Acids, alkalis and salts
Key Vocabulary
litmus – a single indicator for acid and alkali (red – acid, blue – alkali)
phenolphthalein – a single indicator for acid and alkali (colourless – acid, pink – alkali)
methyl orange – a single indicator for acid and alkali (red – acid, yellow – alkali)
pH – a scale from 0–14 measuring how acidic or alkaline a solution is
aqueous – meaning dissolved in water
proton – another term for a hydrogen ion
hydrogen ion – an atom of hydrogen that has lost an electron to have an overall positive charge
acid – a source of hydrogen ions when in solution
alkali – a source of hydroxide ions when in solution
titration – an experiment to determine the volumes of solutions (usually acid and alkali) that react together to produce a pure solution
volumetric pipette – a glass piece of apparatus for measuring one volume accurately
burette – a piece of apparatus used to add and measure volumes of liquid
solubility – a measure of how well a substance dissolves
soluble – something that dissolves in water
insoluble – something that does not dissolve in water
pure – describing a sample that is one single compound or element
excess – describing that there is more than is needed
saturated – a solution in which no more of a solute can dissolve
crystallisation – a technique used to prepare hydrated salts from a solution
residue – the solid collected after filtration
filtrate – solution collected after filtration
precipitate – an insoluble solid formed when two solutions are mixed
decant – to allow solid in a solution to settle at the bottom and then pour off the liquid to leave behind just the solid
Indicator colours
Solution | Methyl Orange | Phenolphthalein | Litmus Solution |
|---|---|---|---|
NaOH(aq) | Yellow | Pink | Blue |
HCl(aq) | Red | Colourless | Red |
NaCl(aq) | Orange | Colourless | Purple |
Word and balanced symbol equations
a. Magnesium + sulphuric acid → magnesium sulphate + hydrogen
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
b. Copper(II) carbonate + nitric acid → copper(II) nitrate + carbon dioxide + water
CuCO3(s) + 2HNO3(aq) → Cu(NO3)2(aq) + CO2(g) + H2O(l)
c. Sodium hydrogencarbonate + hydrochloric acid → sodium chloride + carbon dioxide + water
NaHCO3(aq) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l)
d. Sulphuric acid + potassium hydroxide → potassium sulphate + water
H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l)
e. Nitric acid + ammonia solution → ammonium nitrate
HNO3(aq) + NH3(aq) → NH4NO3(aq)
Preparation of salts:
A salt is an ionic compound formed when the hydrogen ions of an acid are replaced by metal ions or ammonium ions.
Four methods of salt preparation:
Excess solid method – Add excess insoluble solid (metal or base) to an acid, then filter off unreacted solid. Forms soluble salts (not Group I or ammonium salts).
Titration method – Use exact volumes of acid and soluble base with an indicator. Forms soluble salts that are Group I or ammonium salts.
Precipitation method – Mix two soluble salts to produce an insoluble salt.
