AIR POLLUTION
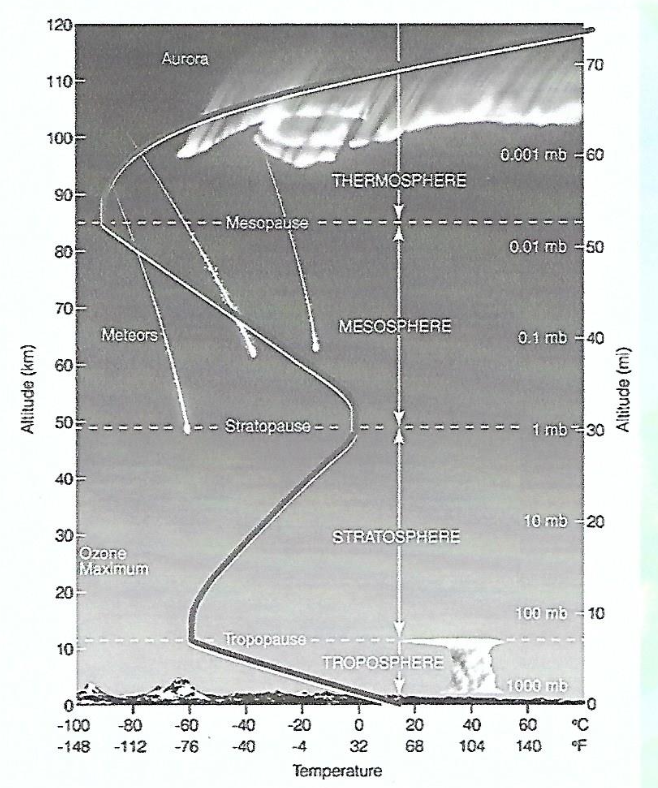
Atmosphere
The atmosphere is very thin compared to the size of the earth. Scaled to the size of an apple, it's no thicker than the skin.
The atmosphere gradually thins out with increasing altitude, without a definite "top."
Layers of the Atmosphere
The atmosphere is divided into layers based on the vertical temperature (T) profile:
Troposphere (convection current; different weathers)
Stratosphere (location of ozone)
Mesosphere (low air density)
Thermosphere (where the International Space Station is found; high heat, low density)
Atmospheric Composition
The composition of the atmosphere by volume:
Nitrogen: 78.09 \%
Oxygen: 20.94 \%
Argon: 0.93 \%
Carbon dioxide: 0.0318 \%
Neon: 0.0018 \%
Air Pollution
Defined as any alteration of the physical, chemical, and biological properties of atmospheric air, or any discharge of liquid, gaseous, or solid substances that create harmful conditions.
Source: RA 8749 (Philippine Clean Air Act)
Sources of Air Pollution
Man-Made:
Motor Vehicles
Industry
Power Plants
Agricultural Sprays
Solid Waste Disposal
Natural:
Plant & Tree Pollens
Bacteria & Spores
Gases & Dusts from Forest & Grass Fires
Fogs
Ozone & Nitrogen dioxide from lightning
Volcanic ash & gases
Sources of Air Pollution (Continued)
Stationary Sources
Any building or immobile structure, facility, or installation which emits or may emit any air pollutant (RA 8749).
Point Sources: Mostly industrial plants (power, processing, and manufacturing).
Area Sources: Large areas generating significant air pollutants (busy roads, construction sites, aircraft operations, forest fires, burning wastes).
Mobile Sources
Any vehicle propelled by combustion of carbon-based or other fuel, used for conveying persons or transporting property (RA 8749).
Biogenic
sources of air pollution from plants and animals
Air Pollutant
Any matter in the atmosphere other than oxygen, nitrogen, water vapor, carbon dioxide, and inert gases in normal concentrations, detrimental to health or the environment.
Includes smoke, dust, soot, cinder, fly ash, solid particles, gases, fumes, chemical mists, contaminated steam, and radioactive substances.
Type of Pollutant
Primary Pollutant
Found in the atmosphere in the same form as emitted.
Examples: sulfur dioxide, nitrogen dioxide, and hydrocarbons.
Secondary Pollutant
Formed in the atmosphere as a result of reactions (hydrolysis, oxidation, photochemistry).
Example: photochemical smog.
