Chapter 3 - Atoms
400 B.C.E. - Democritus
- Argues that if you cut a substance in half, what results is two pieces of the same substance. Eventually, you would reach a point where you could no longer divide it and still have the same substance
- atomos - “indivisible”
Law of Conversation of Mass
Antoine Lavoisier (1743 - 1794)
In chemical reactions, matter is neither created or destroyed
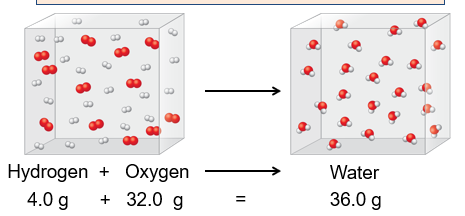
An example: According to the law of conservation of mass, the mass of substances before the reaction (on the left hand side of the arrow) must equal the mass of the substances after the reaction (on the right side of the arrow). We begin with a total of 80.0 grams, therefore, we must end with 80.0 grams. Based on this, we can say that we will form 44.0 grams of carbon dioxide.
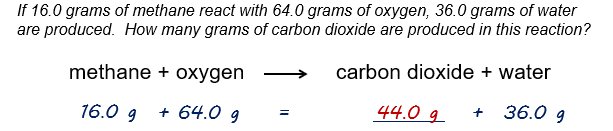
Origins of Atomic Theory
John Dalton (1766 - 1844)
- Elements are made of tiny, indivisible particles called atoms
- The atoms of each element are unique
- Atoms can join together in a whole - number ratios to form compounds
- Atoms are unchanged in chemical reactions
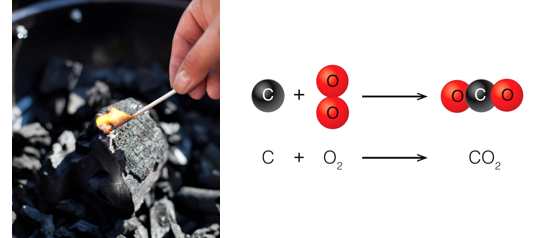
Example: When charcoal burns the charcoal (which is essentially the element carbon) reacts with oxygen in the air to form a new compound, carbon dioxide. From Dalton, we know that carbon atoms are distinct from oxygen atoms. When they combine, they do so in a whole number ratio. One carbon atom combines with two oxygen atoms to produce one molecule of carbon dioxide. The atoms themselves are not changed, but the properties of carbon dioxide are different than the properties of carbon and oxygen.
Three foundational Ideas
The study of chemistry springs from these concepts
- All matter is composed of atoms
- The atoms of each element have unique characteristics and properties
- In chemical reactions, atoms are not changed, but combine in whole - number ratios to form compounds
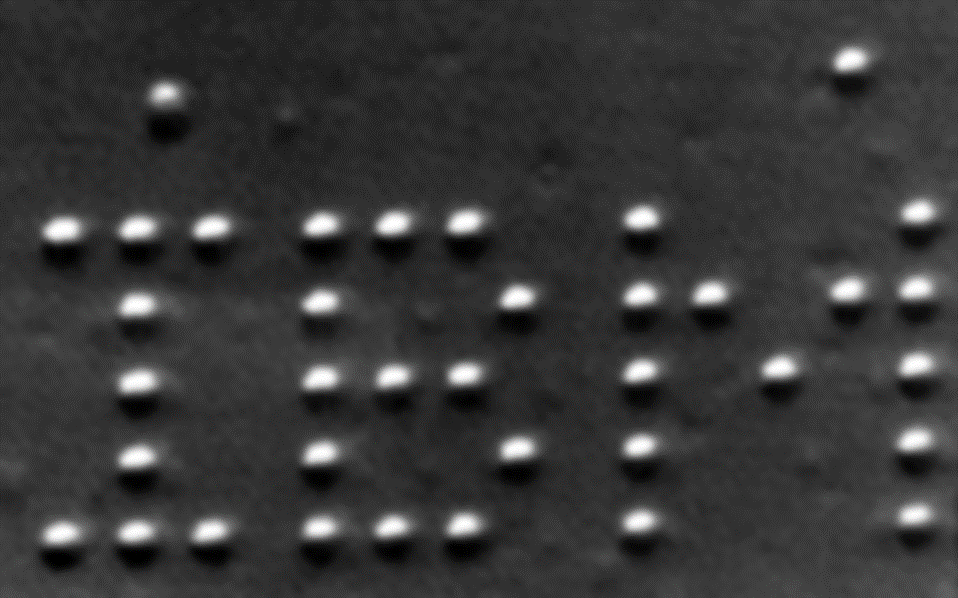
Can we see atoms?