Direct combination – Combine elements directly (limited use, for some anhydrous salts).
Examples and methods:
NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(l)
Method: titration (soluble base + acid, produces Group I salt)
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Method: excess solid (insoluble calcium carbonate + acid, then filtration)
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
Method: precipitation (mix two soluble salts to form insoluble BaSO4)
2Fe(s) + 3Cl2(g) → 2FeCl3(s)
Method: direct combination (metal + non-metal)
CuO(s) + 2HNO3(aq) → Cu(NO3)2(aq) + H2O(l)
Method: excess solid (insoluble copper oxide + acid, then filtration)
2NH3(aq) + H2SO4(aq) → (NH4)2SO4(aq)
Method: titration (soluble base + acid, forms ammonium salt)
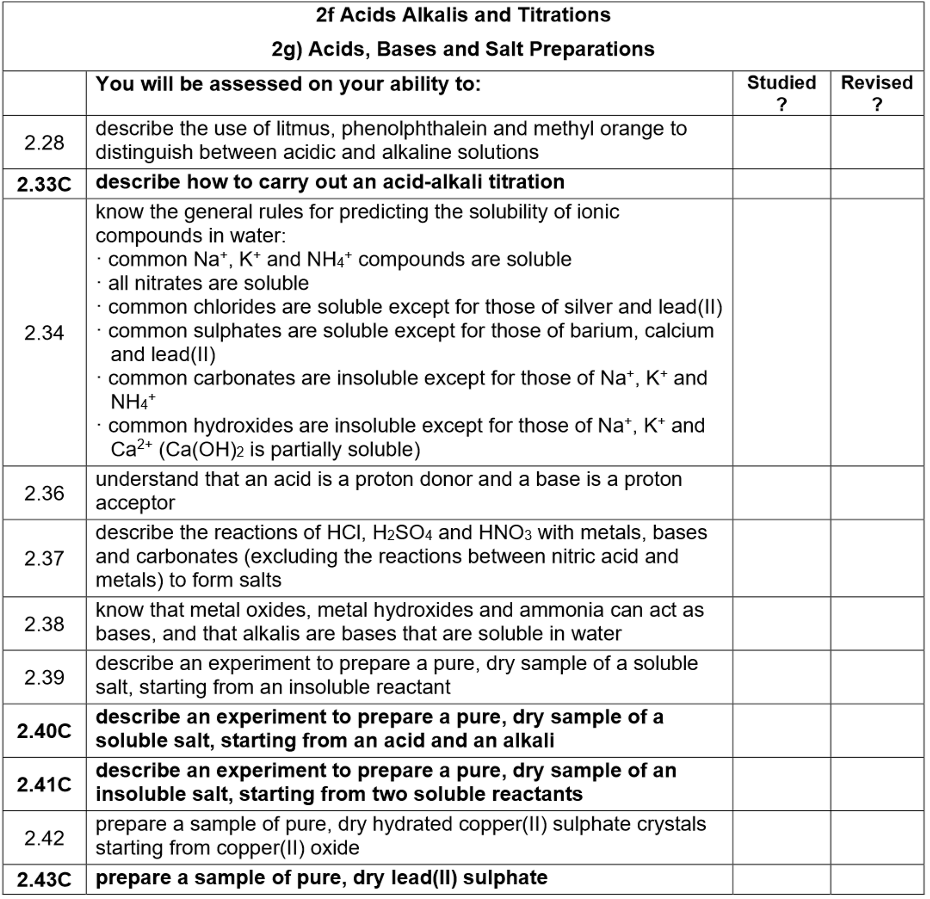
Making soluble salts:
Reactions used with excess insoluble solid:
Acid + Base → Salt + Water
Acid + Carbonate → Salt + Water + Carbon Dioxide
Acid + Reactive Metal → Salt + Hydrogen
Method for preparing a soluble salt using excess insoluble solid
Safety:
Wear goggles, lab coat, hair tied back.
Follow additional teacher instructions.
Procedure
Add 25 cm³ dilute sulfuric acid to a 100 cm³ beaker and gently warm on a tripod and gauze.
Turn off the Bunsen and place the beaker on a heat-proof mat.
Add half a spatula of copper(II) oxide, stirring; continue adding until no more dissolves (solid in excess).
Filter the solution into an evaporating basin.
Crystallisation
Heat the filtrate until its volume is halved to form a hot saturated solution; test by dipping a glass rod (crystals form on cooling).
Allow solution to cool and crystallise.
Separate crystals by filtration and dry in a warm oven or between filter paper.
Questions:
Observations in the experiment:
Black copper(II) oxide disappears as it reacts with the acid.
The solution turns blue as copper(II) sulfate forms.
Some black solid remains when excess is added.
Crystals form when the solution cools.
Word and formulae equations:
Word: Copper(II) oxide + sulfuric acid → copper(II) sulfate + water
Formulae: CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
Why excess copper(II) oxide was added:
To ensure all the acid reacts and the salt solution is pure.
Compounds present in the filtrate:
Copper(II) sulfate dissolved in water (CuSO4(aq))
Reaction using copper(II) carbonate:
Word: Copper(II) carbonate + sulfuric acid → copper(II) sulfate + water + carbon dioxide
Formulae: CuCO3(s) + H2SO4(aq) → CuSO4(aq) + H2O(l) + CO2(g)
Why the salt cannot be made using the metal and acid:
Copper is unreactive and does not displace hydrogen from the acid.
Why the filtrate was heated:
To concentrate the solution and produce a saturated solution for crystallisation.
Making a soluble salt by titration:
Overview:
Used when both reactants are soluble in water.
Suitable for making soluble salts that are Group I or ammonium salts.
Safety:
Wear goggles, lab coat, tie back hair.
Follow teacher’s additional instructions.
