AP Chem unit 3
Topic 1: Intermolecular forces
London dispersion forces: Usually the weakest except in large molecules
found in all molecules
more electrons = more polarizable = stronger LDF’s
Questions: which of these molecules has stronger LDF’s CH4 or C4H10?
C4H10 because it has more electrons than methane and a higher boiling point meaning that C4H10 has strong LDF’s
Rank these non-polar molecules in order of increasing boiling point, C3H8, Ar, and He? (Think of polarizability, the more polarizable it is the higher the boiling point.)
He, Ar, C3H8
Since He has the fewest electrons it’s the least polarizable meaning it has the lowest boiling point, next would be Ar would be next because it has the second least amount of electrons; and the second lowest boiling point. Putting C3H8 after because it has the most electrons and the highest polarizability giving it the highest boiling point.
Dipole-Dipole forces: Moderate in strength, found in polar molecules only.
Dipole-dipole forces are stronger than LDFs but given similar numbers of electrons, the polar molecule should have a higher boiling point than the nonpolar molecule.
Having multiple of one molecule, HCl, H being the partial positive and Cl being the partial negative, we see dipole-dipole forces between the two different molecules.
The H of one molecule will be attracted to the Cl of another molecule.
Question: Which molecule has the higher boiling point. NCl3 or C4H10?
NCl3 is a polar molecule and C4H10 is nonpolar, meaning that NCl4 has LDFs present as well has having dipole - dipole forces while C4H10 only has LDFs; because of this NCl3 has the higher boiling point.
Hydrogen Bonding: Very strong, Hydrogen atoms take up very little space; because of this they’re able to get really close to other atoms. The closer the distance between molecules the stronger the forces of attraction.
Can only bonds with O-H, O-N, or F-H
Explains why polar molecules with F, O, or N usually dissolve in water, because they can hydrogen bond with itself
Question: A chem student observes that NH3 (g) readily dissolves in water, while PH3 (g) does not. Explain this phenomenon in terms of intermolecular forces?
NH3 can hydrogen bond with water,, because it’s central N atom is both small and very electronegative. PH3 has only weak attractions to water. It cannot hydrogen bond to water, because the P atom is larger and is not nearly as electronegative N.
Question: Rank these compounds in order of increasing boiling point. NO2, CO2, and H2O?
CO2, NO2 then H2O
CO2 only has LDFs meaning it has the lowest boiling point out of the three. Next is NO2 because it has dipole-dipole because it is polar molecule, the H2O is last because it has hydrogen bonding making it have the strongest attraction and highest boiling point.
Iconic force: These are ions that stick together because of electrostatic (ionic) forces, this makes iconic forces have the highest melting point and boiling point.
Think about charge differential, then iconic size
Review question: For each following compounds, state which type of intermolecular forces would be present?
HNO3, LDFs and hydrogen bonding
BaSO4, LDFs and ionic forces
CH4, just LDFs
NF3, LDFs and dipole-dipole forces
Rank above structures in order of increasing boiling point.
CH4, NF3, HNO3, then BaSO4
LDFS, dipole-dipole, hydrogen bonding, then iconic forces
Topic 2: properties of solids
Vapor pressure: The pressure exerted by a vapor back down on the liquid from which it evaporated.
As temperature increases, the vapor pressure of a liquid increases.
liquids with weaker intermolecular forces will have a higher vapor pressure because they will evaporate easier. This is because of the low boiling point.
You can change the boiling point of most liquids just by changing the pressure.
Weaker intermolecular forces = higher vapor pressure
Iconic solids: Composed of iconic compounds in a crystal lattice.
Brittle and can be easily shattered because any force that lines up like charges will result in the charges rebelling and breaking the structure.
Conduct electricity when dissolved in water or melted, because charged particles will allow electrons to pass through easily.
Molecular solids: Solids composed of covalently-bonded compounds.