Major Air Pollutants
Particulate Material
Primary source is combustion.
Primary Particles
Construction sites, agricultural fields, unpaved roads, fires, smokestacks
Secondary Particles
Power plants, Industry Vehicles
Dusts
Solid particles entrained by process gases directly from the material being handled or processed.
Direct offspring of parent material undergoing mechanical operation.
Entrained materials used in mechanical operation.
Fumes
Solid particles formed by condensation of vapors by sublimation, distillation, calcination, or chemical processes.
Frequently a metallic oxide; size is 0.03 to 0.3 micrometer.
Mists
Entrained liquid particle formed by condensation of a vapor and by chemical reaction; size is 0.5 to 3.0 micrometer.
Generated by condensation.
Smoke
Entrained solid particles formed by incomplete combustion of carbonaceous materials; size is 0.05 to 1 micrometer.
Sprays
Liquid particle formed by the atomization of a parent liquid.
Settle under gravity and created by mechanical disintegration.
Nitrogen Oxides
Important precursor to smog and acid rain, affecting terrestrial and aquatic ecosystems.
Plays a major role, together with VOCs, in ozone production.
Forms when fuel is burned at high temperatures.
Major emission sources:
transportation
stationary fuel combustion (electric utility and industrial boilers).
Nitrogen monoxide (NO):
Produced whenever air is heated to high temperature, such as in automobile cylinders or high temperature furnaces of power plants and industrial boilers.
Nitrogen dioxide (NO2):
A brownish, highly reactive gas present in all urban atmospheres.
Can irritate the lungs, cause bronchitis and pneumonia, and lower resistance to respiratory infections.
The major mechanism for the formation of NO_2 in the atmosphere is the oxidation of nitric oxide (NO).
Sulfur Oxides
Sulfur Dioxide(SO2):
A colorless gas that is toxic and irritating to the respiratory system
High concentrations affect breathing and may aggravate existing respiratory and cardiovascular disease.
Sensitive populations include asthmatics, individuals with bronchitis or emphysema, children and the elderly.
A primary contributor to acid deposition, or acid rain, which causes acidification of lakes and streams and can damage trees, crops, historic buildings and statues.
Combines with water vapor in the air forming sulfuric acid & sulfates.
Sulfur compounds in the air contribute to visibility impairment
Contributors: Industry Sector 90%, Transport 9%, Open Burning 1%
Usually emitted from the burning of coal and oil in electrical energy generation or heating or from internal combustion engines.
It is also released in the industrial production of sulfuric acid.
Ambient SO_2 results largely from stationary sources such as coal and oil combustion, steel mills, refineries, pulp and paper mills and from nonferrous smelters.
Carbon Oxides
Carbon monoxide (CO):
A colorless, odorless, and poisonous gas produced by incomplete burning of carbon in fuels, primarily by vehicles and with the incomplete burning of fossil fuels.
When CO enters the bloodstream, it reduces the delivery of oxygen to the body's organs and tissues.
Health threats are most serious for those who suffer from cardiovascular disease, particularly those with angina or peripheral vascular disease.
Exposure to elevated CO levels can cause impairment of visual perception, manual dexterity, learning ability and performance of complex tasks.
Most CO emissions are from transportation sources.
Carbon dioxide (CO_2)
Hydrocarbons
Diverse group of organic compounds that contain only hydrogen and carbon (ex: CH_4-methane)
Usually unburned fumes that evaporate from gas tanks & are emitted from exhausts of vehicles.
Some are related to photochemical smog and greenhouse gases
Ozone (O_3)
Stratospheric Ozone (Good ozone)
Essential component that screens out UV radiation in the upper atmosphere
Man-made pollutants (ex: CFCs) can destroys ozone
Ground Level (Tropospheric) Ozone (Bad ozone)
Man-made pollutant in the lower atmosphere
Secondary air pollutant
Component of photochemical smog
A photochemical oxidant and the major component of smog.
Not emitted directly into the air but is formed through complex chemical reactions between precursor emissions of volatile organic compounds (VOC) and oxides of nitrogen (NO_x) in the presence of sunlight.
The reactivity of O_3 causes health problems because it damages lung tissue, reduces lung function and sensitizes the lungs to other irritants.
Lead (Pb)
Exposure to lead (Pb) can occur through multiple pathways, including inhalation of air and ingestion of lead in food, water, soil or dust.
