✭ COVALENT SUBSTANCES
✩1- Covalent Bonding and Geometry
Learning intentions:
[ ] Explain the covalent bonding model
[ ] Draw electron dot diagrams for main group atoms
[ ] Construct Lewis (electron dot) structures for selected diatomic and polyatomic molecules
[ ] Represent the structures of diatomic and polyatomic molecules using structural formulas.
KEY IDEAS OF COVALENT BONDING:
Covalent bonds form between non-metals and other non-metals.
Atoms are particularly stable when they have the same electron configuration as a noble gas (an octet in their outer shell).
Non-metals can share electrons to achieve a stable octet.
A pair of shared electrons is a single covalent bond.
A single covalent bond is represented by a single line.
COVALENT BONDING OCCURS BETWEEN NON-METALS
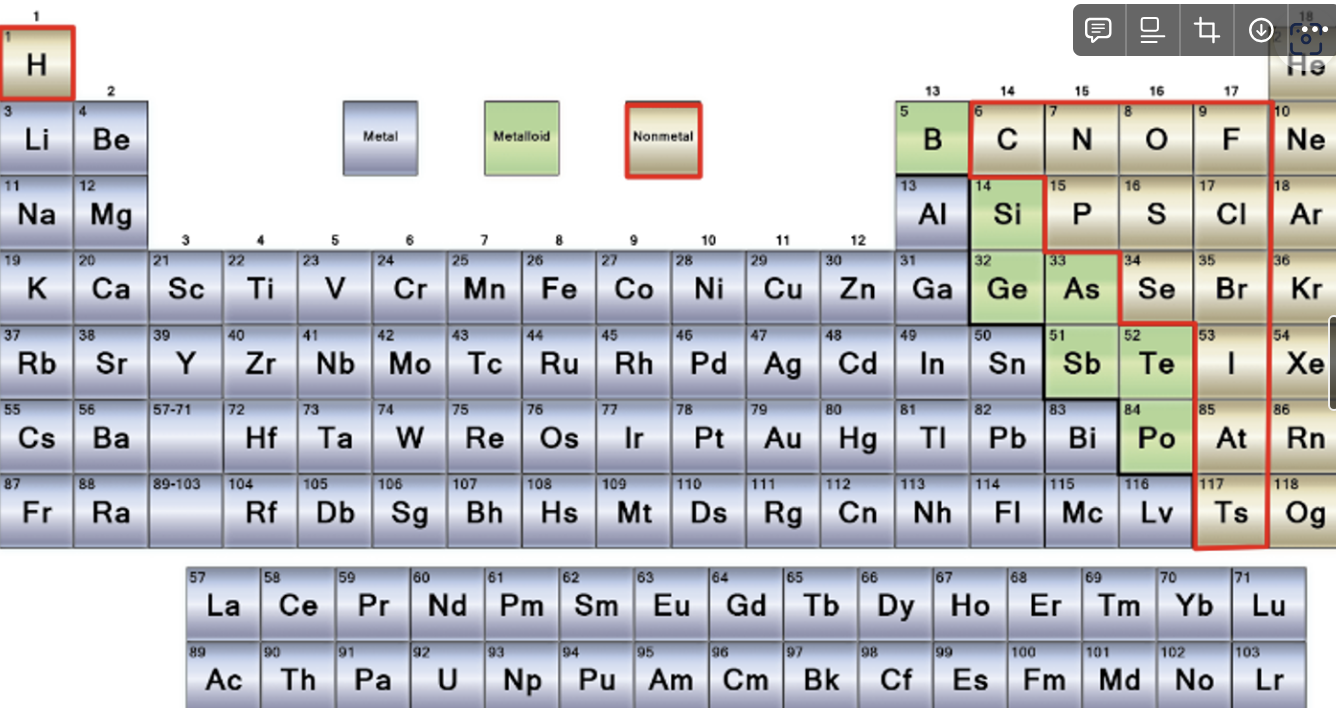 Q: Why are the noble gasses not included?
Q: Why are the noble gasses not included?
A: Noble gases already have a stable “octet” of valence electrons therefore tend to exist in the monoatomic form
LEWIS (ELECTRON DOT) STRUCTURES OF ATOMS
Q: Why are the noble gasses not included?