Intermolecular forces are very weak, so these solids have low melting and boiling points
Covalent Network solids: A repeating network of covalent bonds creates an extremely strong structure that’s very difficult to break.
extremely high melting and boiling points due to their strong covalent bonds, often oriented in multiple directions. Higher than iconic bonding.
Question: Why does carbon dioxide (CO2) have such a low boiling point and silicon dioxide (SiO2) have a high boiling point?
Carbon dioxide is composed of individual molecules containing covalent bonds. Though silicon dioxide has covalent bonds, it consists of a covalent network solid. The repeating lattice bonds in multiple directions gives it extra strength, giving it an extremely high boiling point.
Topic 3: Solids, liquids, Gases
True solids: Crystalline in structure, called a crystal lattice.
Amorphous materials: These are materials that are not crystalline, but have other properties of solids.
i.e. plastic, paper, glass
Gas: Very far a part from each other and have the space to move and bounce around.
because they’re far apart we are able to squeeze the molecules and compress the gas.
All gases have four properties that we need to be aware of, the ones we will be manipulating.
Pressure: Determined by how often the gas molecules collide with the container walls. Usually measured in atmospheres (atm).
Volume: The amount of space the gas takes up, usually measured in liters
Temperature: A Measure of the average kinetic energy (mass and velocity) of the molecules.
Number of Gas molecules: Usually measured in moles.
Topic 4: Fundamental gas law, ideal gas law, Dalton’s law of Partial Pressures
Fundamental gas law:
Boyle’s Law: The pressure of a gas is inversely proportional to its volume, when one volume increases the pressure decreases.
P1V1=P2V2
Question: A balloon is filled with 30 liters of Helium gas at 1 atm. What is the volume when the balloon rises to an altitude where the pressure is only 0.25?
P1V1=P2V2
(1atm)(30liters) = (.25atm)(?)
V2=120liters
Charle’s law: The volume of a gas is directly proportional to its temperature, when volume increases so will the temperature and vise versa.
V1/T1 = V2/T2
This only works if were talking about the temperature in Kelvins, C+273=K
Question: A balloon in an air-condition room at 27C, is inflated to a volume of 4.0L. It’s then heated to a temperature of 57C. What is the new volume of the balloon if the pressure remains the same?
V1/T1 =V2/T2
4.0L/300k = (?)/330k
Cross multiplication: 300(V2) = 1320
V2=4.4L
Gay-Lussac’s Law: The pressure of a gas is directly proportional to it’s temperature, if pressure increases then so will temperature and vise versa.
P1/T1 = P2/T2
temperature has to be in kelvins, C+273=k
Questions: A tire has a pressure of 20.0 p.s.i when the temperature is -17C. What will be the tire pressure when the temperature goes up to 30C?
P1/T1 = P2/T2
20.0 p.s.i/ 256k = (?)/ 303k
Cross multiplication = 256k x P2 = 6060
P2 = 23.67 p.s.i
Hard part about solving gas law problems is knowing what law to use.
Question: We have a sealed zippered plastic bag that has 1.00 liters of air sealed inside at a room temperature of 21C. When the bag is placed into a freezer at a temperature of -18C, what will it’s volume be?
Charle’s law = V1/T1 = V2/T2
1.00L/294k = (?)/ 255k
Cross multiplication: 294k x V2= 255
divide by 294k
V2= 0.867L
Combined gas law: The first three gas laws put together.
(P1)(V1)/T1 = (P2)(V2)/T2
Use if all three factors are changing at the same time.
Question: A container with an initial volume of 1.0 L is occupied by a gas at a pressure od 1.5 atm at 25C. By changing the volume, the pressure of the gas increases at 6.0 atm as the temperature is raised to 100C. What is the new volume?