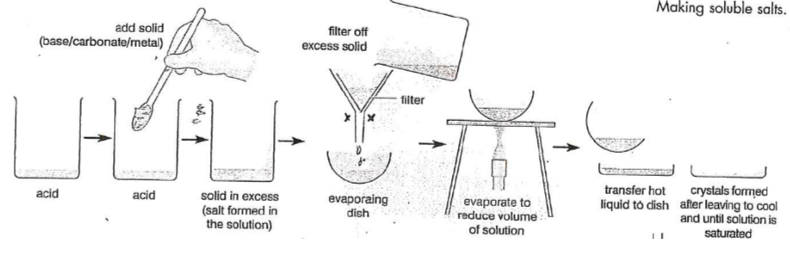
Method
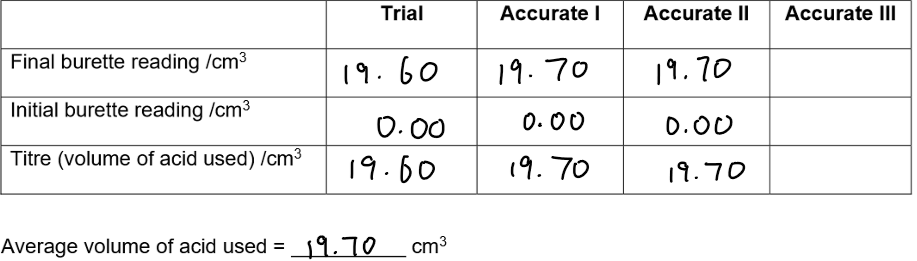
Measure 25.0 cm³ sodium hydroxide into a conical flask and add 3–4 drops of phenolphthalein.
Fill and secure a burette with 0.1 mol/dm³ hydrochloric acid; run some into a waste beaker to fill the tip.
Record the initial reading.
Titrate acid into alkali while swirling until the indicator changes colour (trial titration).
Record the final reading and calculate the titre.
Repeat to obtain two concordant results (±0.10 cm³), adding acid slowly near the end-point.
Calculate the average titre to 2 d.p using only accurate results.
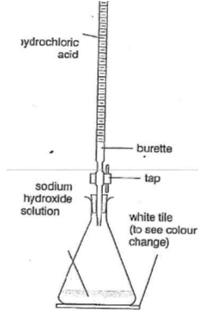
Results:
Change in colour of the indicator
Analysis
Word and balanced symbol equation:
Word: Hydrochloric acid + Sodium hydroxide → Sodium chloride + Water
Symbol: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Calculating concentration of sodium hydroxide:
Use: n=c×v to find moles of HCl used (convert cm³ to dm³).
Use the mole ratio from the equation (1:1) to find moles of NaOH.
Calculate concentration of NaOH: c=nv
Obtaining a pure dry sample of the salt:
Evaporate the water from the sodium chloride solution.
Allow crystals to form on cooling (crystallisation).
Filter off the crystals and dry them between filter paper or in a warm oven.
Determining the concentration of sodium hydroxide solution by titration
Safety:
Wear goggles, lab coat, and tie hair back.
Method
Measure 25 cm³ sodium hydroxide into a conical flask and add 3–4 drops of phenolphthalein.
Fill a burette with 0.05 mol/dm³ sulphuric acid and record the initial reading.
Titrate acid into alkali while swirling until the indicator changes from pink to colourless (trial titration).
Record final burette reading to calculate the titre.
Repeat to obtain two concordant results (±0.10 cm³).
Calculate the average titre and use it to determine the concentration of sodium hydroxide.
Results:
Change in colour of the indicator
Analysis
Word equation:
Copper(II) oxide + sulfuric acid → copper(II) sulfate + waterBalanced symbol equation:
CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)Concentration of sodium hydroxide:
Use titration data: moles NaOH = volume × concentration
Moles H2SO4 = moles NaOH × (mole ratio 1:1 or 1:2 depending on acid)
Concentration NaOH = moles ÷ volume (dm³)
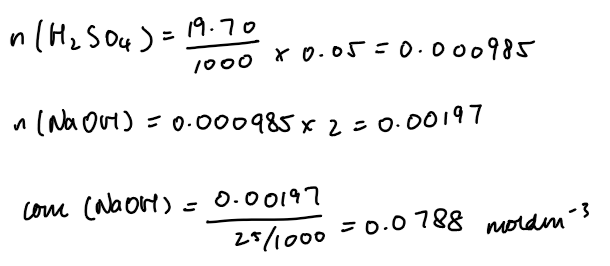
Obtaining salt crystals from solution:
Gently heat to form a saturated solution.
