01: Introduction to Cellular Metabolism Principles of Metabolic Regulation
Section Objectives
Define: metabolism (anabolism, catabolism)
Describe the process of regulatory feedback
Explain the role of negative feedback
Describe the control mechanisms that regulate metabolism
Describe the role of diet and nutrition in the production of energy
Describe the role of ATP and Coenzyme A as carriers
Describe the biochemical role of redox cofactors
Niacinamides
Flavins
Life Needs Energy
Recall that living organisms are built of complex structures.
Building complex structures that are low in entropy is only possible when energy is spent in the process.
The ultimate source of this energy on Earth is sunlight
Metabolism Is the Sum of All Chemical Reactions in the Cell
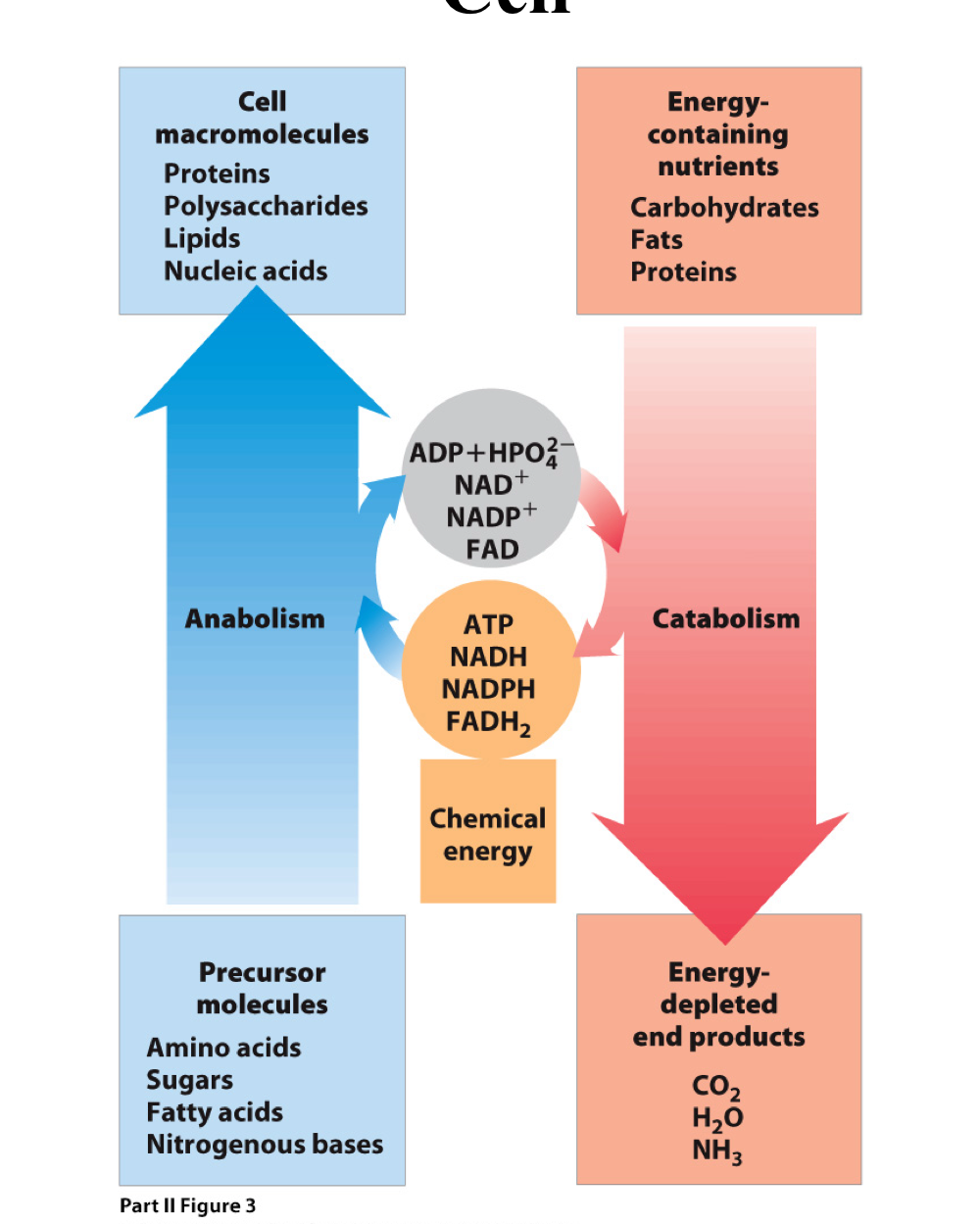
Metabolism
Definition: Primary metabolism is a collection of reactions responsible for
the generation of energy for the cells
the use of this energy, along with simple organic precursor molecules, to make more complicated molecules for the cell
Anabolism: subset of those reactions that lead to the synthesis of complex molecules from simple precursors
in the definition of primary metabolism
Catabolism: subset of those reactions that lead to the breakdown of energy- yielding molecules
in the definition of primary metabolism
Metabolism is an orderly progression of chemical transformations
Each reaction is linked with another
Pathway: The set of linked reactions from precursor to final product, along with enzymes, cofactors, and regulatory factors
Enzyme + cofactor = Holoenzyme
Not all enzymes need cofactors
Pathways require some energy input
Pathways are tightly regulated and coordinated with other pathways
Pathways can be
Linear
Branched
Circular
Regulatory Feedback
Feedback: When a portion of the output of a system (or a process) returns as input for that system (or process), it is called feedback