In the early 1990s, the scientists at IBM labs were not only able to visualize atoms on a metal surface, but, using a technique called scanning tunneling microscopy, were actually able to manipulate atoms, and create the letters “IMB” from individual atoms.
Scientists can also visualize the arrangement of atoms
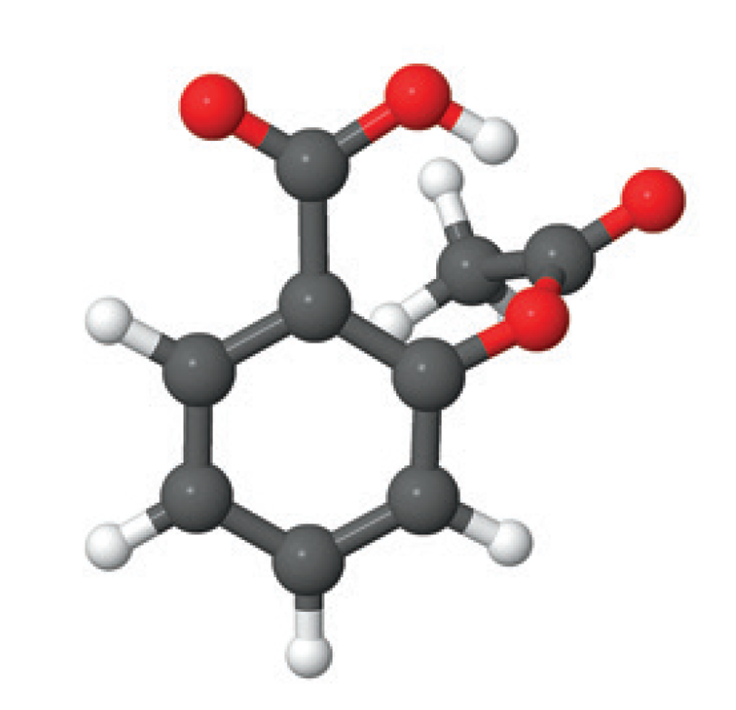
Scientists can use X - ray crystallography to visual atoms. In this technique, a solid is bombarded with a form of energy called X - rays. Based on the patterns the X - rays form as they pass through the material, scientists are able to visualize the arrangement of atoms in the solid. For example, this image shows the x-ray structure of the pain - killer aspirin.
Periodic Table of the Elements
Dmitri Mendeleev organized the 60 known elements in the late 1860s based on their atomic masses, such as fluorine, chlorine, bromine, and iodine, into columns. By doing this, he was also able to predict the existence of several elements, such as germanium and gallium, which had not been discovered yet. His table became the basis for the modern periodic table of the elements, which organizes all known elements based on their masses and properties.
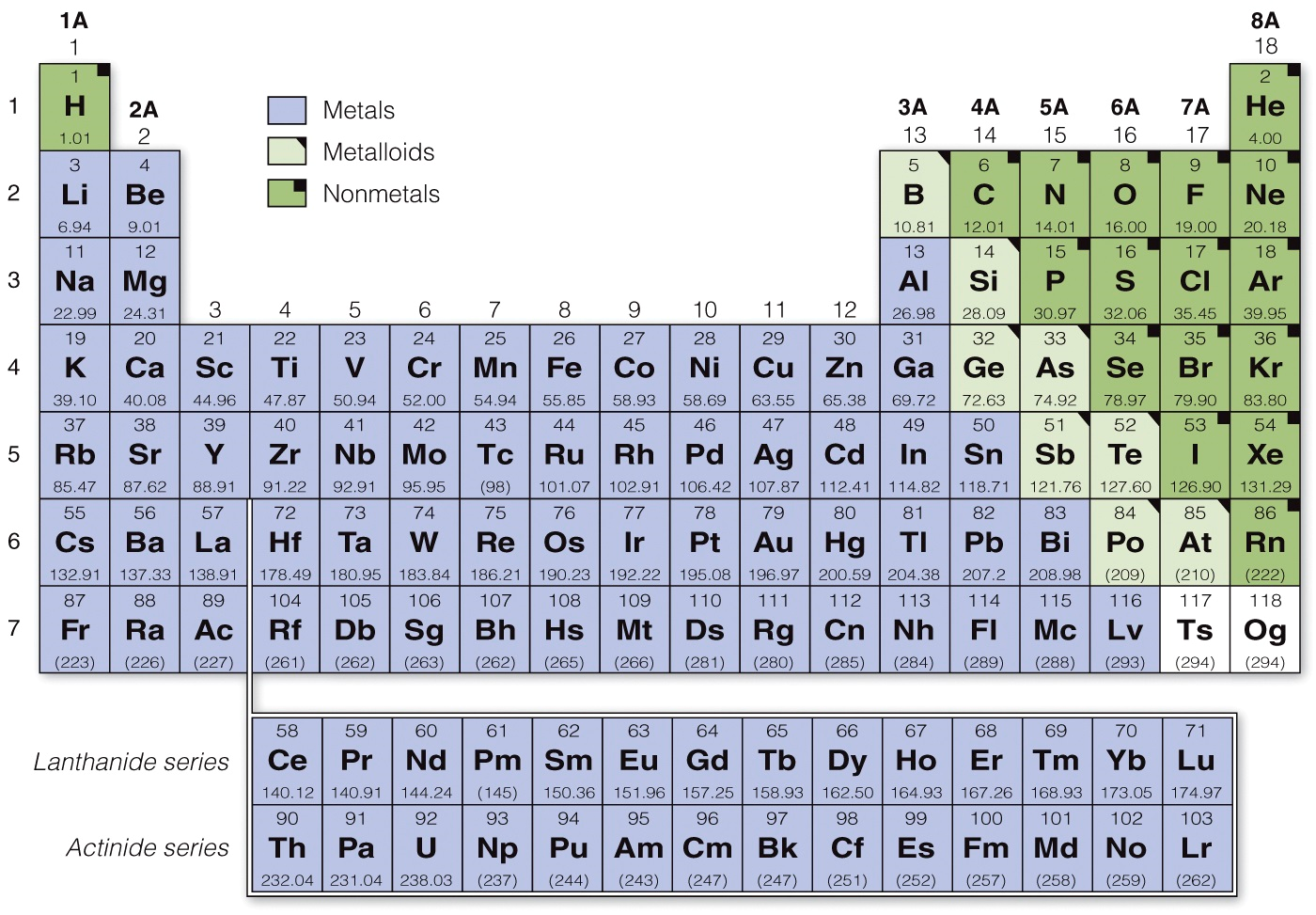
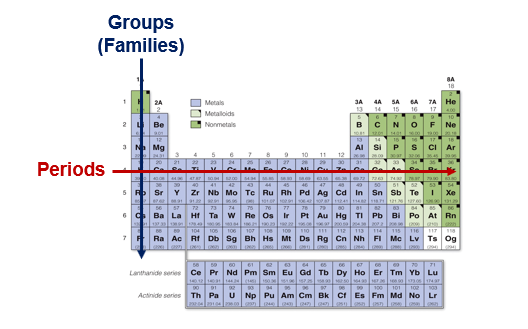
Periodic means “cyclical” and indicates how the table is organized. As we move from left to right across a horizontal row of the periodic table, we cover the entire spectrum of chemical properties. The rows are called periods. Elements that are in the same column have similar properties, and they are called groups, or families.