Excessive Pb exposure can cause seizures, mental retardation and/or behavioral disorders.
Low doses of Pb can lead to central nervous system damage.
Recent studies have also shown that Pb may be a factor in high blood pressure and in subsequent heart disease in middle-aged males.
Lead gasoline additives, non-ferrous smelters, and battery plants are the most significant contributors to atmospheric Pb emissions.
Criteria Air Pollutants
EPA uses six "criteria pollutants" as indicators of air quality
EPA established for each of them a maximum concentration above which adverse effects on human health may occur.
Nitrogen Dioxide: NO_2
brownish gas irritates the respiratory system originates from combustion (N2 in air is oxidized); NOx sum of NO,NO2, other oxides of N
Ozone: ground level O_3
primary constituent of urban smog
reaction of VOC + NO_x in presence of heat +sun light
Carbon monoxide: CO
reduces bloods ability to carry O_2
product of incomplete combustion
Lead: Pb
cause learning disabilities in children, toxic to liver, kidney, blood forming organs
tetraethyl lead - anti knock agent in gasoline
leaded gasoline has been phased out
Particulate Matter: PM10 (PM 2.5)
respiratory disorders
Sulfur Dioxide: SO_2
formed when fuel (coal, oil) containing S is burned and metal smelting
precursor to acid rain along with NO_x
National Ambient Air Quality Guideline for Criteria Pollutants
Criteria Pollutants - there are already a set of national ambient air quality guideline values or standards that need to be met to protect the environment.
Total Suspended Particulates (TSP) - respiratory disorder
Sulfur Dioxide (SO_2) - acid rain
Nitrogen Dioxide (NO_2) - respiratory system originates from combustion
Carbon Monoxide (CO) - reduces bloods ability to carry O2
Lead (Pb) - learning disabilities in children, toxic to liver, kidney, blood forming organs
Ozone (O_3) - urban smog
Ambient Air Quality Guideline Values
Concentration of air over specified periods classified as short-term and/or long-term which are intended to serve as goals or objectives for the protection of health and/or public welfare.
In general, used as a basis for taking positive action in preventing, controlling, or abating health impacts from air pollution.
National Ambient Air Quality Guideline Values
Ambient Air Quality
The general amount of pollution present in a broad area; and refers to the atmosphere's average purity as distinguished from discharge measurements taken at the source of pollution
Ambient Air Quality Monitoring
The measurement of a representative sample and is indicative of a portion of the atmosphere.
In theory, the pollutant concentrations measured in ambient air could be entirely different if the monitoring were located a short distance away.
The ambient network - is designed to determine the concentrations expected to occur in the area covered by the network.
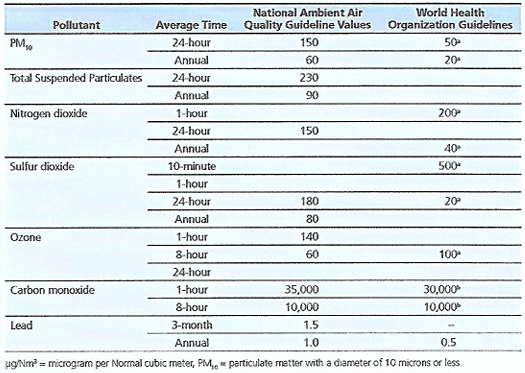
Table 19 Philippine National Ambient Air Quality Guideline Values vs. World Health Organization Guidelines (\mu g/Nm^3)
Ambient Air Quality Monitoring Station Network
Criteria pollutants
particulate matter (PM10)
sulfur dioxide
carbon monoxide
nitrogen dioxide
Total Suspended Particulates
ozone
lead
Non-criteria pollutants
benzene
toluene
xylene.
Measures meteorological data
wind direction
vertical wind speed
wind speed
Temperature
relative humidity
net radiation
barometric pressure
solar radiation (400-1100 nm)
UV radiation (295-385 nm).
Philippine National Ambient Air Quality Guideline Values versus World Health Organization Guidelines (\mu g/Nm^3)
AIRSHED
Refers to areas with common weather or meteorological conditions and sources of air pollution which affect the interchange and diffusion of pollution in the surrounding atmosphere.