Allow to cool to crystallise.
Filter to collect crystals.
Dry crystals in oven or between filter paper.
Optional: evaporate all water for anhydrous solid.
Insoluble Salts and Precipitation Reactions
Insoluble salts are made by precipitation, mixing two soluble salts to form an insoluble solid (precipitate) and a soluble salt. Partners ‘swap’ in the reaction.
General reaction:
soluble salt + soluble salt → insoluble salt + soluble saltExample reaction:
Word equation: lead(II) nitrate + sodium sulphate → lead(II) sulphate + sodium nitrate
Symbol equation: Pb(NO3)2(aq) + Na2SO4(aq) → PbSO4(s) + 2NaNO3(aq)
Ionic equation: Pb2+(aq) + SO42-(aq) → PbSO4(s)
Rule: Only combine the ions that form the precipitate for the ionic equation.
Solubility rules:
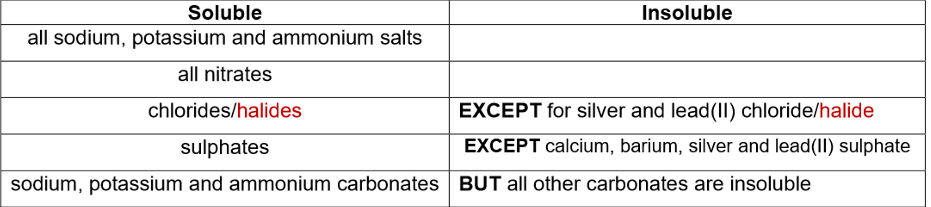
Example: barium chloride + magnesium sulfate
Word equation: barium chloride + magnesium sulfate → barium sulfate + magnesium chloride
Symbol equation: BaCl2(aq) + MgSO4(aq) → BaSO4(s) + MgCl2(aq)
Ionic equation: Ba2+(aq) + SO42-(aq) → BaSO4(s)
Table to predict which of the following mixtures would produce a precipitate:
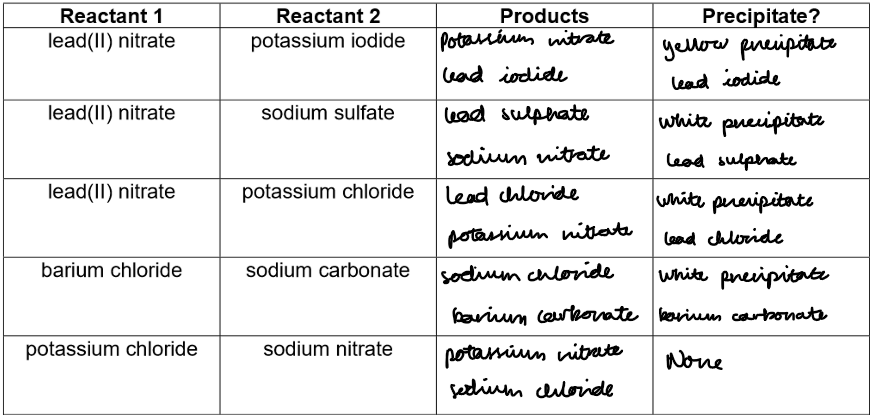
Making Insoluble Salts by Precipitation Reactions
Purpose: Mix solutions of soluble salts to see if an insoluble precipitate forms. Record observations and ionic equations.
Safety: Wear goggles, lab coat, hair tied back. Follow teacher instructions.
Method:
Add 1 cm³ of salt 1 to a test tube using a dropping pipette.
Add a few drops of salt 2 with a separate pipette.
Note if a precipitate forms.
Dispose of the mixture in the correct waste container.
Repeat for all combinations and fill in the results table with ionic equations for any precipitate.
Reaction type:
A⁺B⁻(aq) + C⁺D⁻(aq) → A⁺D⁻(s) + C⁺B⁻(aq)Precipitate forms if A⁺D⁻ is insoluble.
If both products are soluble, ions remain separate, no precipitate occurs.
To obtain a dry precipitate:
Mix solutions and filter.
Wash the residue on the filter paper to remove soluble salts.
Dry in a warm oven or between filter papers.
Observation: Precipitate formation confirms a precipitation reaction.