The periodic table uses an accepted set of one - and two - letter abbreviations for different elements. In order to understands these chemicals descriptions, it is important that you know the symbols and names for the most common or important elements. Most of the symbols align with the English words, but some are derived from the Latin words for these elements.

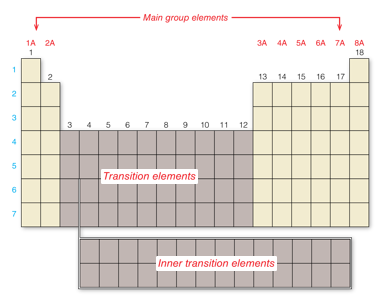
The blocks of elements on the left and right sides of the periodic table are the main group elements. We can predict many properties of these elements based on their location on the periodic table. The elements in the middle block are called the transition elements. At the bottom of the periodic table are two additional rows, called the inner transition elements. Although these elements actually belong within the last two rows of the table, they are typically shown below to make the table easier to read. The periodic table contains seven periods.
In the modern system, the numbers 1 - 18 designate the columns that contain the main - group and the transition elements
An older but very useful system numbers the main group columns as 1A - 8A
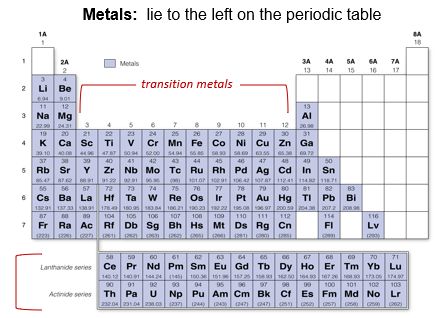
Metals
The elements on the left - hand side of the periodic table are metals. Metals are usually solid at room temperature, they may be molded into different shapes, and they conduct heat and electricity. The transition elements in columns 3 - 12 of the periodic table are often called the transition metals which are harder and less reactive than the metals of the columns 1 -2. The inner transition elements are also metals. The first row is the lanthanide series, which contains heavier, naturally occurring metals called the rare earth metals. The second row is called the actinide series, with the first few elements are mostly man - made, although trace amounts of a few have been found in nature.