A: Noble gases already have a stable “octet” of valence electrons therefore tend to exist in the monoatomic form
LEWIS (ELECTRON DOT) STRUCTURES OF ATOMS
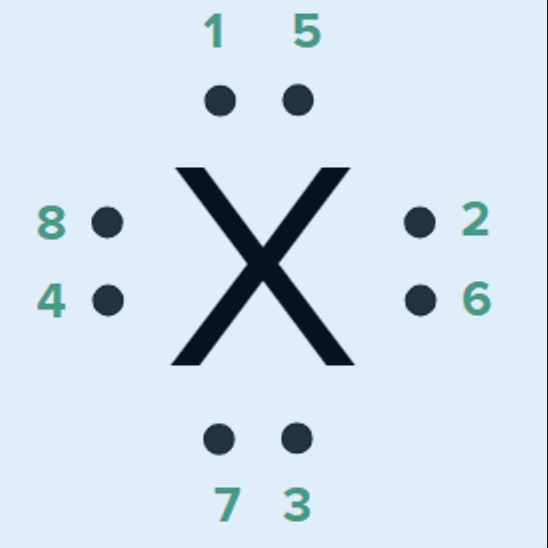
![]() An atom will add electrons to its octet through sharing until it has an octet of electrons
An atom will add electrons to its octet through sharing until it has an octet of electrons
![]()
Unpaired electrons NOT involved in bonding are “lone pairs” or “non-bonding pairs”
![]()
LEWIS VS STRUCTURAL FORMULA
VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR)
Electron pairs in molecules repel each other and try to position themselves as far away from one another as possible.
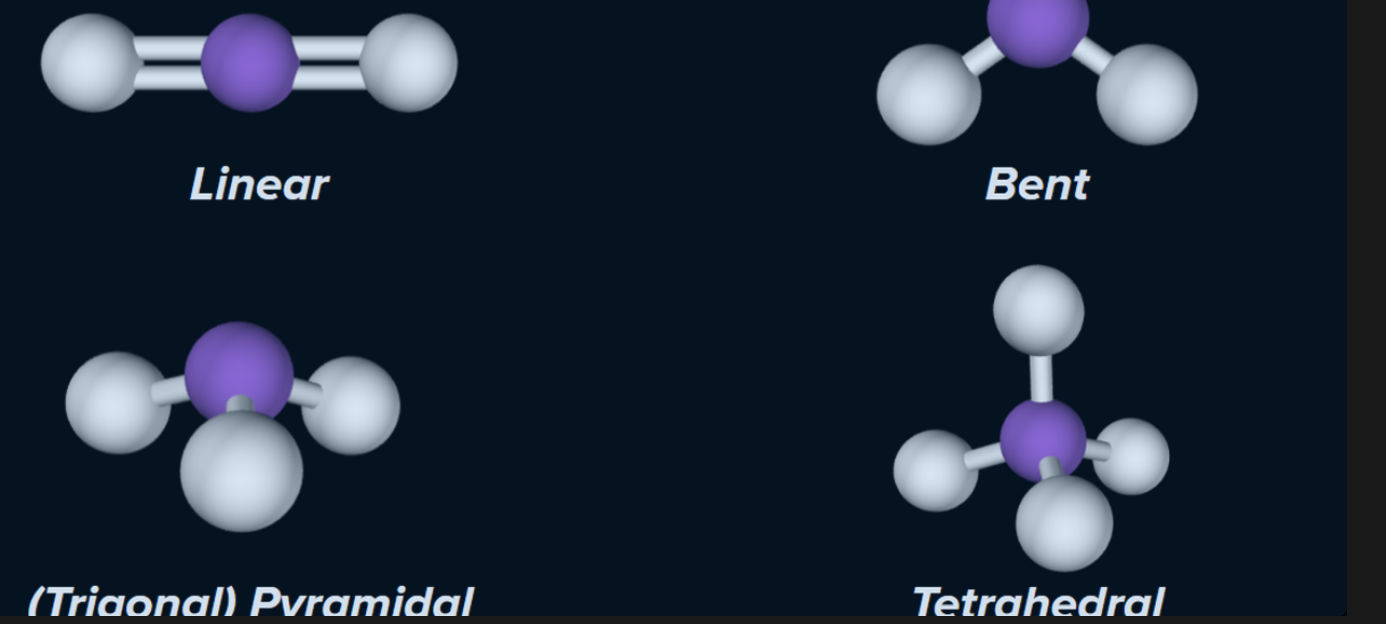
The shape of the molecules is determined by the number of atoms that the central atoms are bonded to and the number of lone pairs on the central atom
SHAPES YOU NEED TO KNOW
✩2- Polarity of Covalent Molecules
Learning Intentions:
[ ] Distinguish between polar and nonpolar bonds based on differences in electronegativity
[ ] Predict the polarity of diatomic and polyatomic molecules by making links to shape and polarity of bonds
[ ] Identify and explain the presence of intermolecular forces.