(P1xV1)/T1 = (P2xV2)/T2
(1.5 atm)(1.0L)/(298k) = (6.0 atm)(?)/(373k)
Cross multiplication: 1788 (V2) = 559.5
divide by 1788
V2= 0.313 liters
Ideal gas law:
PV=nRT
P: Pressure (atm)
V: Volume (Liters)
n: number of moles of gas
R: Universal Gas Constant (.0826 Liters x atm/moles x Kelvin)
T: Temperature (kelvins)
Questions: A rigid steel container with a volume of 20.0 liters is filled with nitrogen gas to a finale pressure of 200 atm at 27C. How many moles of N2 gas does the cylinder contain?
PV=nRT
(200atm)(20.0L) = (n)(.0821 Lxatm/molxk)(300k)
cross multiplication: 4000 = 24.63n
divide by 24.63
n= 162 moles
Question: What volume will 12.0 grams of Oxygen gas (O2) occupy at 25C and pressure of 0.520 atm?
PV=nRT
12.0g O2 × 1mol/32g = 0.375mol O2
(0.520atm)(V) = (0.375mol)(.0821 Lxatm/molxk)(298k)
cross multiplication: 0.520 (V) = 9.175
divide 0.520
V=17.6 liters
Question: In grams per liter, calculate the density of oxygen gas (O2) at 25C and a pressure of 1.00 atm.
to find number of moles you take grams and divide by the molar mass, in this equation we’ll substitute n with grams divided by the molar mass. g/MM
PV = g/MM RT
rearrange so we get grams per liter int he equations
P(MM)/RT = g/V
(1.00atm)(32.00g/m)/ (Universal constant)(298k) = g/V
1.31g/L
Partial Pressure of a Gas:
Dalton’s law of partial pressure: Partial Pressure(gas) = X(gas) x P (total)
The pressure that a specific gas is responsible for in a gas mixture
X = mole fraction the gas is occupying in the mixture
P = The total pressure in the container
Question: A flask has a total pressure of 1.25 atm. If the flask contains 7.2 moles of water vapor and 12.9 moles of carbon dioxide gas, then what is the partial pressure of (a) water vapor? (b) Carbon Dioxide?
P(gas) = X(gas) x P(total)
a
PH2O = (7.2 mol H2O / 20.1 total mol) x 1.25 atm = 0.448 atm
b
PCO2 = (12.9 mol CO2 / 201.1 total mol) x 1.25 atm = 0.802 atm
do the partial pressure add up to the total pressure. 0.488 atm + 0.802 atm = 1.25 atm
Topic 5: Kinetic - molecular theory
Raise the temp the molecules move faster
Heat and temp. are related but not the same thing
Temperature: A numerical measure of the average kinetic energy in the molecules of a material
Measured in kelvins, shows us the kinetic energy of the molecules.
Heat: The form of energy transferred between two systems at different temperatures, also called thermal energy.
measure in joules.
Grahams law of Effusion: The rate of movement of gas particles is inversely proportional to their molecular mass.
Rate of A over Rate of B = the square root of the molecular mass of B over the square root of the molecular mass of A
Should choose lighter gas as mass A
Lighter gases move faster and heavier gases move slower.
Effusion: The escaping of molecules through a very tiny hole in a material.
Helium balloon losing helium throughout the tiny holes of the balloon.
Grahams law tells us that heavier molecules move slower.
Question: Which gas effuses faster: Nitrogen or Helium? how much faster?
Nitrogen’s atomic mass is 28.01 because it’s diatomic, Helium’s atomic mass is 4.00
Helium will be rate A and Nitrogen will be Rate B
Rate A He square root of 28.01
= divided by = 2.65
Rate B N2 square root of 4.00
Helium effuses 2.65 times as fast as nitrogen gas.
Question: Helium can effuse 2.245 times as fast as a gas with what molecular mass?
Rate A He square root of x
= divided by = 2.245
Rate B x square root of 4.00
setting the 2.245 over one to cross multiply give us square roots of x = 4.490
square both sides we get x=20.2 amu
Gas would be neo because it’s atomic mass is close tot 20.2 amu
Topic 6: Deviation of ideal gas law
Ideal gas
A gas that has molecules that take up no space
A gas that has molecules with no intermolecular attractions for each other
Under some circumstances, real gases can approximate ideal conditions.