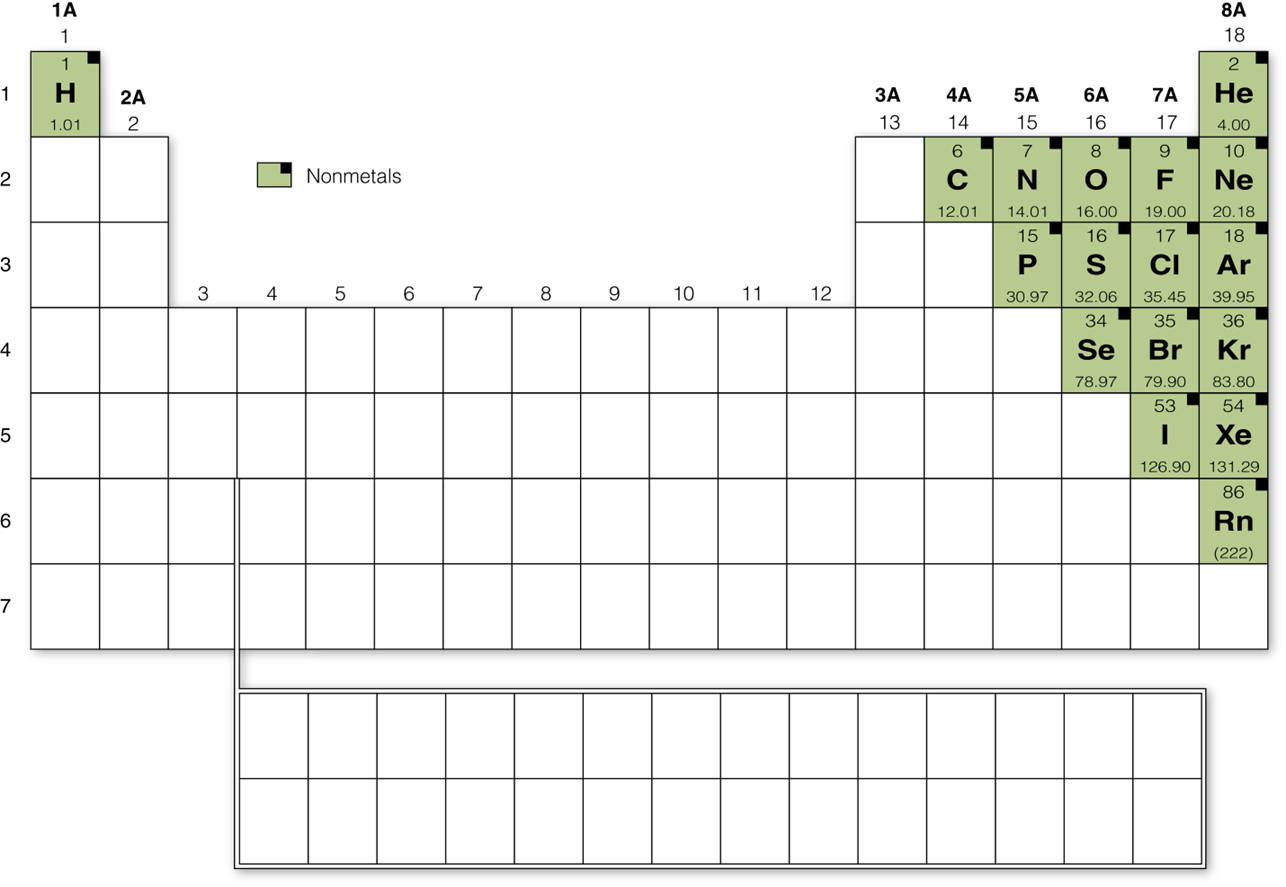
Nonmetals
The nonmetals are found on the upper - right side of the periodic table. The physical properties of the nonmetals vary widely. Nonmetals form a rich variety of compounds, from simple gases to plastics to biomolecules. These are primary atomic building blocks for all plant and animal life.