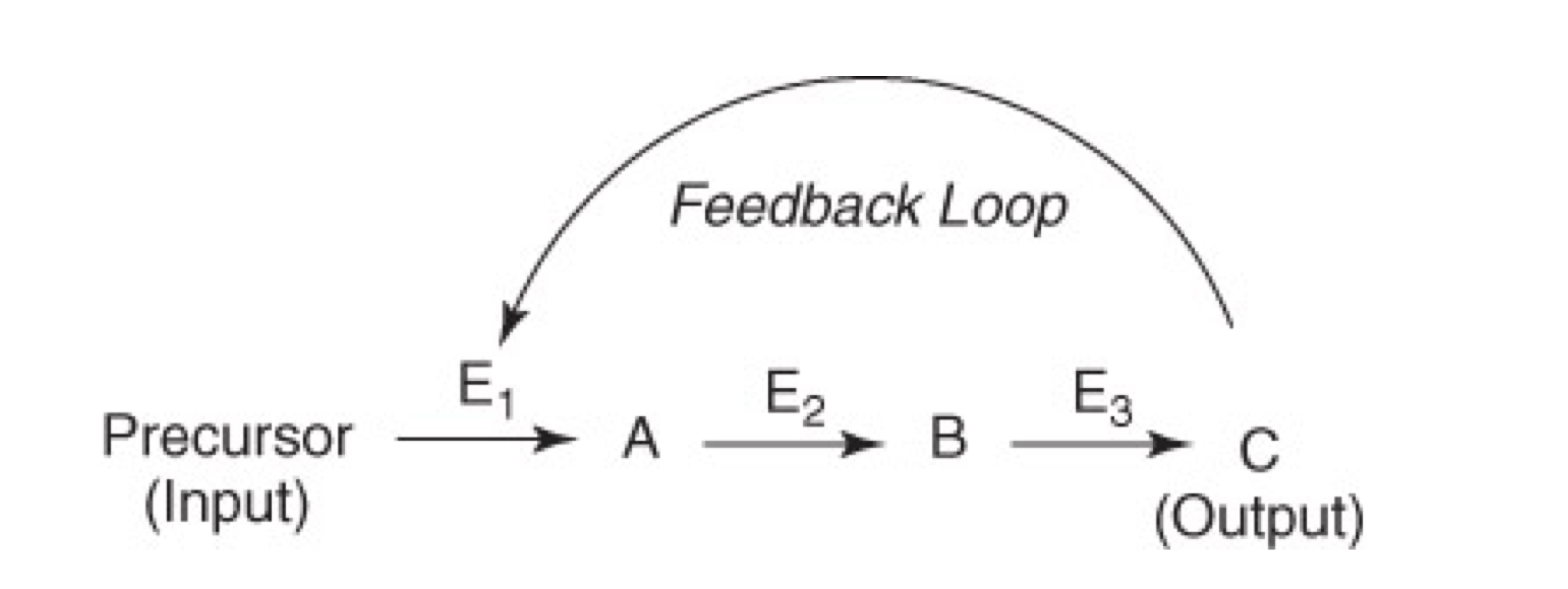
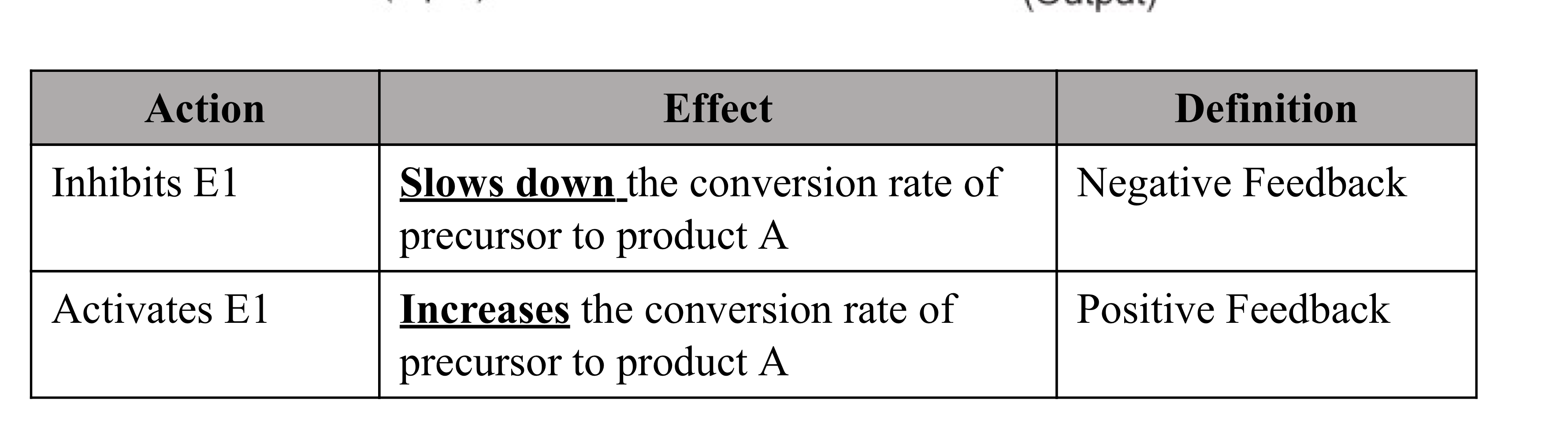
Negative Feedback
Negative feedback is more common in physiology and biochemistry, than positive feedback
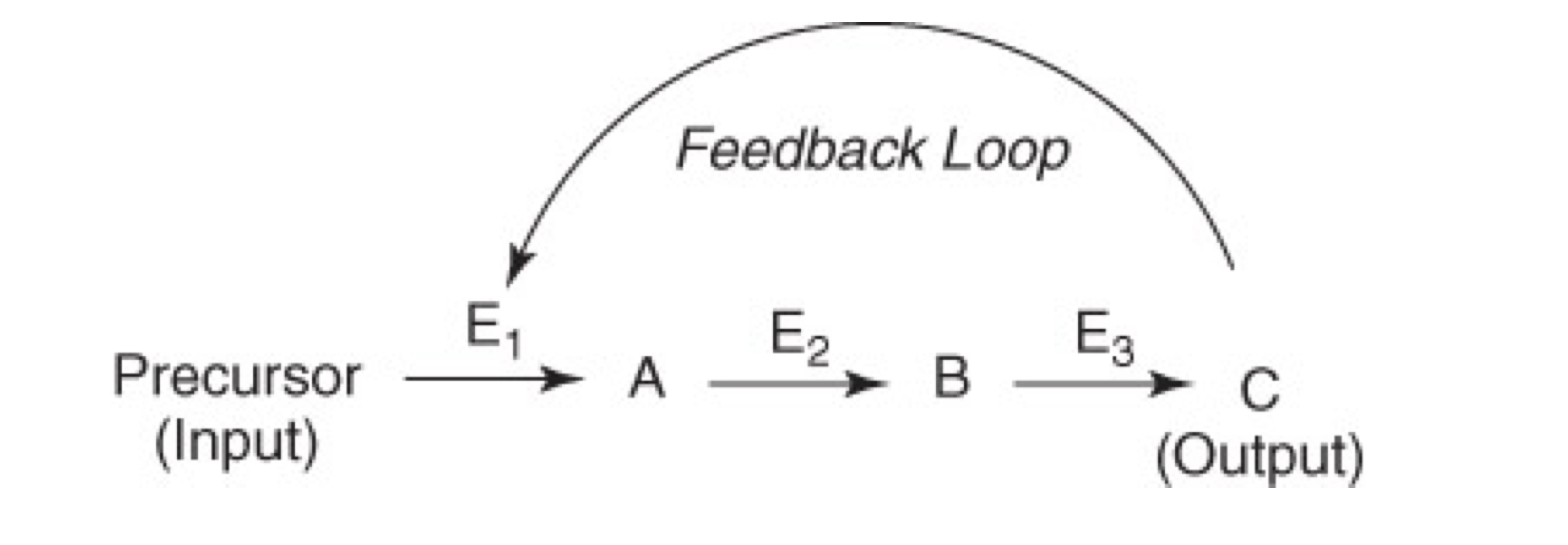
Important features of negative feedback are:
The first unique step of a pathway is regulated
Committed step; no going back
End product is an inhibitor of the process/pathway
Intermediate products can also inhibit enzyme(s)
Product A on E1
Product B on E1 and E2
or Product C on E1, E2, and E3
The end product C may or may not resemble the precursor (or substrate) for the enzyme
Inhibition of enzymes that control metabolic pathways results in greater efficiency
Inhibition is ideally reversible
Product of the pathway binds to the enzyme and changes enzyme
conformation
Competitive
Non-competitive
Allosteric inhibition
Reaction “stops” as a result
Negative feedback is a regulated process
Only necessary reactions proceed
Unnecessary, wasteful loss of energy and materials is avoided
Metabolic Control Mechanisms
Metabolism is regulated by several mechanisms
The most important ones are:
Concentration
Compartmentalization
Enzyme activation and deactivation
Reciprocal regulation of competing pathways
Concentration
Concentration mechanisms include the following factors:
Enzyme levels
Enzyme synthesis is controlled by genes
Elevated enzyme levels increases Kcat
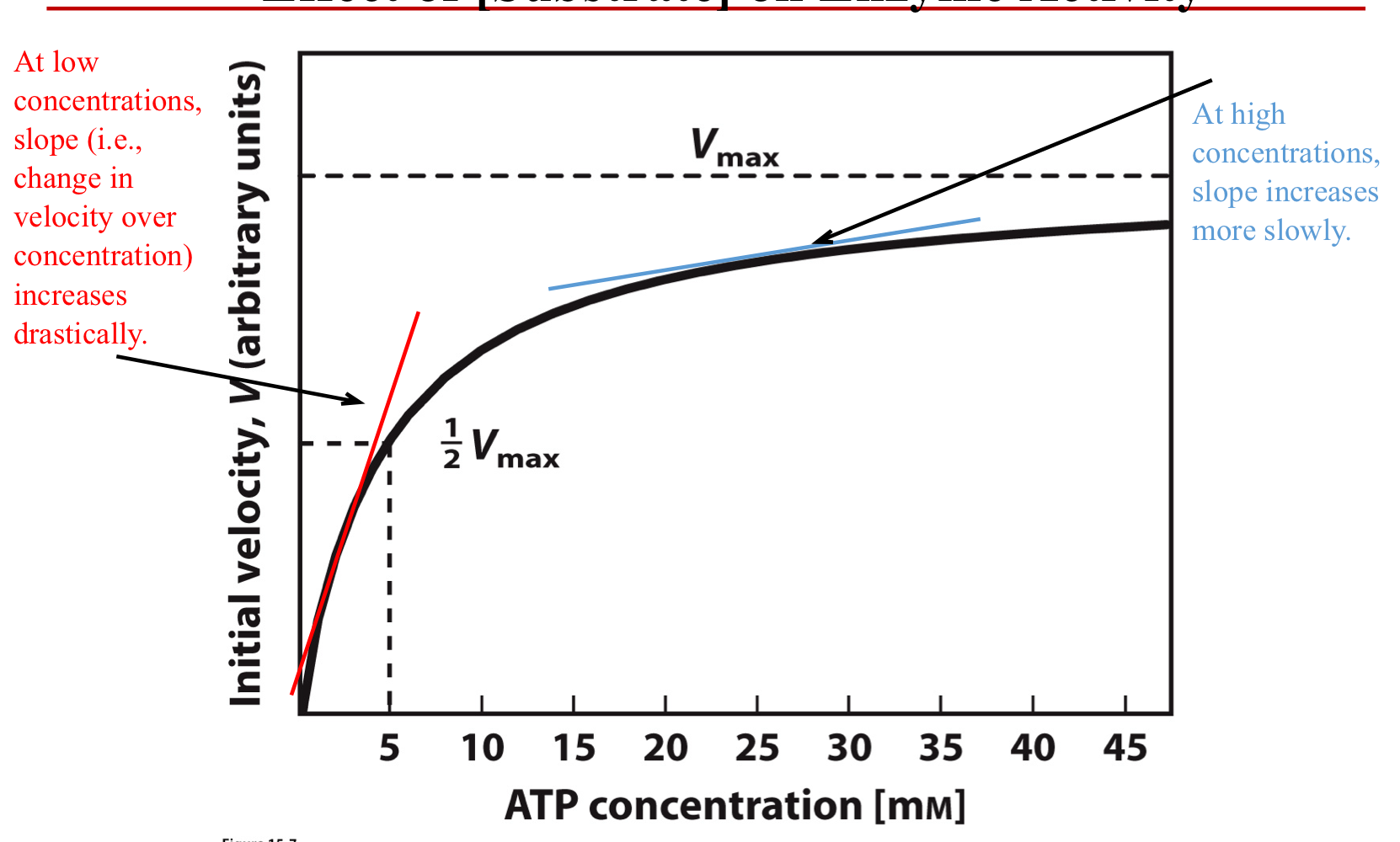
Substrates
Availability of substrate
High conc. of substrate will lead to more product
Rate of reaction depends upon [S]
Cofactors
Availability of cofactors
Presence of cofactors will favor product formation
Effect of [Substrate] on Enzyme Activity
Compartmentalization
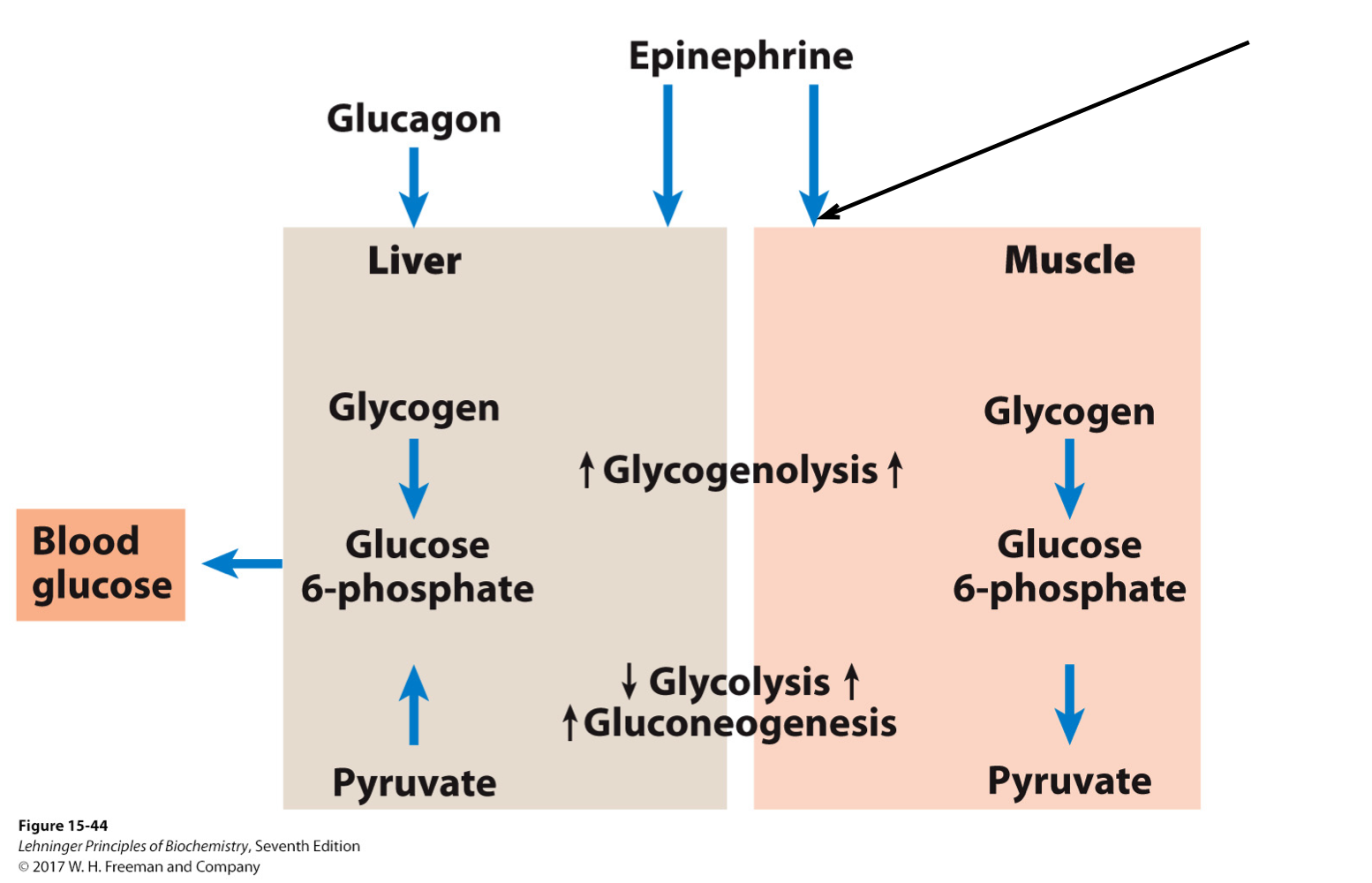