Very small molecules, such as He, N2, and Ne take up very little space and can be close to ideal.
Under certain conditions other molecules can be close to not having a attraction to each other. these conditions include high temperature and low pressure.
Van der Waals equation:
Compensates for the difference between real gases and ideal gases.
not an equation that will be asked to solve, but we must know why it’s used and the conditions real gases approximate ideal gases
(P+n²a over V²) (V-nb) = nRT
A is a Factor that corrects for intermolecular forces.
B is a Factor that correct for the size of the particles themselves.
Topic 7: Solutions and mixtures
Mixtures: materials that contain more than one substance, there are two types Heterogeneous and Homogeneous.
Heterogeneous: The individual phases, components, and visible to the naked eye.
Homogenous: Components are mixed together uniformly, cannot see the individual components that make up the mixture.
commonly called solutions
focusing on solids in water
Concentration: how much solute is dissolved in the solid
Solute is solid and solvent is usually water
Focusing molarity
Molarity: is the primary unit of solution concentration in chemistry.
Molarity = Moles of solute divided by the liters of solution
Question: A student produces a solution by adding 0.711g tin(II) chloride to enough distilled water to make 75.0mL of solution. Calculate the molarity of this solution.
Convert 0.711g SnCl2 to moles. 0.711g SnCl2 × 1mol/189.62g SnCl2 = .00375 mol
Convert 75.0mL to Liters. 75.0mL x 1liter/1000mL = .0750 L
divide .00375mol/.0750L = Molarity (M) = 0.0500
Question: What is the molarity of a solution that was prepared by dissolving 14.2g NaNO3 into enough water to make 350mL of solution.
Convert 14.2g NaNO3 into moles. 14.2g NaNO3 × 1mol/85.0g = 0.167 moles
Convert 350mL into Liters. 350mL x 1Liter/1000mL = 0.35 Liters
divide 0.167moles/0.35Liters = M = 0.477M
Sometimes you’re given the molarity and either grams or moles and the volume that you have to solve for the other.
Question: A small beaker contains 50.00 mL of 0.250 M NaOH solution. If this solution is allowed to dry, how many g of NAOH(s) can be retrieved?
Convert to 50.00 mL to Liters. 50.00 × 1Liter/1000mL = 0.05000 L NaOH
Convert to moles. 0.05000L NaOH x 0.250moles/1Liter = mol = 0.0125mol
Then you convert 0.0125mol to grams. 0.0125 x 40.0g/1mol=0.500g NAOH
The 0.250 M means Moles over liters which is why we can use it as a conversion factor to find moles in this equation.
remember molarity x liters = moles, the 0.05000 × 0.250 = 0.0125
Question: how many grams of NaBr are needed to prepare 700mL of 0.230M NaBr solution?
Convert to 700mL to liters. 700ml x 1L/1000mL = 0.7L
Convert to moles. 0.7L x 0.230moles/1L = 0.161 moles
Then convert Moles to grams. 0.161 × 102.9g/1mol = 16.6g NaBr
Remember that molarity x liters = moles
Question: A chemist needs to produce 500.0mL of 0.250 M HCl solution. How many ml of concentrated 12.0M HCl solution should be used to create the desired solution?
Convert to moles. 500.0mL x 0.250M = 0.125mols
Use moles to determine to volume. Rearrange the molarity equation so that L = moles divided by molarity. 0.125mol/12.0M = L = 0.0104 L
Convert L to mL. 0.0104 × 1000 = 10.4mL
Another equation to make this easier
M1V1=M2V2 also known as the dilution equation, this equation cuts out the conversion of L to mL because if you put ml in for one part of the equation then mL will come out and vice versa.
plug numbers into equation. (0.250M)(500.0mL) = (12.0M)(V2=X)
solve and you still get V2=10.4mL
Topic 8: Representations of Solutions, Electrolytes, Nonelectrolytes and dissociation into ions
Components of solution:
Solvent, what is doing the dissolving
solute, what is being dissolved
water is the universal solvent
Dilute: A solution with a relatively low amount of solute
Concentrated: Have a relatively high amount of solute in solution
Saturated: Concentrated solutions that have dissolved the maximum amount of solute for that temperature.
you can tell when there is solids that sinks down to bottom of the container.