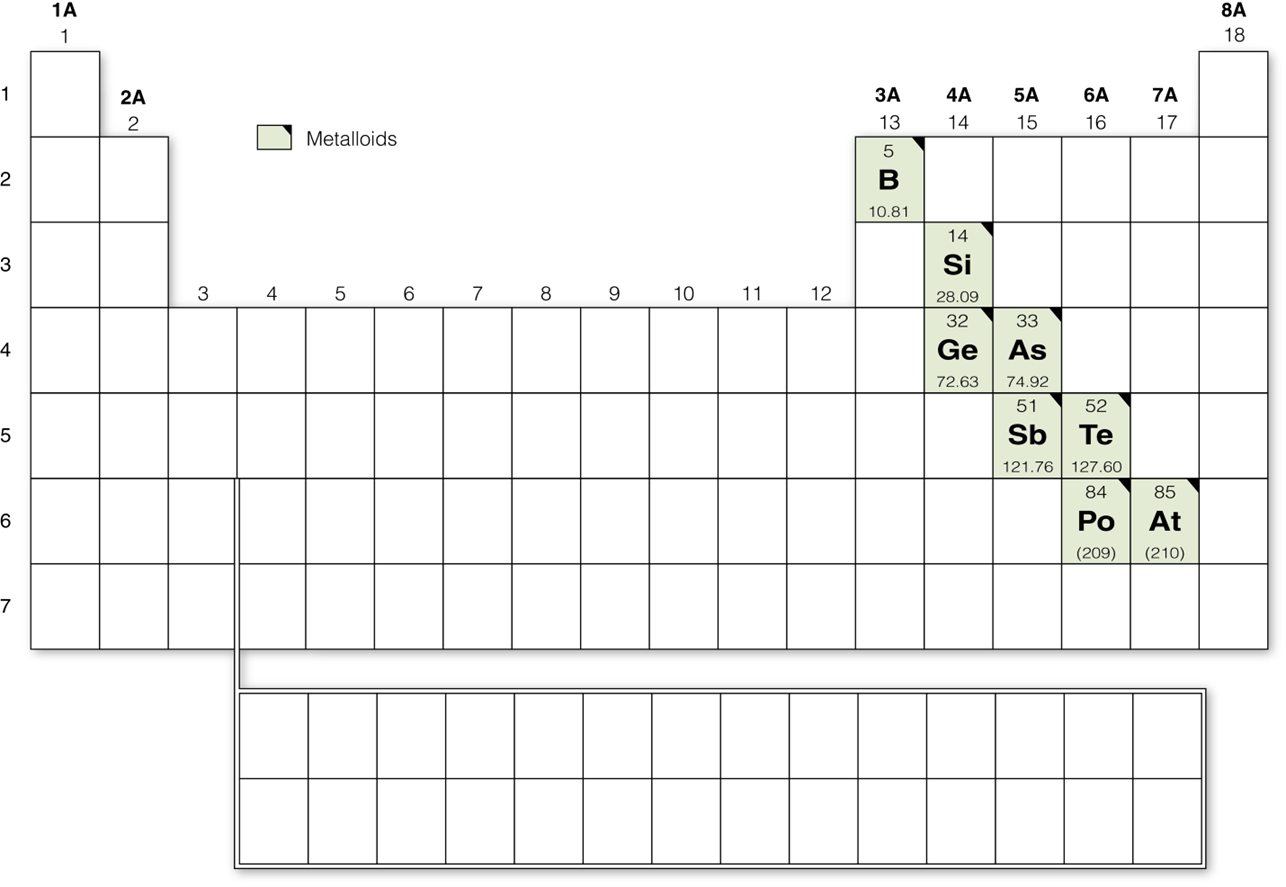
Metalloids
Between the metals and nonmetals, there is a stair step pattern of elements called the metalloids. These are semi conductors, meaning they conduct electricity, but not as efficiently as metals do. These are essentially the components of modern electronics.
Group 1A: Alkali Metals
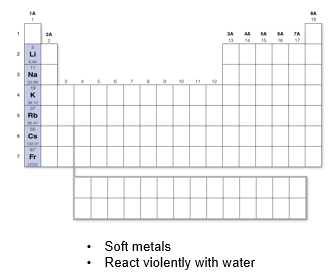
React violently with water or even with moisture in the air
Group 2A: Alkaline earth metals
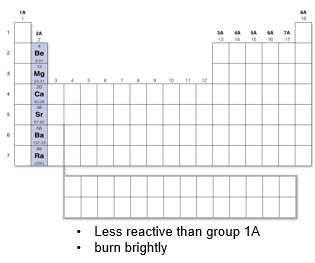
Burn brightly when combined with oxygen
Group 7A: Halogens
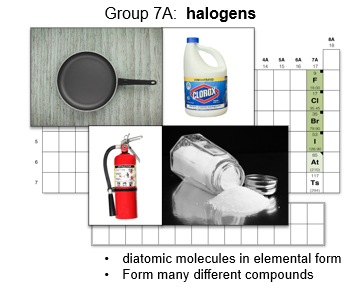
Substances such as bleach, Teflon, fire retardants, antiseptics, and table salt include halogens in their composition
Group 8A: Noble gases
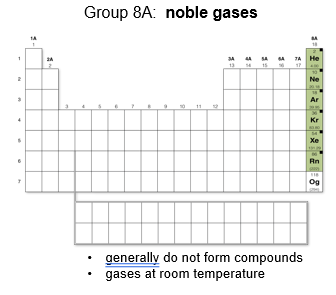
These gases generally do not react with other elements to form compounds.