Different or opposing pathways are placed in different cellular compartments i.e. cell organelles, or different organs (muscle v. liver)
Nucleus: DNA replication, synthesis of mRNA
Mitochondria: Krebs’ cycle, fatty acid oxidation
Cytosol: glycolysis, fatty acid synthesis
The transport systems that ferries material across the membranes are also regulated
Affects concentration of enzymes, substrates and cofactors on either side of the membrane
E.g. OATP (Anion Transporter Proteins), P-glycoprotein
Control of Carbohydrate Metabolism in the Liver vs the Muscle
Enzyme Activation and Deactivation
The activation/deactivation of enzymes directly impacts their catalytic
activity
Catalytic activity is influenced by substrates or inhibitors
Covalent modifications
Non-covalent modifications
Enzymes with mutually opposing roles may co-exist in the same organelles
Their activity is controlled by the process of reciprocal regulation
E.g. phosphatases and kinases in the cytosol
These enzymes have opposite types of actions. Kinases load phosphate groups on molecules, while phosphatases help unload those phosphate groups.
Phosphate groups ... think ATP, ADP!
Phosphorylation of Enzymes Affects their Activity
Phosphorylation is catalyzed by protein kinases.
Dephosphorylation is catalyzed by protein phosphatases
or they can be spontaneous.
Typically, proteins are phosphorylated on the hydroxyl groups of Ser, Thr, or Tyr.
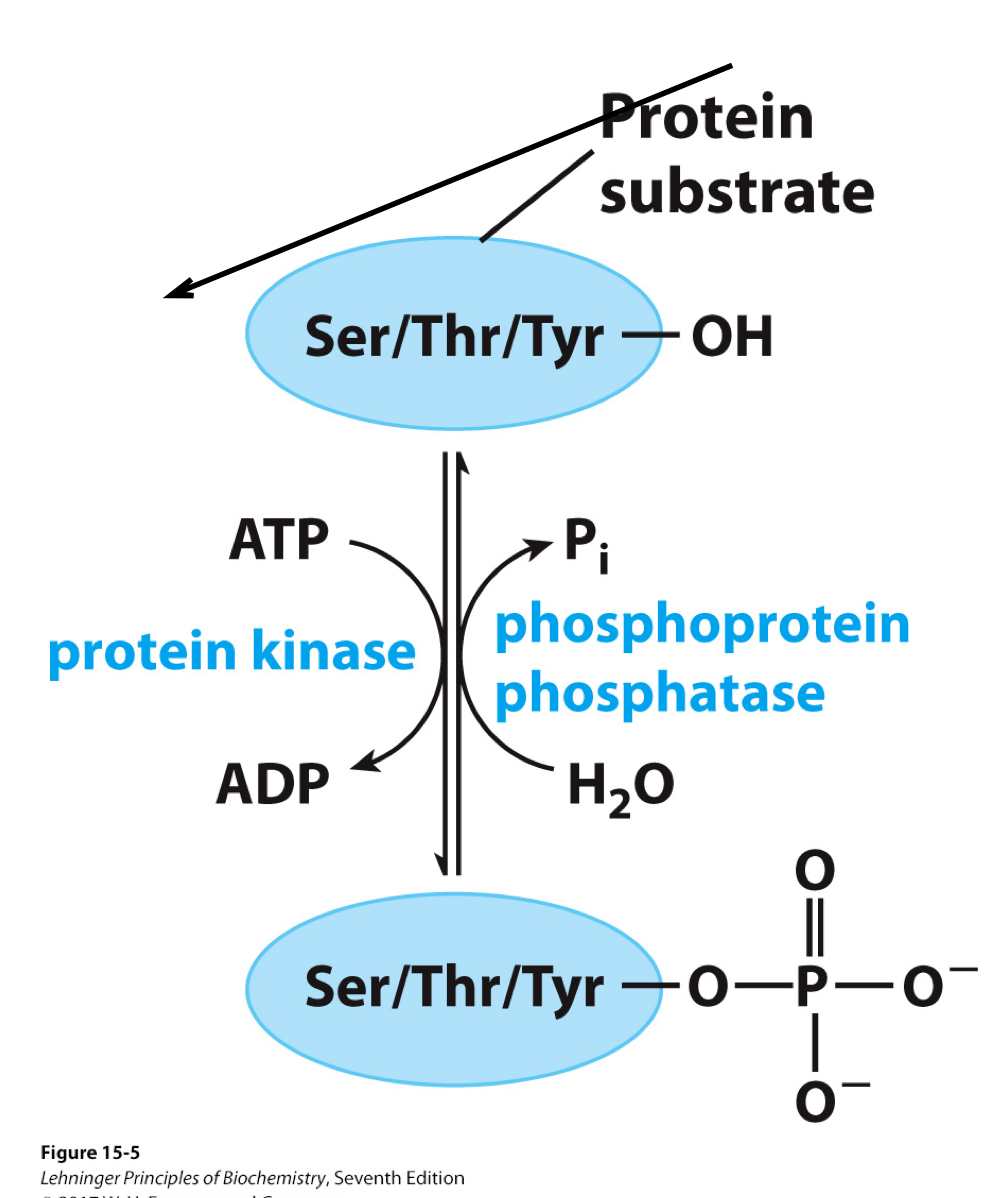
Reciprocal Regulation
The cell contains many enzymes for biosynthesis of molecules
These molecules are necessary for the cell’s function
Some enzymes may have opposing activities
Compartmentalization may not always work
Enzyme activity carefully controlled to prevent wastage
Control is done by reciprocal regulation
Definition: Depending upon the immediate physiological state, one set of enzymes have to be shut off when the other set of enzymes is active.