Supersaturated: Concentration that has temporarily dissolved more than the maximum amount of solute for that temperature.
usually an unstable and it’ll flash crystallize.
Mole fraction (X): X(substance) = Moles(substance) divided by Moles(total)
is another way to calculate concentration. Aside from Molarity.
X(substance) = mole fraction of the substance - a ration with no unit.
Moles(substance) = moles of the substance
moles(total) = Sum of the moles of all the components in the solution including the solvent.
Question: Calculate the mole fraction of sucrose (C12H22O11) in a solution where 10.00g of sucrose is dissolved in 400.0g of water
find the moles for the 10.00g of sucrose. 10.00g C12H22O11 × 1mol/342.30g = 0.02921mol
find the moles for the 400.0g of water. 400.00g H2O x 1mol/18.01g = 22.20mol H2O
Moles of sucrose divide by total moles of solution. 0.02921/0.02921 + 22.20 = 1.214 × 10^-3
Question: Mole fraction of MgCl2 and H2O if 2 moles of MgCl2 are dissolved in 1000g of H2O.
Convert H2O to moles. 1000g H2O x 1mol/18.01g = 55.53 mol H2O
Use mole fraction equation 2/2+55.53 = 0.034
Electrolytes, Nonelectrolytes and dissociation into ions
aqueous: dissolved in water, (aq) written after substance means that that substance has been dissolved into water.
NaCl(s) means it’s a solid, NaCl(aq) means it’s dissolved into water
since NaCl is ionic it’s components will break apart when dissolved leaving it as. Na+(aq) +Cl-(aq)
Ionic compounds are strong electrolytes, meaning when they dissolve in water they dissociate completely into their component ions.
Happens when polar molecules (water) surround ions and pull them away from ionic crystal lattice.
When an ion is positively charged then the negatively charged oxygen in the water will surround that ion and pull it away from the lattice. When an ion is negatively charged than the positively charged Hydrogen in the water will pull it away from that lattice structure.
This is called an ion-dipole force, only happens when the ion-dipole force is stronger than the ionic force in the compound.
Na attracts the oxygen and Cl attracts the hydrogen
When writing chemical equations, write soluble ionic compounds in ionic form.
Zn(NO3)2 = ZN^+2 + 2NO3
Na2S = 2Na+1 + S^-2
Compounds that should be written in ionic form when in aqueous solution
Strong acids
HCl, HBr, HI, HNO3, H2SO4, HClO4
dissociate completely and written in ion form
Strong bases
LiOH, NaOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, Ba(OH)2
dissociate completely and written in ion form
If you see a compound with N and H they’re weak bases
All other molecular compounds, including water, are nonelectrolytes and hardly ionize at all.
Strong electrolytes conduct electricity very well when dissolved in water.
Topics 9 and 10: Chromatography, Distillation and Solubility
Ways to separate mixture:
Distillation: Separating two mixtures with different boiling points by heating them up so one will boil away and another will stay in the container, the one that boils away will be condensed into another container.
Chromatography: Running a mixture through a column, the components will pass through the column at different rates. depending on if you put something on the column to mediate the process will can also show you if molecules are polar or non-polar.
How do we know somethings going to dissolve into something else:
“Like dissolves Like”
Polar molecules like NH3 will dissolve easily in polar solvents like H2O
Nonpolar molecules like CH4 will dissolve easily in nonpolar solvents like CCl4
Don’t use like dissolves like as an answer because it doesn’t explain the chemistry.