Uncovering Atomic Structure
- The atoms of each element are unique
- Atoms combine in whole - number ratios to form compounds
- Atoms are not created or destroyed in chemical reactions
Dalton stated before that atoms could not be broken into smaller pieces, but this turned out to be untrue. Later discoveries showed that atoms are composed of even smaller components called subatomic particles which the arrangement of determines the identity and behavior of atoms.
Describing particles
We describe atoms and subatomic particles based on two key properties - their mass and their charge.
We see the effects of charged particles through the familiar phenomenon we call electricity and electrical energy, a form of energy that involves the motion of charged particles
1800: The year that changed chemistry
In 1800, the Italian Scientist Alessandro Volta built the first battery (more formally called an electrochemical cell) - a device capable of moving electronically charged particles. Volta built this device by placing alternating plates of zinc and copper on either side of pieces of cardboard soaked in sulfuric acid. When he connected the two sides with a metal wire, the result was an electrical current, the flow of charges particles from one side of the battery to another.
- Using this controlled electrical energy of the battery, scientists later discovered that you can use electrical energy to separate some compounds, such as water, into their elements.
- This allowed scientists to discover many new elements in a few short years. Much of the information on Mendeleev’s periodic table was a direct result of Volta’s discovery.
Electrons
In addition, scientists began to identify charged particles, and to measure how charged particles interact with each other. One way they did this was to connect a battery to two plates.
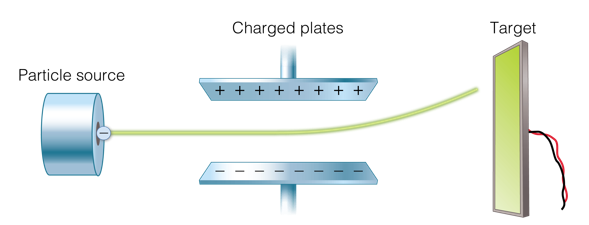
This produced positive charges on one plate, and negative charges on the other plate. They then firect particles between the plates. As they passed through the plated, particles curved toward the oppositely charged plates. Based on this, scientists were able to determine the charges on different particles.
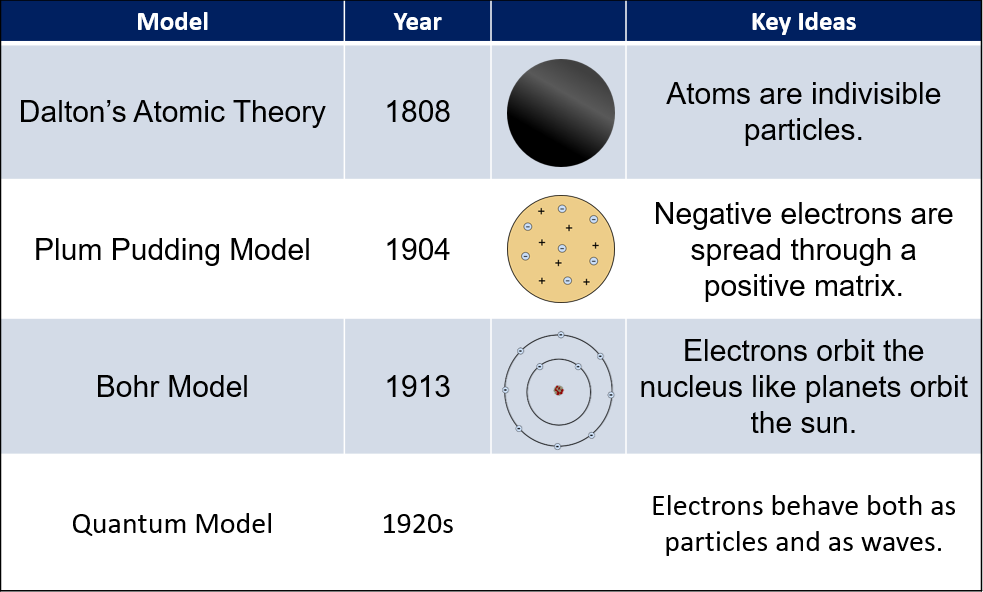
Plum Pudding Model
Thomson and other scientists knew that atoms contained charged particles, but did not know how they were arranged. They knew that atoms contained small negatively charged electrons, and to balance there had to be something that contained positive charges. He hypothesized that the small, negative electrons were spread throughout the positive atomic substance, like blueberries through a muffin, which was called the plum pudding model.