INTERIM AIRSHEDS
3 Metro Manila Airshed
NCR
Region 3 except Nueva Ecija
Region 4A except Quezon
Metro Cebu Airshed
Cagayan de Oro City, Geothermal Airshed
Davao Airshed
Naga City Airshed
BLIST Airshed (Baguio, La Trinidad, Itogon, Sablan, Tuba)
Agusan Del Norte Airshed
Zamboanga City Airshed
AIR QUALITY INDEX (AQI)
Index for reporting daily air quality.
It tells you how clean or polluted your air is, and what associated health effects might be a concern for you.
The AQI focuses on health effects you may experience within a few hours or days after breathing polluted air.
suspended particulates
sulfur dioxide
photochemical oxidants or ozone
carbon monoxide
nitrogen dioxide
SIX LEVELS OF HEALTH CONCERN
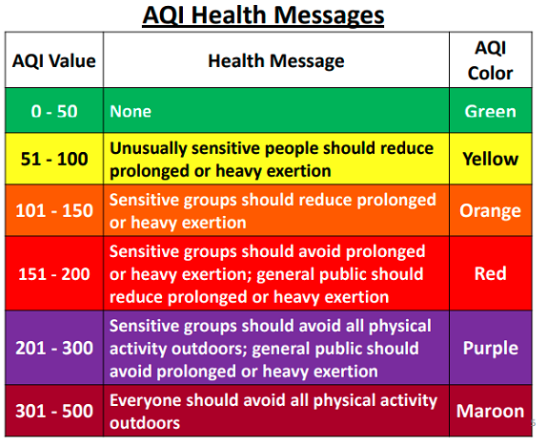
"Good" GREEN 0-50
Air quality is considered satisfactory, and air pollution poses little or no risk.
"Moderate" (Fair) YELLOW 51-100
Air quality is acceptable; however, for some pollutants there may be a moderate health concern for a very small number of people. For example, people who are unusually sensitive to ozone may experience respiratory symptoms.
"Unhealthy for Sensitive Groups" ORANGE 101-150
Although general public is not likely to be affected at this AQI range, people with lung disease, older adults and children are at a greater risk from exposure to ozone, whereas persons with heart and lung disease, older adults and children are at greater risk from the presence of particles in the air.
"Unhealthy" (Very Unhealthy) RED 151-200
Everyone may begin to experience some adverse health effects, and members of the sensitive groups may experience more serious effects.
"Very Unhealthy" (Acutely Unhealthy) PURPLE 201-300
This would trigger a health alert signifying that everyone may experience more serious health effects.
"Hazardous" (Emergency) MAROON 301-500
This would trigger a health warnings of emergency conditions. The entire population is more likely to be affected.
PHOTOCHEMICAL SMOG (aka LA smog)
A mixture of pollutants that are formed when nitrogen oxides and volatile organic compounds (VOCs) react to sunlight, creating a brown haze above cities. - EPA
Primary pollutants
Nitrogen oxides and VOCs, combine to change in sunlight in a series of chemical reactions to create what are known as secondary pollutants.
Secondary pollutants
The secondary pollutant that causes the most concern is the ozone that forms at ground level.
Many other hazardous substances are also formed, such as peroxyacetyl nitrate (PAN).
SMOG FORMATION
Morning rush-hour traffic results in the production of large amounts of NO:
N_2+O_2\rightarrow2NO
NO reacts with molecular oxygen (O2) from the atmosphere to form NO2:
2NO+O_2\rightarrow2NO_2
Later in the morning, NO_2 reacts with ultraviolet light to form atomic oxygen (O):
NO_2 \rightarrow NO + O
Abundant O_2 in the atmosphere reacts with atomic oxygen (O) to form ozone:
O_2+O\rightarrow O_3
THERMAL INVERSION
A condition when warm air becomes trapped between two layers of cold air and acts like a lid on the valley.
Cooler air is unable to mix with the warm air above and cannot escape because of the surrounding mountains. Cool air is trapped, sometimes for several days, and accumulates pollutants.
If thermal inversion continues, the levels of pollution can become dangerously high.
GLOBAL WARMING
Increase in temperature of earth due to greenhouse effect of gases
Greenhouse effect
The warming thought to occur from the increase of greenhouse gases.