Question: Which of the following molecules would be expected to dissolve in water.
CF4 or NaCl
CF4 is perfectly balanced out making it nonpolar so it won’t dissolve in polar molecules
NaCl Is polar so it will dissolve in water which is also polar
Solubility of solids Vs. temperature:
Normal relationship between the solubility of solids and temperature is a positive correlation, As you raise temperature the solid becomes more soluble.
Solubility of gases Vs. temperature
As you raise the temperature of a solution the amount of gas that will dissolve decreases giving solubility of a gas and temperature a negative correlation.
Solubility of Gases Vs. pressure:
The more pressure a container of solution has the more gas will dissolve in the solution
Henrys law: The solubility of a gas in a liquid is directly proportional to the pressure of the gas directly over the solution
carbo dioxide in soda when the cap is closed puts pressure on the liquid which dissolves the carbon dioxide. When you release the pressure the solubility of the gas decreases and you’ll start to see fizz or bubbles in the soda.
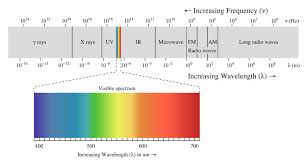
Topic 11: Waves, Light and Photons (spectroscope and electromagnetic spectrum)
Every color of correlates to a wavelength
Visible light: Is a small part of the electromagnetic spectrum, ROYGBIV, right of visible light we get infrared, microwaves and radio waves. Left of visible light we get UV, X-rays, and Gamma rays.
UV and Gamma have more energy than visible light.
Infrared and radio waves have less energy than visible light.
 Max Planck: Studied electromagnetic radiation and the nature of light.
Max Planck: Studied electromagnetic radiation and the nature of light.
Any time of electromagnetic energy can only be emitted only in quantized form
energy and light can only exist in “packets” we call photons
Types of radiation and their effects:
UV or visible light: Causes electrons to transition or jump to different energy levels
Infrared radiation: Causes molecules to vibrate
Microwave radiation: Causes molecules to rotate
Topic 12: Wavelengths and how we calculate them
We can calculate the wavelength by taking the distance from on crest on the wave to another crest.
we measure wavelength in meters and the symbol for wavelength is λ.
short wavelengths have higher frequency
always in motion
Frequency: How many wave cycles hit a point per second, the symbol for frequency is v.
Electromagnetic waves move at the speed of light.
We can use this equation, c = λv, to relate the relationship between wavelength and frequency.
C is speed of light and it’s equation is 3.00 × 108 m/s
λ is wavelength in meters, m.
v is frequency, wave cycles per second measured in Hertz
Wavelength and frequency have an inverse relationship.
Question: An FM radio station broadcast on a frequency of 96.9 MHz. Determine the wavelength of the radio waves emitted by this station’s antenna.
c = λv
Don’t forget to convert megahertz to hertz.
(3.00 × 108 m/s) = (λ)(96,900,000 s^-1)
3.09m=λ
Question: A red laser pointer emits light at a steady wavelength of 670nm. what frequency light is emitted by this pointer.
c = λv
(3.00 × 10^8 m/s) = 670 × 10^-9 m) (v)
4.5 × 10^14 Hz
Equation to figure out the energy that is being packed into a photon of light.
E = hv
E is energy of a single photon is joules, v is frequency in wave cycles per second, h is planks constant which is 6.63 X10^-34J-s
energy and frequency have a directly proportional relationship
if we know the frequency we can figure out the energy of a photon and vice versa
Question: If an AM radio station broadcast on a frequency of 1340 kHz, determine the energy ore photon in its electromagnetic waves.
E = hv
E = (6.63 x 10^-34 J-s)(1340 × 10³)
kHz is 1,000 Hertz
E = 8.88 x 10^-28 Joules
Topic 13: Beer-Lambert Law and spectrophotometry
Spectroscopy: Using color intensity to determine the concentration of a solution.
We use a spectrophotometer to determine concentration