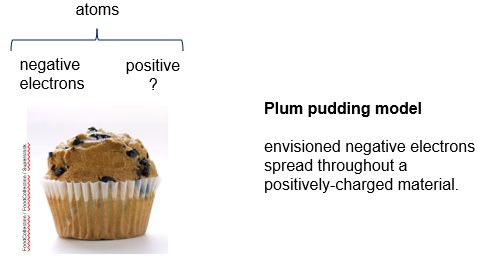
This model did not last long, and Ernest Rutherford, one of Thomson’s former students, showed that the model was incorrect
In 1909, Ernest Rutherford and his students were studying the behavior of positively charged particles called alpha particles. Rutherford expected the alpha particles to pass through the film and hit behind it, and some did. However, to his surprise, a small number of the particles reflected off the film, and back toward the alpha particle source.
This led Rutherford to conclude that the plum pudding model was not correct and he postulated that most of the atom was empty space, with a very dense nucleus at the center of each atom. So, the very dense nucleus was the reason that sometimes the particles were deflected back to the source.
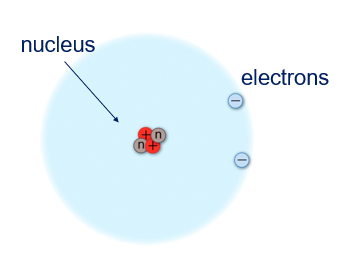
We now know that Rutherford was correct. We draw atoms as a nucleus surrounded by orbiting electrons; however, these pictures are never drawn to scale. Although the nucleus contains almost all of the mass of the atom, it is incredibly dense, packing the mass into a very tiny volume. The volume of the nucleus to the volume of the atom has been compared to the size of an insect inside a football stadium.
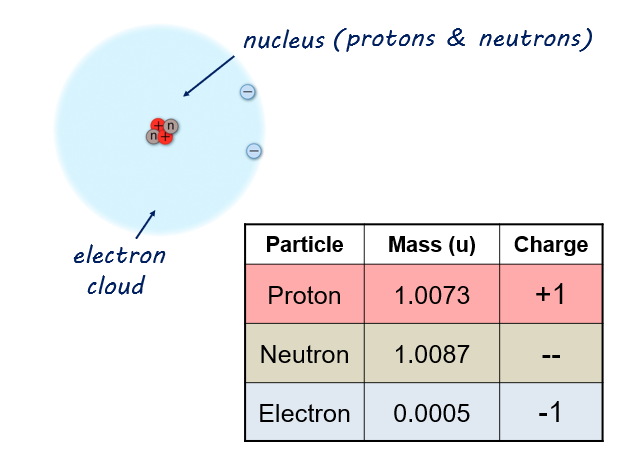
This is a picture of an atom, with the protons and neutrons on the inside, and electrons on the outside.
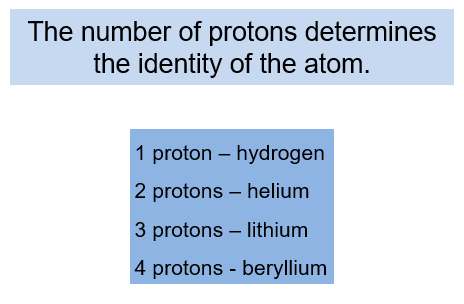
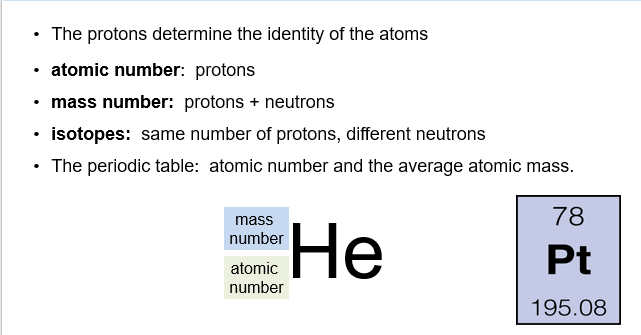
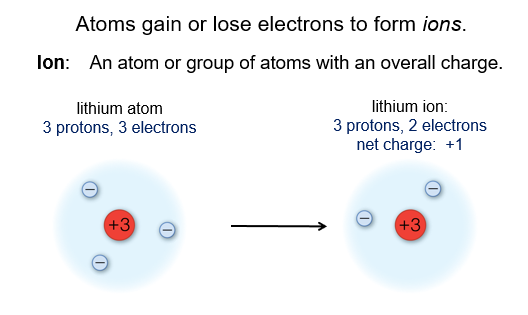
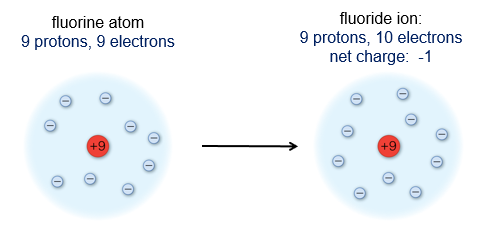
Of these particles, it is the number of protons that determines the identity of the atom. For example, any atom that has just one proton in its nucleus is a hydrogen atom.