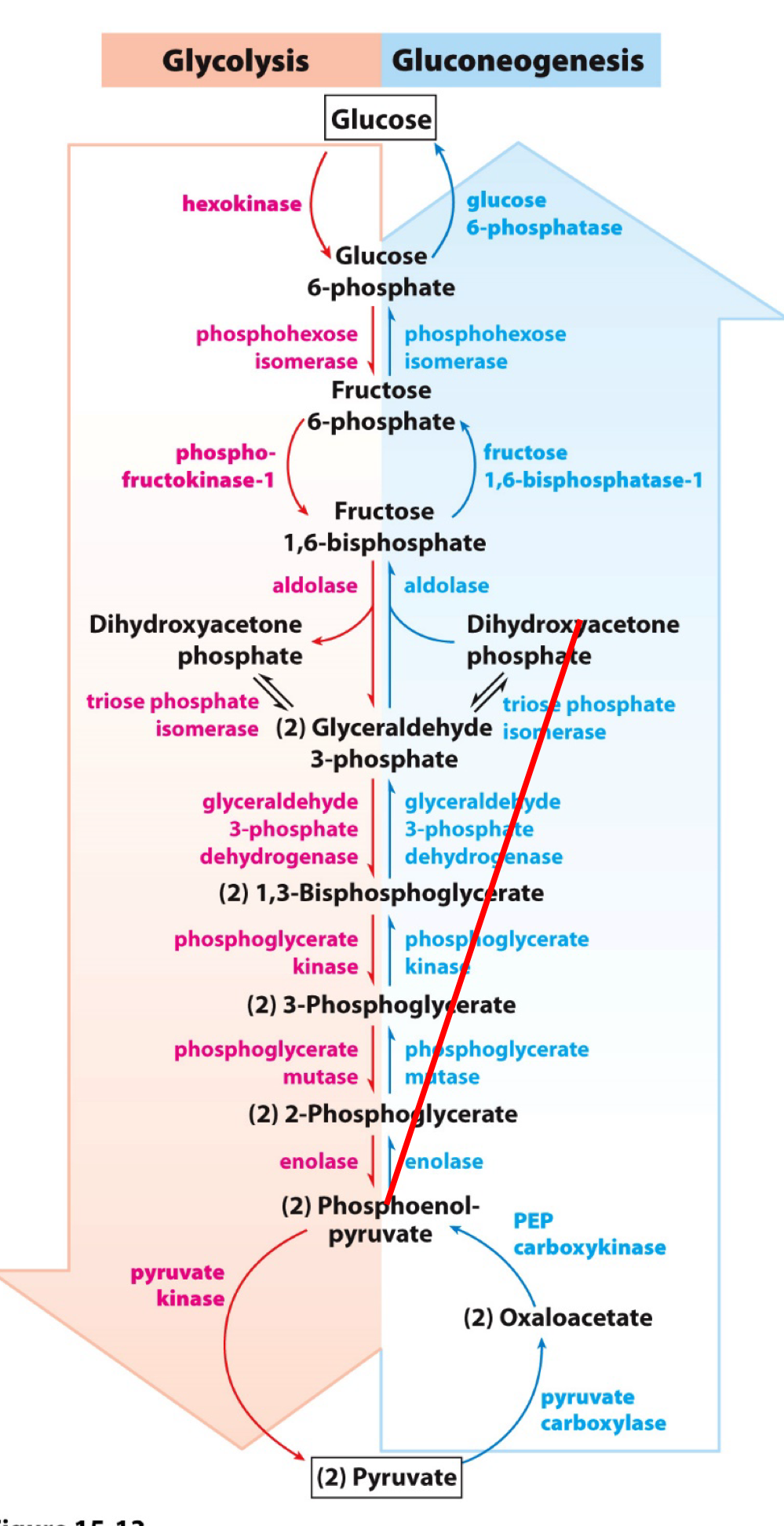
Glycolysis vs Gluconeogenesis
Regulated enzymes often correspond to points in the pathways that have the same substrate and product, but a different enzyme.
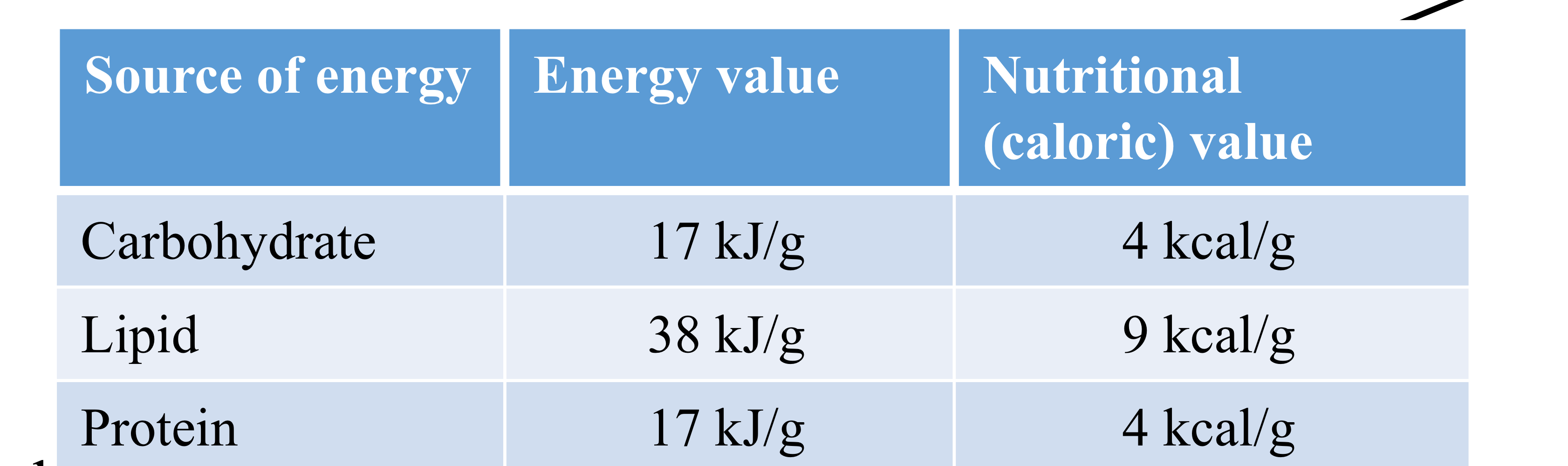
Major Food Components
Food is a complex mixture of
Carbohydrates, Lipids (fats), Proteins (major components)
Vitamins and minerals (minor components)
Carbohydrates (sugars)
Complex sugars (polysaccharides)
Simple sugars (monosaccharides and disaccharides)
Lipids (fats)
Triglycerides (aka Triacylglycerols)
Proteins
Amino acids
Diet and Nutrition
The body needs energy for its various functions
Energy is obtained from a proper diet
Oxidation of carbohydrates and lipids releases energy
Amino acids are needed to build nucleic acids and proteins
Enzymes!