[ ] Describe the relative strength of covalent bonds and the various intermolecular forces.
POLAR AND NON-POLAR BONDS
Non-polar:
between the same element (+ C-H and other similar electronegativity bonds)
Polar:
different electronegativities (a slightly negative charge (𝛿-) and the other end will have a slightly positive charge (𝛿+). This is known as having a dipole.
POLAR MOLECULES
traits
asymmetric
on the side is more negative compared to the other
dipoles of individual bonds to not “cancel out”
NON-POLAR MOLECULES
contains all non-polar bonds
symmetrical arrangement around the central atom
symmetry causes the individual bond polarities to cancel out
✩3- Intermolecular Forces
Learning Intentions
[ ] Be able to identify intermolecular forces present between molecules.
[ ] Be able to compare the relative strengths of intramolecular bonds (covalent bonds) and intermolecular forces (dispersion, dipole-dipole, and hydrogen bonding).
KEY TERMINOLOGY
Intramolecular Bond ⇒ within molecules + strong (ionic & metallic)
Intermolecular Forces ⇒ between molecules
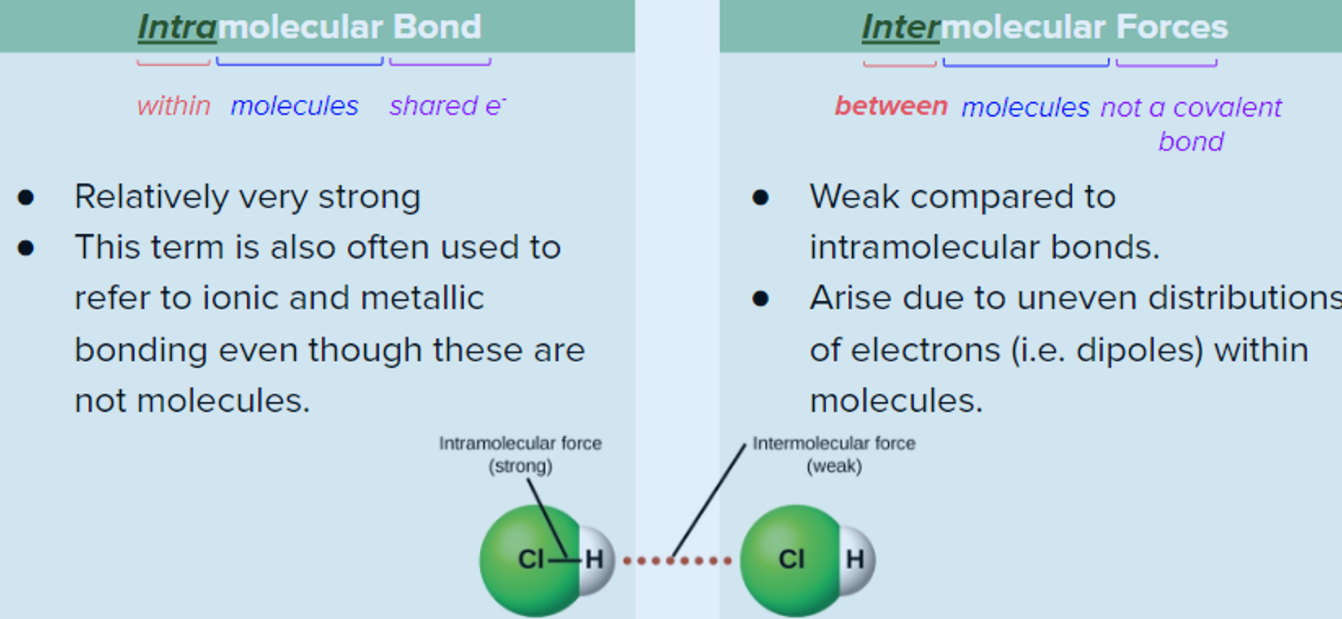
⬜TYPES OF INTERMOLECULAR FORCES
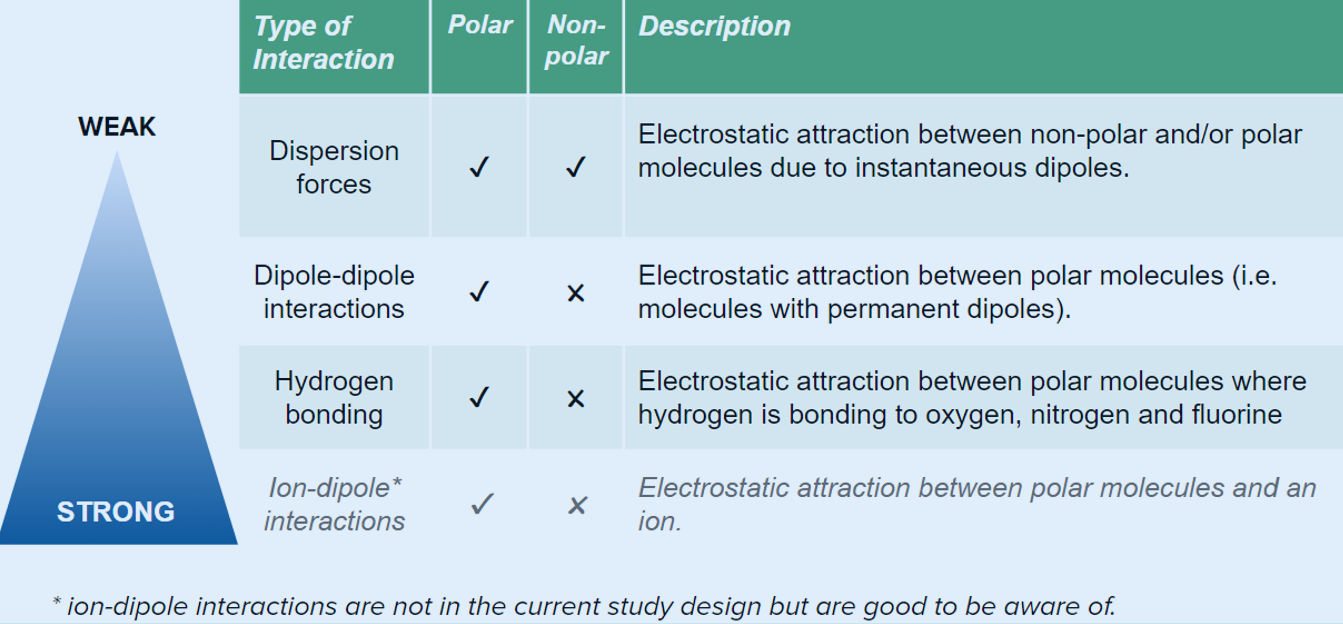
Polar has intermolecular forces of:
Dispersion Force (electrostatic attraction between nonpolar molecule and/or polar molecule
Dipole-Dipole (Electrostatic attraction between two polar molecules)
Hydrogen bonds (Electrostatic attraction between polar molecules where hydrogen is bonded to N,O,F)
Non-polar has intermolecular forces of
Dispersion Force (electrostatic attraction between a non-polar and/or polar molecule
◽DIPOLE-DIPOLE INTERACTIONS
Electrostatic attractions between the positive end of one polar molecule and the negative end of another.
◽INSTANTANEOUS (temporary) DIPOLES
Has no polarization
Instantaneous dipole Molecule A induces a temporary dipole in Molecule B.