The atomic number is the integer value normally seen above the atomic symbol of the periodic table. The atomic number also tells us the number of electrons in the neutral atom.
The mass number is the sum of the number of protons and the number of neutrons in an atom.
Isotopes
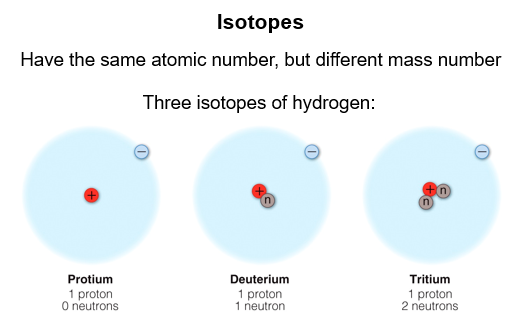
It is possible for atoms to have the same atomic number, but a different mass number. They are called isotopes. The mass number on the periodic table is the average mass number.
Writing Atomic Symbols
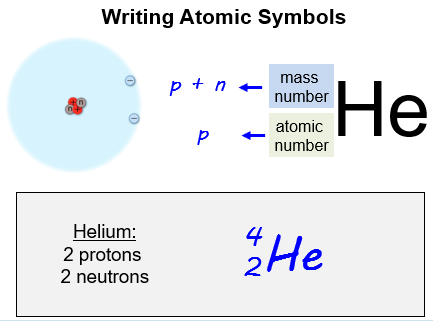
If we want to show the atomic number and the mass number of an atom, we typically write these numbers alongside the chemical symbols. We place atomic numbers at the lower left - hand side of the atomic symbol, and the mass number at the upper left - hand side of the atomic symbol.
Example:
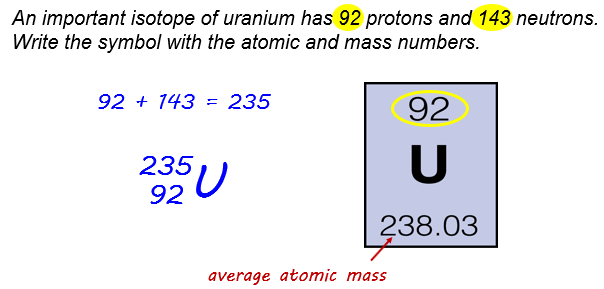 Average Atomic Mass
Average Atomic Mass
Average atomic mass: A weight average of the different isotopes of an element
In order to understand what a weighted average means, let’s consider a simple example involving poker chips:
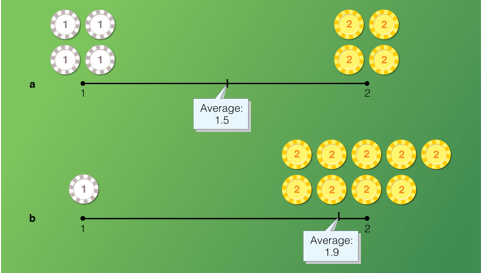
The average is closest to the side of the chips in which we have more of.
To mathematically figure the average atomic weight:
 An example:
An example:
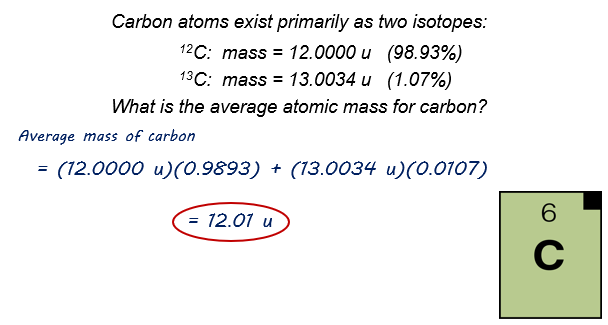
Summary