Vitamins, minerals, are not synthesized by the body
They have to be obtained from the diet
Water is a major component of the cell
Transport of molecules, ions
Dissolution of molecules, ions
Solvent medium for cellular reactions
Stages of Digestion of Food
Polymeric foodstuff is broken down to monomeric components (Stage I)
Enzymes in the mouth and gut; acid in the stomach; bile salts in the intestine
Monomeric components are converted to simple metabolic intermediates (Stage II)
Components are transported to the cell by the blood; conversion occurs within the cell
Degradation of simple intermediates to CO2, NH3, water and urea (Stage III)
Catalyzed by enzymes
Energy-generation via metabolic pathways
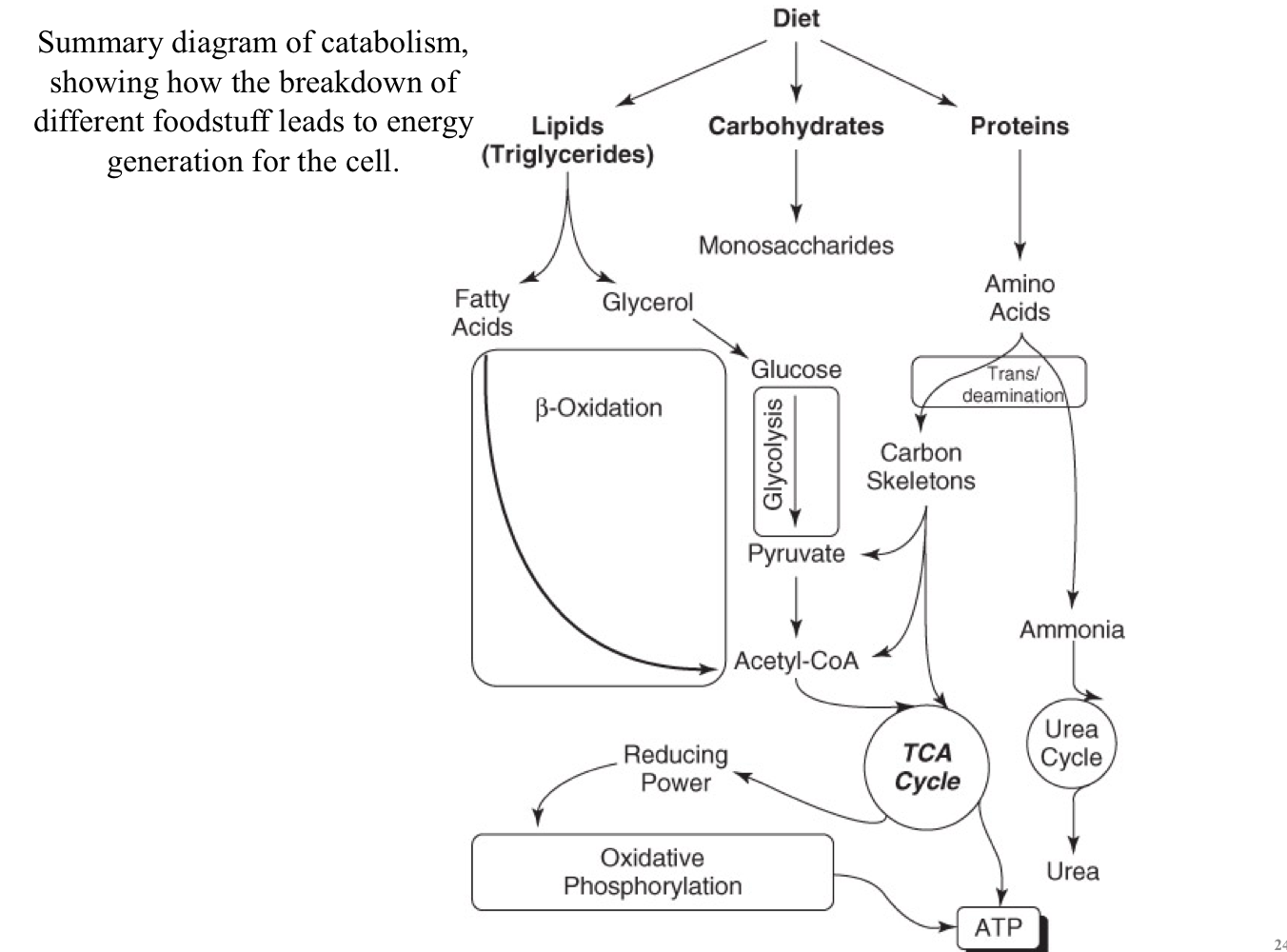
Metabolic Pathways for Digestion
Carbohydrates, lipids, and proteins are sources of energy
Metabolic pathways involved in the breakdown to release energy
Polysaccharides are hydrolyzed to mono- and disaccharides
Triglycerides are hydrolyzed to “free” fatty acids and glycerol
Proteins are hydrolyzed to amino acids
Energy and ATP
Activated group carriers reduce energy barriers
