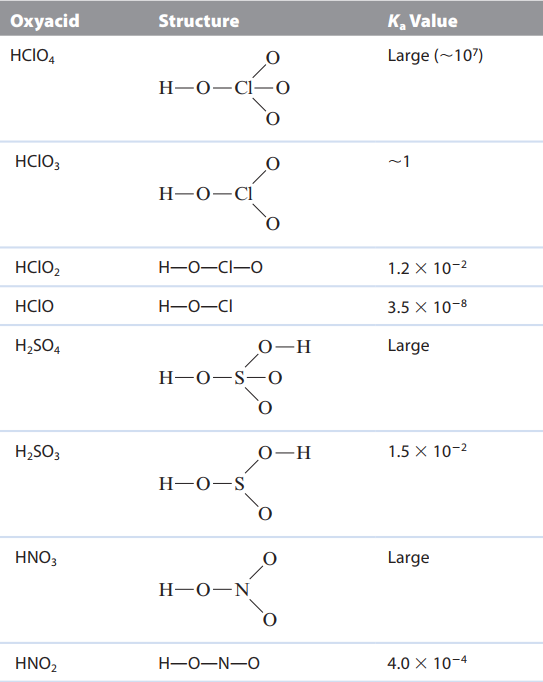
ex. H2SO4 \n oxyacids - acidic proton is attached to an oxygen atom
ex. H3PO4, HNO2, HOCL
Organic acids - acids with a carbon atom backbone, commonly contain the carboxyl group \n ex. acetic acid (CH3COOH), benzoic acid (C6H5COOH)
monoprotic acids - acid having one acidic protons
Vinegar contains acetic acid and is used in salad dressings. What if acetic acid was a strong acid instead of a weak acid? Would it be safe to use vinegar as a salad dressing?
\n amphoteric - can behave either as an acid or as a base \n Water is the most common amphoteric substance
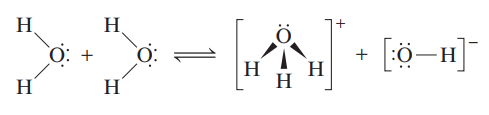 Kw - ion-product constant (or the dissociation constant for water)
Kw - ion-product constant (or the dissociation constant for water)
a neutral solution, where H = OH
an acidic solution, where H > OH
a basic solution, where H < OH
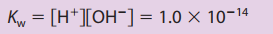
pH scale - represent solution acidity \n 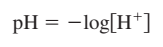 acid–base equilibria - must focus on the solution components and their chemistry \n ”focus on the major species, those solution components present in relatively large amounts”\
acid–base equilibria - must focus on the solution components and their chemistry \n ”focus on the major species, those solution components present in relatively large amounts”\
Calcium hydroxide, Ca(OH)2, often called slaked lime
“Kb always refers to the reaction of a base with water to form the conjugate acid and the hydroxide ion“ \n 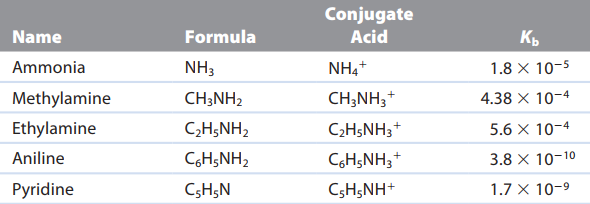
polyprotic acid always dissociates in a stepwise manner, one proton at a time
triprotic acid - acid having three acidic protons
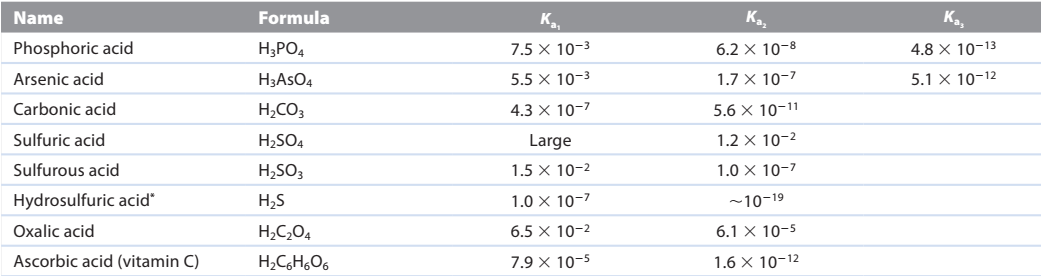
What if the three values of Ka for phosphoric acid were closer to each other in value? Why would this complicate the calculation of the pH for an aqueous solution of phosphoric acid?
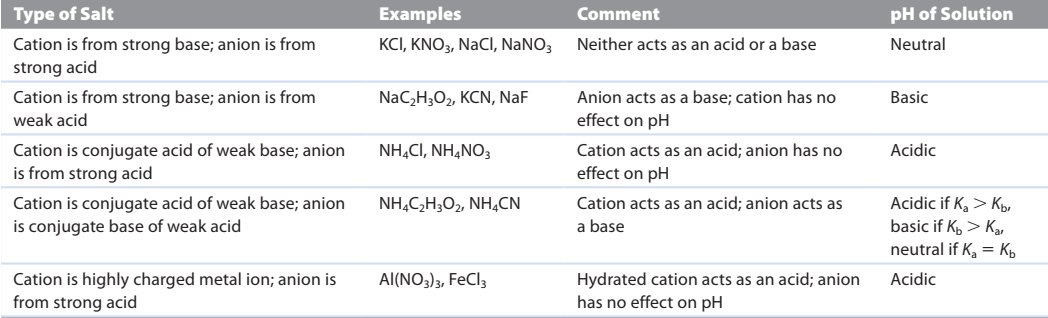
Salt or ionic compound
Salts that consist of the cations of strong bases and the anions of strong acids have no effect on [H+] when dissolved in water
salts in which the anion is not a base and the cation is the conjugate acid of a weak base produce acidic solutions
 Lewis Acid-base model - An even more general model for acid–base behavior \n Lewis acid - an electron-pair acceptor
Lewis Acid-base model - An even more general model for acid–base behavior \n Lewis acid - an electron-pair acceptor
Lewis base - an electron-pair donor \n 
common ion effect - shift in equilibrium position that occurs because of the addition of an ion already \n involved in the equilibrium reaction \n buffered solution - resists a change in its pH when either hydroxide ions or protons are added \n 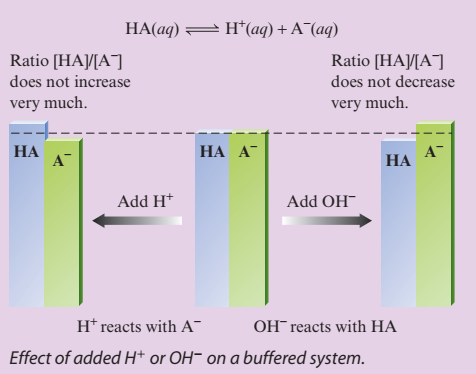
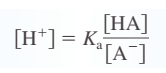
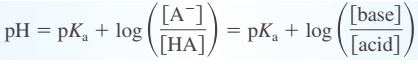
buffering capacity - amount of protons or hydroxide ions the buffer can absorb without a significant change in pH
“The pH of a buffered solution is determined by the ratio [A-]y[HA]. The capacity of a buffered solution is determined by the magnitudes of [HA] and [A-]“
pKa of the weak acid to be used in the buffer should be as close as possible to the desired pH \n \n \n \n \n \n \n \n
\n \n
\n \n \n \n
\n \n \n \n \n \n \n