Called such because the effect is similar to what happens in a greenhouse where the glass allows light to enter but retards the loss of heat.
CLIMATE CHANGE
Is the variation in the Earth's global climate or in regional climates over time.
A change of climate which is attributed directly or indirectly to human activity that alters the composition of the global atmosphere and which is in addition to natural climate variability observed over comparable time periods. - UNFCCC
GREENHOUSE GASES
Those gases that can potentially or can reasonably be expected to induce global warming, which include carbon dioxide, oxides of nitrogen, chloroflourocarbons, and the like.
Philippine Clean Air Act (RA 8749)
Carbon dioxide (CO_2)
Enters the atmosphere through burning fossil fuels (coal, natural gas and oil), solid waste, trees and wood products, and also as a result of certain chemical reactions (e.g., manufacture of cement).
Methane (CH_4)
Emitted during the production and transport of coal, natural gas, and oil. Methane emissions also result from livestock and other agricultural practices and by the decay of organic waste in municipal solid waste landfills.
Nitrous oxide (N_2O)
Emitted during agricultural and industrial activities, as well as during combustion of fossil fuels and solid waste.
Fluorinated gases
Synthetic, powerful greenhouse gases that are emitted from a variety of industrial processes.
Hydrofluorocarbons
Perfluorocarbons
Sulfur hexafluoride
Nitrogen trifluoride
Sometimes used as substitutes for stratospheric ODS.
Typically emitted in smaller quantities, but because they are potent greenhouse gases, they are sometimes referred to as High Global Warming Potential gases ("High GWP gases").
KYOTO PROTOCOL
It is a protocol to the United Nations Framework Convention on Climate Change (UNFCCC) aimed at fighting global warming.
The Protocol was initially adopted on December 11, 1997 in Kyoto, Japan, and entered into force on February 16, 2005.
The first major international agreement towards GHGE (Greenhouse gasses emission) reduction.
Industrialized countries agreed to reduce emissions of six green house gases baskets to 5.2% below 1990 levels between 2008 - 2012.
Carbon dioxide (CO_2)
Methane (CH_4)
Nitrous Oxide (N_2O)
Hydrofluorocarbons (HFCs)
Sulphur hexafluoride (SF_6)
perfluorocarbons (PFCs)
Mechanisms to reduce the cost of meeting the above target.
International Emissions Trading (Article 17)
Allows the trading of assigned amounts within or among industrialized countries to meet quantified emission limitation or reduction commitments.
"Emissions Trading"
or the selling of excess allowable emission of carbon dioxide of a country to another country that is still behind its target reduction of GHG emission.
OZONE LAYER DEPLETION
Ozone - a molecule that consists of three atoms of oxygen (0_3).
Occurs both in the Earth's upper atmosphere and at ground level.
Ozone layer
Layer found in the stratosphere with high concentration of ozone molecules
Located 20-40 km from the earth's surface
Acts as a shield that envelops the entire earth and protects all life forms on earth from the harmful UV radiation emitted by the sun.
1985 - Discovered a significant thinning of the ozone layer over the Antarctic occurred during the Southern Hemisphere spring.
Some regions of the ozone layer showed 95% depletion.
Antarctica - earth's southernmost continent, underlying the south pole
Has also been found to be occurring farther north than previously.
Measurements in arctic regions also suggest thinning of the ozone layer.
Arctic - region around the earth's north pole.
The presence of ozone in the atmosphere shields the earth from the harmful effects of UV light radiation.
OZONE DEPLETING SUBSTANCES
Those substances that significantly deplete or otherwise modify the ozone layer in a manner that is likely to result in adverse effects of human health and the environment such as, but not limited to, chloroflourocarbons, halons and the like
RA 8749 (Philippine Clean Air Act)
Class I
Chlorofluorocarbons (CFCs)
Commonly known as "freon"
Contains chlorine, fluorine and carbon
Discovered by Thomas Midgley in 1928
Chlorine reacts with ozone in the following way to reduce the quantity of ozone present:
ClO+O_3\rightarrow ClO+O_2
ClO + O \rightarrow Cl + O_2
These reactions both destroy ozone and reduce the likelihood that it will be formed because atomic oxygen (O) is removed as well.