Preparation of a sample of pure, dry, lead(II) sulphate (PbSO₄) (DEMO)
Reaction:
lead(II) nitrate + sodium sulphate → lead(II) sulphate + sodium nitrate
Pb(NO₃)₂(aq) + Na₂SO₄(aq) → PbSO₄(s) + 2NaNO₃(aq)Forms a white precipitate.
Method:
Pour 10 cm³ of 0.5M Pb(NO₃)₂ into a beaker.
Add 15 cm³ of 0.5M Na₂SO₄ and stir; white PbSO₄ precipitate forms.
Weigh filter paper and place in a funnel over a small conical flask.
Filter the mixture, washing solid from the beaker and glass rod.
Wash the residue with distilled water to remove soluble impurities.
Place filter paper with residue in a warm oven to dry.
Reweigh dry solid + filter paper to get actual mass of PbSO₄.
Calculations / Analysis:
Given results:
Mass of filter paper = 0.105 g
Mass of filter paper + PbSO₄ = 1.313 g
Mass of PbSO₄ = 1.313 − 0.105 = 1.208 g
Moles of Pb(NO₃)₂
Concentration = 0.5 M, Volume = 10 cm³ = 0.010 dm³
Moles of Pb(NO₃)₂=C×V=0.5×0.010=0.005 mol
Moles of Na₂SO₄
Concentration = 0.5 M, Volume = 15 cm³ = 0.015 dm³
Moles of Na₂SO₄=0.5×0.015=0.0075 mol
Pb(NO₃)₂ is the limiting reagent (0.005 < 0.0075), Na₂SO₄ is in excess.
Theoretical moles of PbSO₄
Mole ratio Pb(NO₃)₂ : PbSO₄ = 1:1
Theoretical moles of PbSO₄=0.005 mol
Theoretical mass of PbSO₄
Relative formula mass: PbSO₄ = 207 + 32 + (16×4) = 303 g/mol
Theoretical mass=0.005×303=1.515 g
Percentage yield
% yield=actual mass theoretical mass×100=1.2081.515×100≈79.7%
Class practical: Preparation of a pure, dry sample of strontium carbonate (SrCO₃)
Strontium carbonate is an insoluble salt formed by mixing solutions of strontium chloride and sodium carbonate:
SrCl2(aq)+Na2CO3(aq)→SrCO3(s)+2NaCl(aq)
Method:
Pour 10 cm³ of 0.1 M SrCl₂ into a 100 cm³ beaker.
Add 15 cm³ of 0.1 M Na₂CO₃ and stir; white SrCO₃ precipitate forms.
Weigh filter paper and place in funnel over a conical flask.
Filter the mixture, washing any solid from the beaker and rod.
Wash the residue on the filter paper with distilled water several times.
Place the filter paper with residue in a petri-dish and pat dry.
Reweigh dry solid + filter paper to obtain actual mass of SrCO₃.
Results:
Mass of filter paper / g | Mass of filter paper + SrCO₃ / g | Mass of SrCO₃ / g |
|---|---|---|
1.09 | 1.22 | 0.13 |
Analysis
1. Limiting and excess reagent
Moles of SrCl₂:
n=C×V=0.1×0.010=0.001 mol
Moles of Na₂CO₃:
n=0.1×0.015=0.0015 mol
Limiting reagent: SrCl₂ (0.001 mol < 0.0015 mol)
Excess reagent: Na₂CO₃
2. Maximum mass of SrCO₃
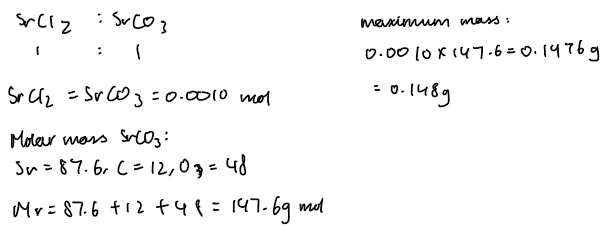
3. Percentage yield
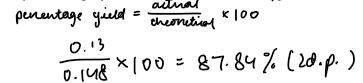
4. Why is the yield not 100%?
Incomplete drying of the precipitate leaves water in the solid.
Some product may be lost during transfer or filtration.
Contamination from soluble salts not fully washed away.
5. Why was the solid residue washed?
To remove any soluble impurities (NaCl) that could contaminate the pure SrCO₃.
6. Alternative methods of drying
Dry in a warm oven or drying oven.
Dry between pieces of filter paper.
Air-dry in a desiccator.