◽HYDROGEN BONDING
‼not a covalent bond to a hydrogen atom
Hydrogen bonding is an electrostatic attraction between
a hydrogen covalently bonded to N, O, F
a N, O, F
DISPERSION FORCES
◽STRENGTH OF DISPERSION FORCES DEPENDS ON
Number of Electrons (more electrons forms stronger dispersion forces)
Shape of Molecule (same-size molecules that can get closer will have stronger dispersion forces)
Greater Surface Area available to interact means stronger dispersion
✩4- Properties of Covalent Molecules
Learning Intentions
[ ] Use structure and bonding to explain the properties of covalent molecules
DIFFERENCE BETWEEN PHYSICAL AND CHEMICAL PROPERTIES
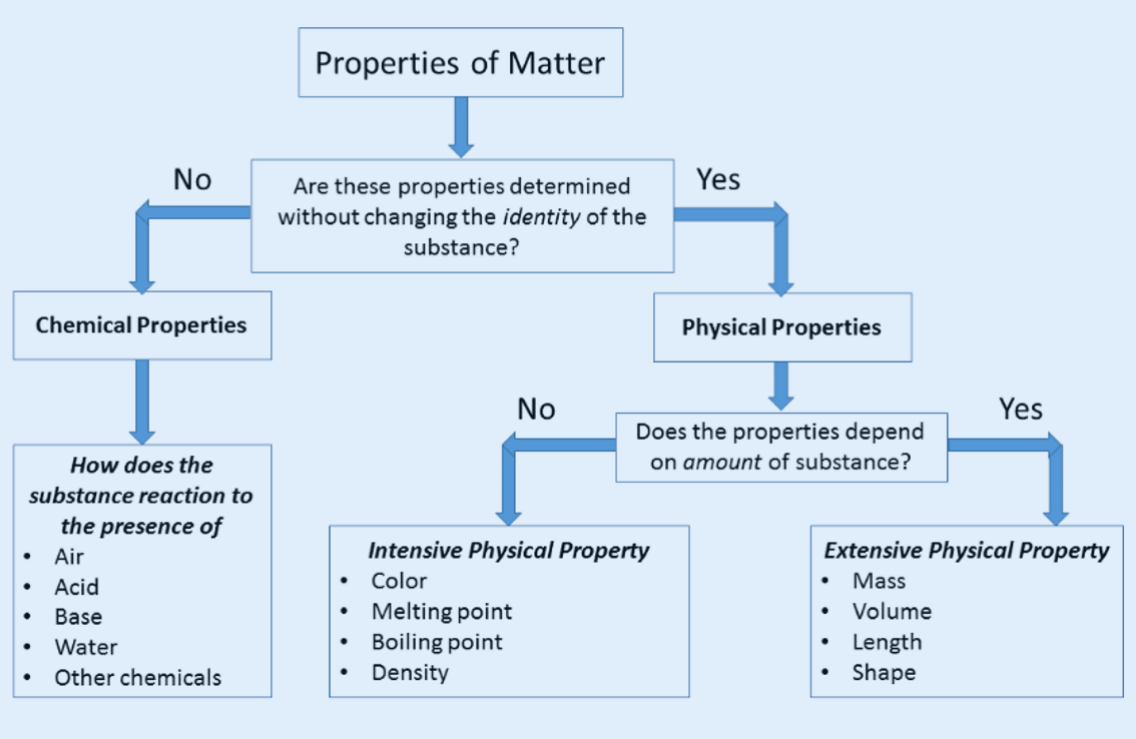
PROPERTIES OF COVALENT MOLECULES
Property | Reason | |
|---|---|---|
Melting/Boiling point | Low | Weak intermolecular forces do not take a lot of energy to overcome |
Hardness | Soft | Weak intermolecular forces do not take a lot of energy to overcome |
Electrical Conductivity (Solid) | Do not conduct | No free-moving chard particles |
Electrical Conductivity (Liquid) | Do not conduct | No free-moving charged particles |
MELTING AND BOILING POINTS
Q: Which force(s) do you think are overcome when covalent molecules (not covalent network) solids melt?
A: The forces that are overcome when covalent molecules melt are the intermolecular forces that hold the individual molecules together in the solid state. To melt the solid, enough energy but be added to overcome these intermolecular forces and allow the molecules to move freely past each other. The intermolecular force's strength determines the substance's melting point as substances with stronger intermolecular forces have higher melting points because more energy is required to overcome these forces.