Makes substrates more reactive
Electrophilic and Nucleophilic reactions
Acid-base catalysis
Enables the conversion of substrates to products
Many are co-substrates
Derived from vitamins
E.g. Adenosine Triphosphate (ATP), Coenzyme A (CoA), S-Adenosyl Methionine (SAM)
Pro-tip #1: Think of these molecules as good leaving groups
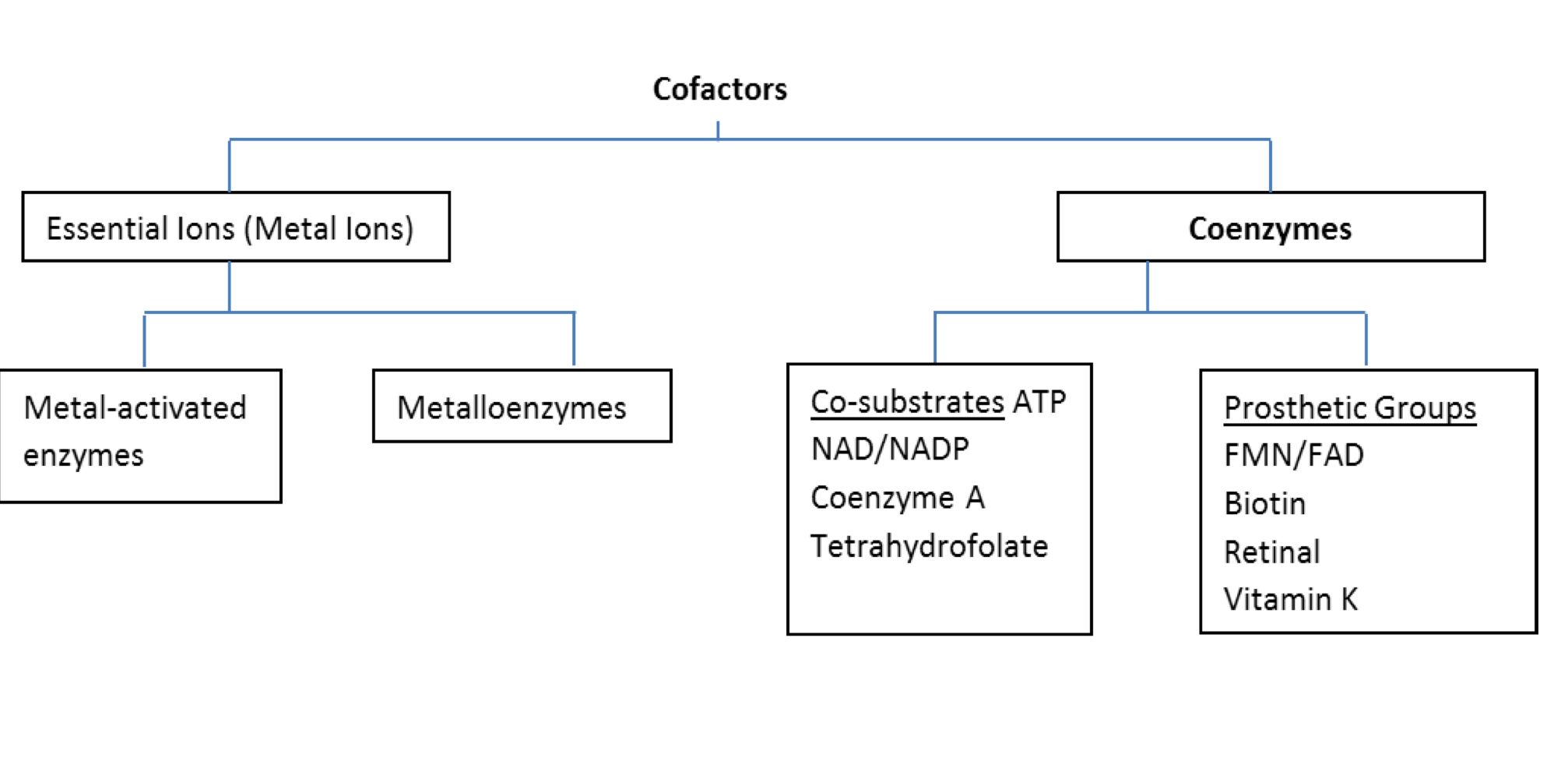
Adenosine Triphosphate (ATP)
Adenosine Triphosphate
Adenine (Nitrogen base)
Ribose (pentose sugar)
Phosphate groups
3: triphosphate (ATP)
2: diphosphate (ADP)
1: monophosphate (AMP)
Hydrolysis of phosphate bonds releases huge amounts of energy
7.3 kcal/mol (30.5 kJ/mol)
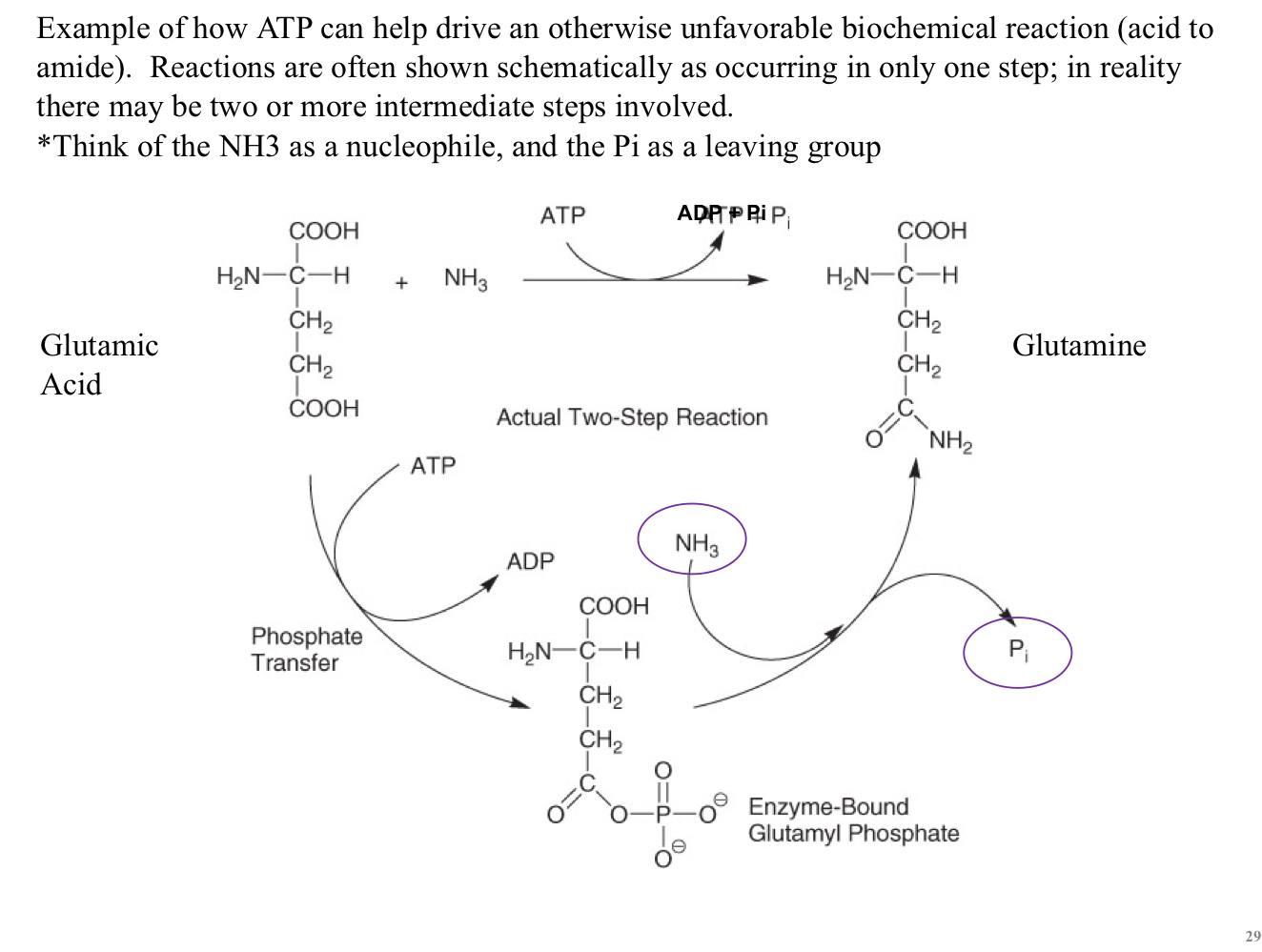
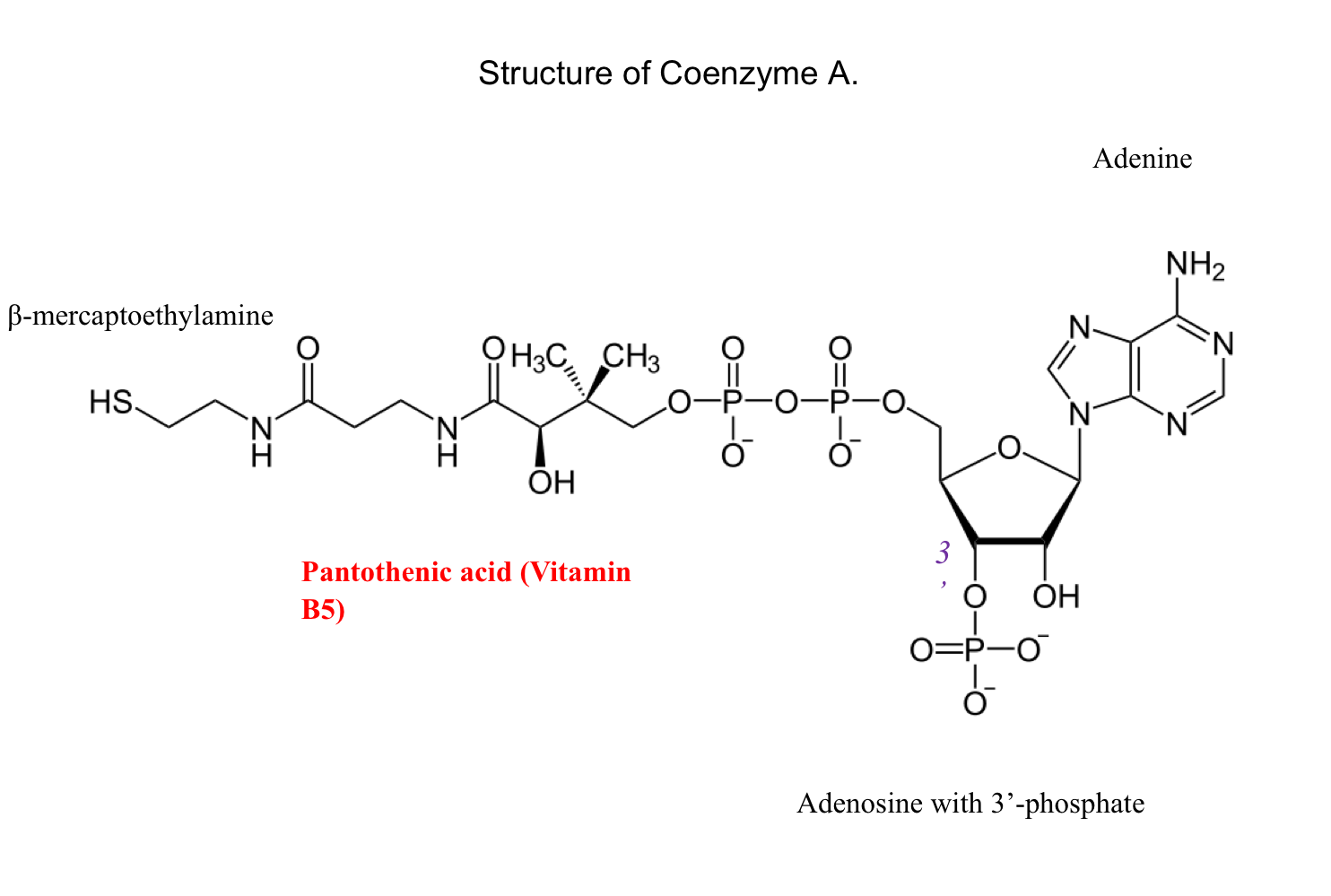
CoEnzyme A
Coenzyme A (CoA, CoASH) transfers activated acyl groups
The R group in carboxylic acids are acyl groups
“Acetyl” is a name for a specific type of acyl group
Acetyl CoA is an important energy molecule
E.g. fatty acid metabolism
Redox Cofactors
Metabolism involves sequential oxidation of molecules obtained from our diet
Oxidation-Reduction reactions: Redox