Halons
Carbon tetrachloride
Methyl chloroform
Methyl bromide
Class II
Hydrochlorofluorocarbons (HCFCs)
Effects
Thinning of the ozone layer resulting to the loss of the blocking effect of the ozone layer against the UV radiation
Allows entry of greater concentrations of UV-B (which increases the risk of skin cancer, cataracts and suppressed immune system; can also damage terrestrial plant life, single-cell organisms and aquatic ecosystems.
MONTREAL PROTOCOL - Montreal Protocol on Substances that Deplete the Ozone Layer (Sept. 16, 1987)
Landmark international environmental accord that was negotiated, entered into force, and amended in record time, in response to scientific information on the damage to the ozone layer by synthetic chemicals.
Established a formula for determining calculated levels of consumption and production of controlled substances based on the ozone depleting potential of each substance.
Philippines - September 14, 1988.
The DENR through the EMB-POD (Environmental Management Bureau- Philippine Ozone Desk)
ACID DEPOSITION
The accumulation of potential acid-forming particles on a surface.
Natural causes:
Volcanoes, lightning, etc.
Man-made causes/human activities:
Burning of fossil fuels (coal, oil)
Use of internal combustion engine.
Results when sulfur dioxide (SO2) and oxides of nitrogen (NOx) are converted to sulfuric and nitric acid, respectively, with the reaction of water and oxidizing agents, and return to the surface as either dry or wet deposition
EFFECTS
Damage to ecosystem
Declining Aquatic Animal Populations
Thin-shelled eggs prevent bird reproduction because calcium is unavailable in acidic soil
Forest decline (Ex: Black forest in Germany - 50% is destroyed)
Damage to properties
Buildings and monuments made from materials containing limestone (calcium carbonate, CaCO)
Sulfuric acid (H2SO4) reacts with (CaCOs) to form gypsum (CaSO4), which is more soluble and is eroded over years of contact with acid rain.
Corrosion of metal surfaces
ACID RAIN
Unpolluted rainwater is slightly acidic (5.6 pH for unpolluted rain water) because of the carbon dioxide from air dissolved in it.
CO2 + H2O => H2CO3
Form of precipitation resulting from the acid-forming reactions.
Scotland - pH = 2.4 in 1974
Northeastern USA-pH=4-4.5
Contains high levels of sulfuric or nitric acids
INDOOR AIR POLLUTION
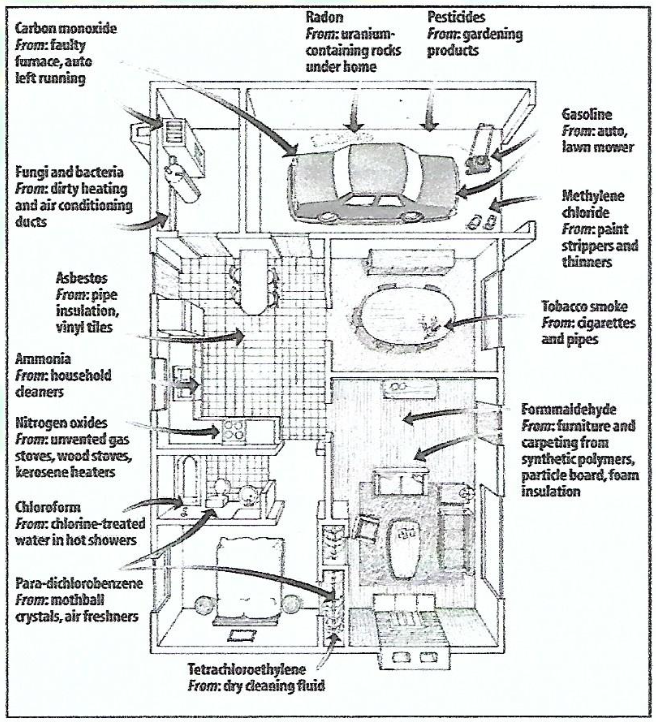
Pollutants can be 2-5X greater than outdoors
Radon
Emit alpha particles
Harmful when ingested/inhaled
Risk for lung cancer
Cigarette smoke
Hydrocarbons
Carbon dioxide
Carbon monoxide
Particulate matter
Cyanide
Radioactive materials
Carbon monoxide
Nitrogen dioxide
Formaldehyde
Airborne pesticide residues
Lead
Asbestos
Many disease-causing microorganisms.