RECALL:
✨strength of the dispersion force depends on
number of electrons (more electrons = stronger dispersion force)
shape of the molecules (same shape = closer = greater SA available to interact)
✨strength of permanent Dipole-Dipole interactions depends on
the difference in electronegativity between the atoms in a bond
(limited to simple covalent bonding, not giant covalent molecules)
Explanation for Melting and Boiling points
In covalent compounds, stronger intermolecular forces will have higher melting/boiling points
MELTING OF DIFFERENT MATERIALS
Metals:
needs to overcome strong metallic bonding between cations and delocalised electrons (REQUIRED LOTS OF ENERGY)
Ionic:
need to overcome strong ionic bonding between cations and anions (REQUIRES LOTS OF ENERGY)
Covalent Molecules
Needs to overcome weak intermolecular forces between molecules (covalent bonds are more broken but REQUIRES LESS ENERGY)
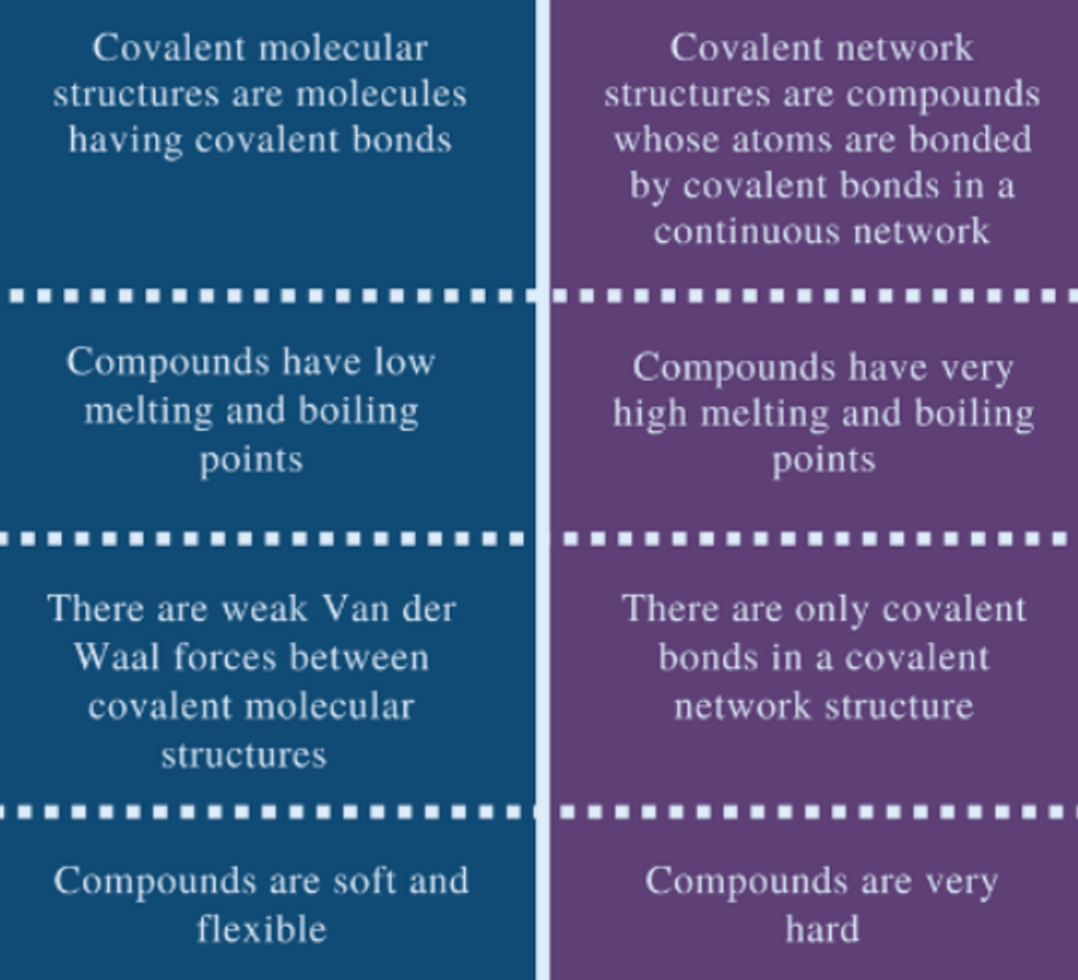
COVALENT MOLECULAR VS COVALENT NETWORK