Redox reactions involve electrons
Recall: OIL RIG; LEO GER
Electron transport chain in the mitochondria
Helper molecules in the cell assist in redox reactions
Organic molecules (possess ionizable groups)
Metals (accept and donate electrons)
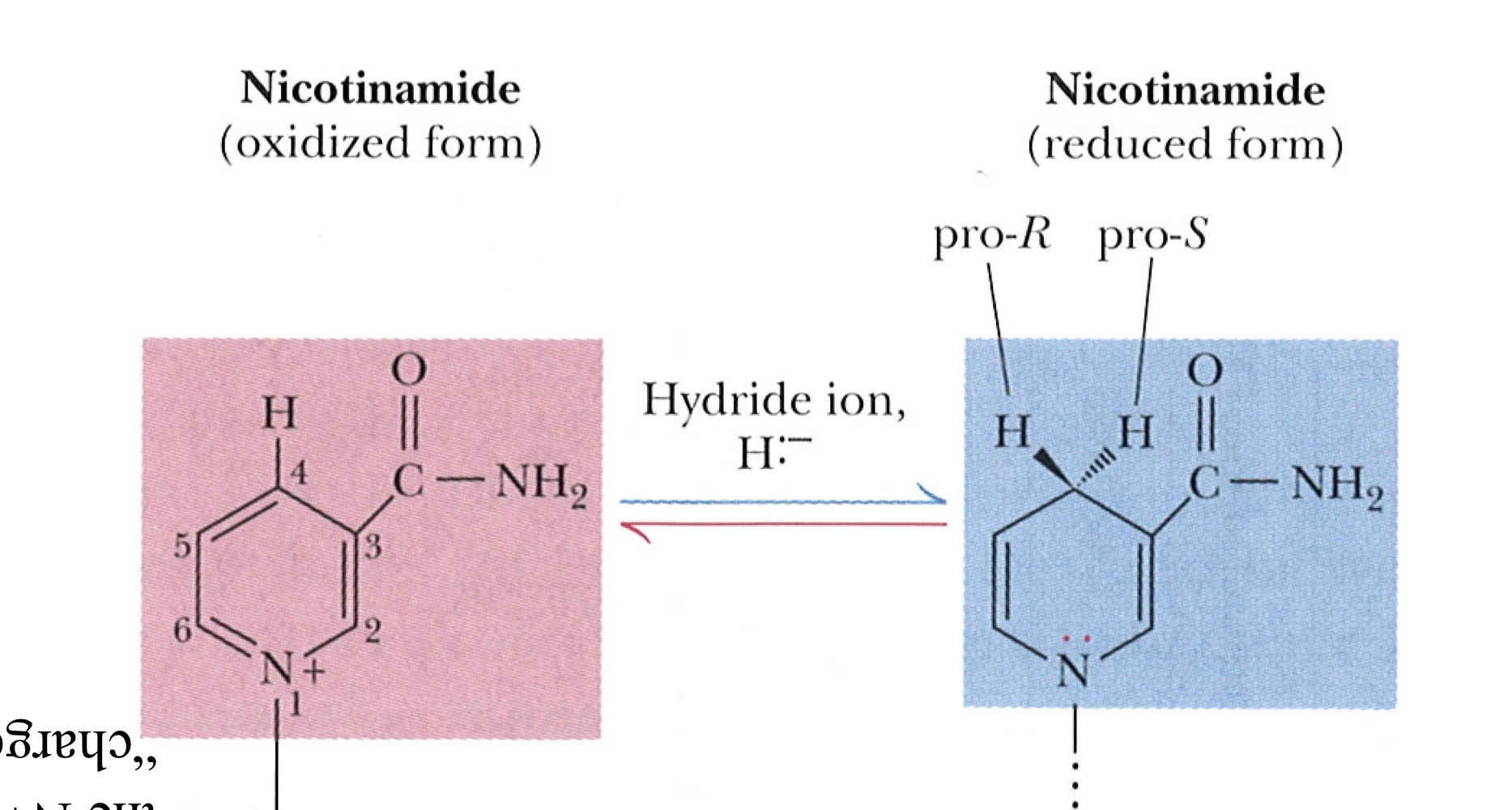
Nicotinamide Adenine Dinucleotide (NAD+ and NADH)
Flavin Adenine Dinucleotide (FAD and FADH2)
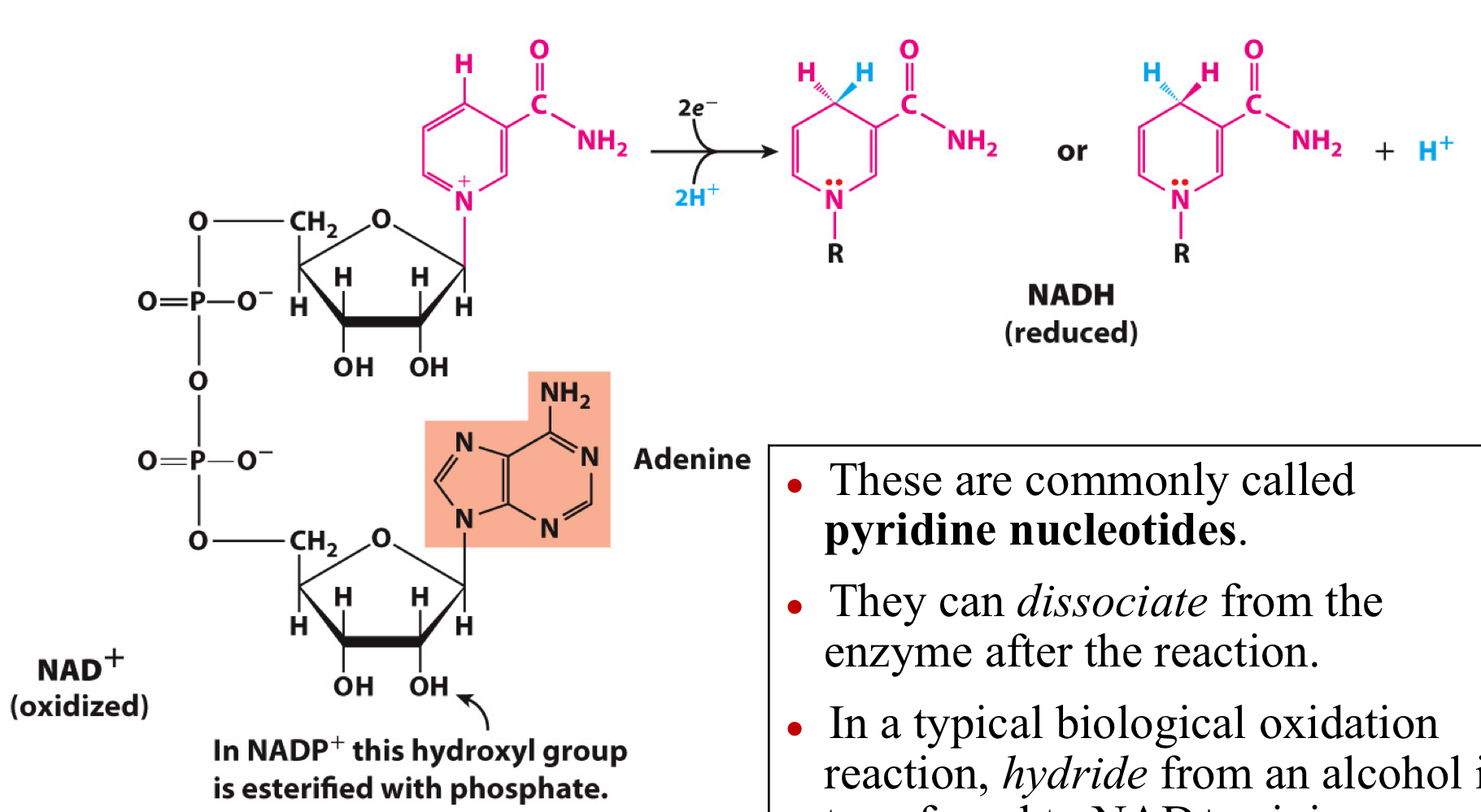
NAD and NADP are Common Redox Cofactors
These are commonly called pyridine nucleotides.
They can dissociate from the enzyme after the reaction.
In a typical biological oxidation reaction, hydride from an alcohol is transferred to NAD+, giving NADH
Nicotinamide adenine dinucleotide, NAD+, and its phosphorylated analog NADP+ undergo reduction to NADH and NADPH
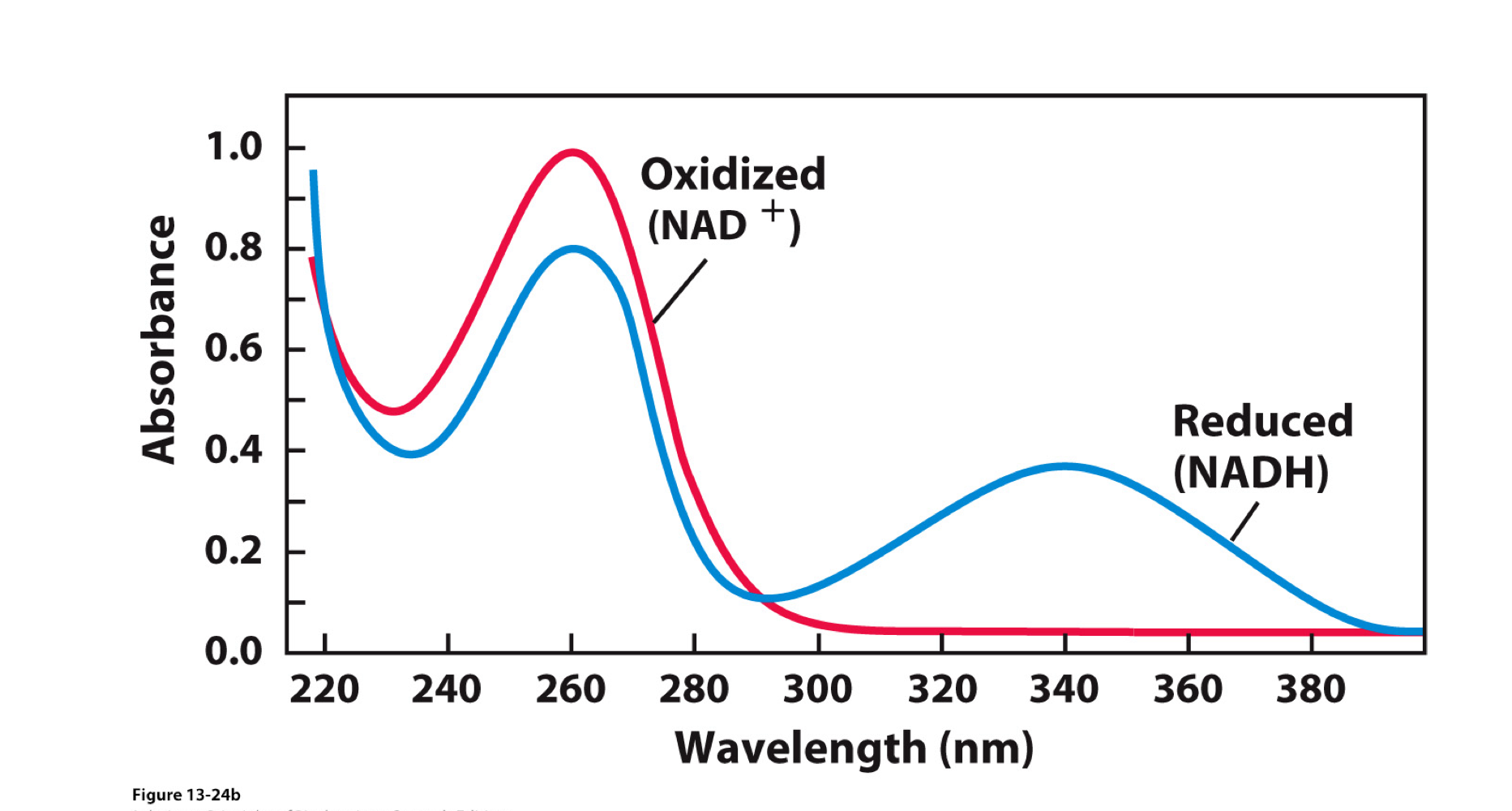
Formation of NADH can be Monitored by UV-Spectrophotometry
Measure the change of absorbance at 340 nm
Very useful signal when studying the kinetics of NAD-dependent dehydrogenases
Lesson in Quantum Chemistry
Most organic molecules, including the reduced fuels, are in the singlet spin
state.
All electrons are paired into electron pairs.
Molecular oxygen is in the triplet spin state
Two electrons are unpaired.
Direct electron transfer from a singlet reduced species to a triplet-oxidizing species is quantum-mechanically unfavorable.
This is why direct oxidation (spontaneous combustion) of biomolecules does not occur readily.
Cofactors, such as transition metal ions and FAD, are able to catalyze consecutive single-electron transfers needed for utilization of O2.
Flavin Cofactors Allow Single Electron Transfers
Permits the use of molecular oxygen as an ultimate electron acceptor
flavin-dependent oxidases
Flavin cofactors are tightly bound to proteins.
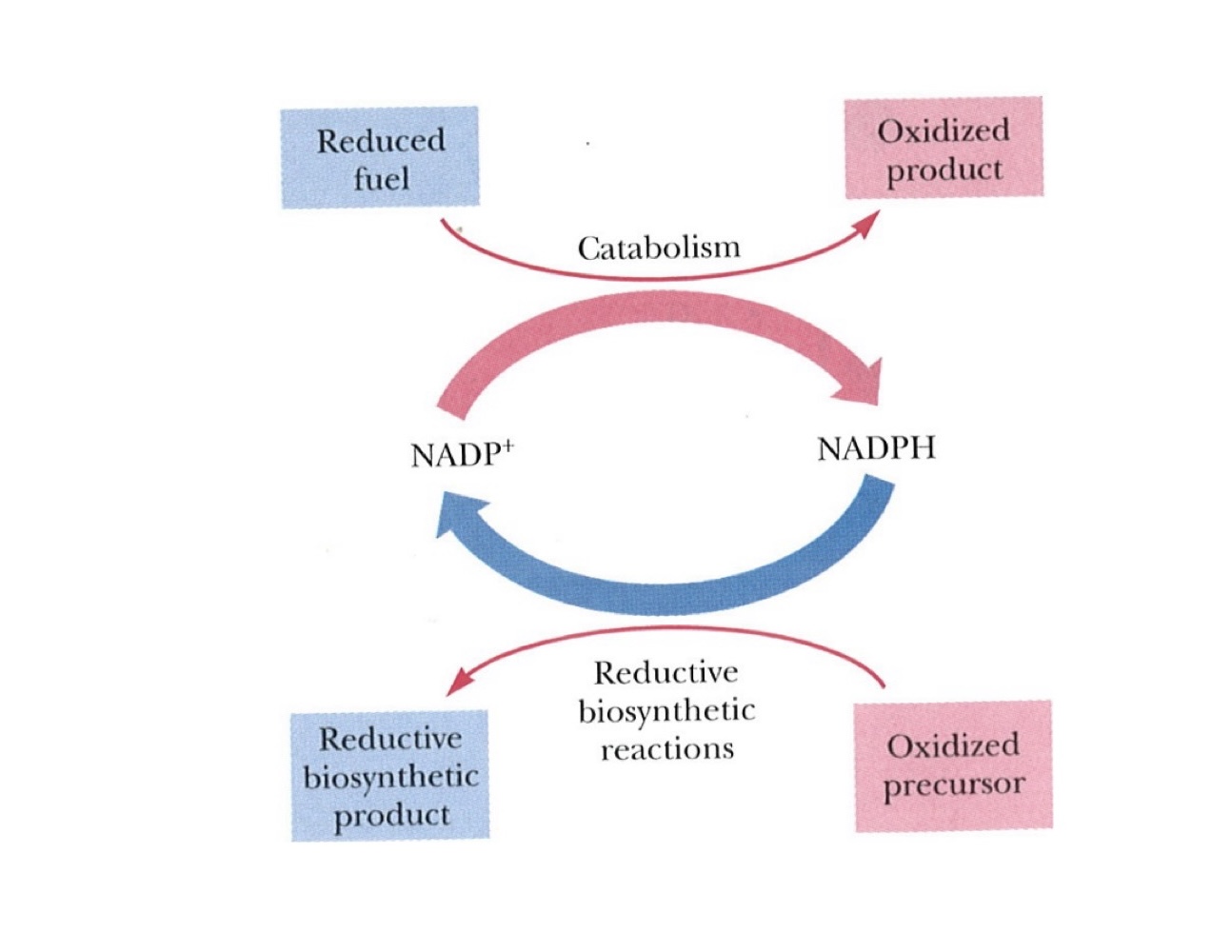
Nicotinamide
Co-substrate derived from niacin (Vitamin B3)
NADH (Nicotinamide Adenine Dinucleotide)
NADPH (Nicotinamide Adenine Dinucleotide Phosphate)
Structure of Nicotinamide
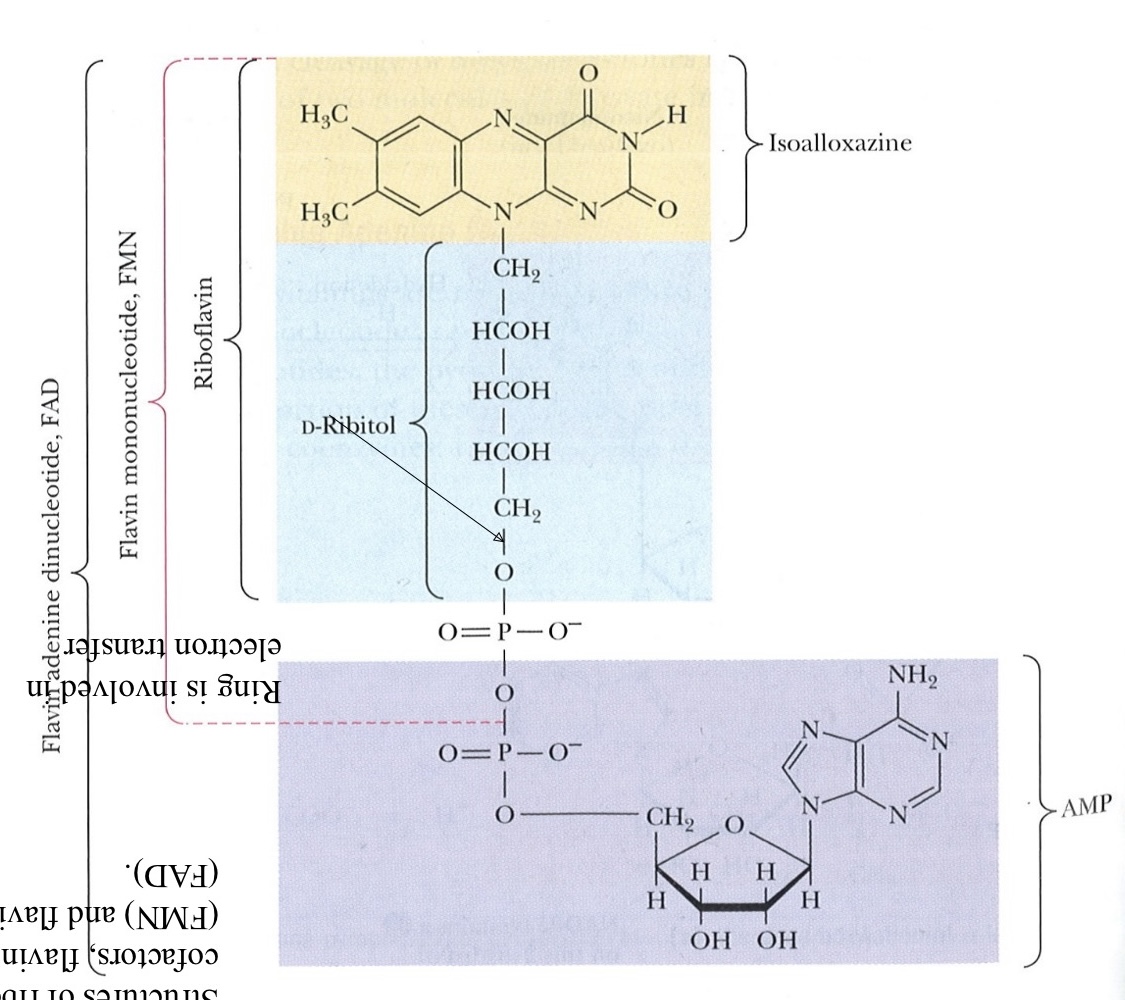
Flavins
Derived from riboflavin (Vitamin B2)
FAD (Flavin Adenine Dinucleotide)
FADH2 is the reduced form
FMN (Flavin Mono Nucleotide)
FMNH2 is the reduced form
Flavins participate in redox reactions
1 e- or 2 e-
Conjugated central ring serves as an electron sink
Pro-tip #3: “Electron Sink” Think of it as a sink where electrons are “drained”
Structure