Compounding Theory
Day 1
What is Compounding?
The process in which the technician uses bulk ingredients to prepare a prescribed medication to treat a specific patient’s medical condition
It involved preparing, mixing, assembling, altering, packaging, and labeling a prescription according to a formulated recipe and prescription by a licensed prescriber who individuals the appropriate quantity and dosage form for his or her patient
Situation Requiring Compounding | Example |
|---|---|
Dose for pediatric patient smaller than commercially available dose | preparing 10mg capsules from 30 mg tables |
Patient cannot swallow solid dosage form | preparing a suspension from tablets |
Dose for veterinary application that is not | preparing a thyroid medication for a cat |
Medication has unpleasant taste | preparing a flavor-masking syrup for a pediatric patient |
Oral medication causes adverse effect | Preparing a gel for a patient who has had |
Types of compounding
Sterile
Uses aseptic technique to prepare solutions free of microorganism
Medication prepared in cleanroom environment
Mainly parenteral and ophthalmic medication
Non Sterile
No cleanroom
Prepared specifically for an intended medication and patient
Hospice Medications for patient who have trouble swallowing
Veterinary Medication depending on the breed and size
Use guideline to keep medications as free of contamination as possible
Only allowed for medications/dosage forms not commercial available
Most common is detailed for a specific patient
Hospice medication
Manufacturing vs Compounding
What is manufacturing?
Manufacturing: when a pharmaceutical company makes a product, applies for a DIN, has an establishment license and notice of compliance
Manufacturers must comply with all federal laws like the Food and Drugs Act and Food and Drug Regulations
Compounders must comply with the provincial regulatory bodies and provincial requirements.
Compounding is made for an individual; manufacturing is mass produced.
Guidelines from Health Canada to determine if its compounding or manufacturing
Is there a demonstrated patient-healthcare professional relationship?
Is there third party reselling of the product outside of the patient-healthcare professional relationship?
Is the activity regulated, and facility possibly inspected by the province?
Is the product being made in anticipation of a prescription?
Is an identical product made commercially?
Types of Compounding
Nonsterile Compounding
Levels of complexity: simple, moderate, and complex according to USP 795 standards
Complex: requires special calculations, decision-making, training, procedures, equipment, environment
Moderate: required some special calculations or procedures to determine
Simple: Reconstituting and combining commercial products; Making a preparation that has a USP compounding monograph that contains specific quantities of all components, procedures and required equipment
Nonsterile Compounding Laws, Regulations, and Standards
NAPRA Model Standards for Pharmacy Compounding of Non-Sterile Preparations
Apply to all non-sterile compounding by pharmacy personnel; however, not every standard will apply in every practice setting.
Represents the minimum requirements to be applied when compounding a non-sterile preparation.
Expected that pharmacists will review the prescription for the non-sterile preparation and use personal expertise to determine whether the compounded preparation is appropriate for that particular patient
Responsibility of the pharmacist and/or pharmacy technician who is designated as the pharmacy supervisor to determine whether the appropriate knowledge and resources to develop the formulation and/or the appropriate equipment and competency to compound the preparation are available.
Excludes mixing, reconstituting or any other manipulation that is performed in accordance with the directions for use on a label of a drug approved by Health Canada
NAPRA requires a risk assessment to be taken to identify the appropriate level of requirement to minimize contamination of each compounded product and to provide adequate protection for personnel
Steps in Conducting risk assessment:
Conduct a risk assessment for compounding non-sterile products, covering risk to preparation and risk to person(s)
Document the risk assessment, clearly explaining how risk to preparation and risk to person(s) have been mitigated
Implement the level of requirements in accordance to the risk
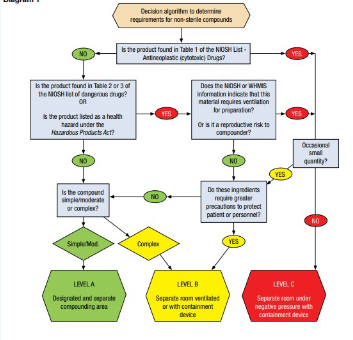
NAPRA’s Level of Risk for Non sterile compounding
LEVEL A: simple and moderate compounds as defined in USP 795
LEVEL B: complex compounds as defined in USP 795; small quantities of ingredients or preparations that require ventilation and are compounded occasionally
LEVEL C: Hazardous drugs classified by National Institute for occupational Safety and Health (NIOSH) as group one
Hazardous materials classified by WHIMIS
NIOSH group 2 & 3 drug for which large quantities of active pharmaceutical ingredients are used routinely
Requirements for all levels of non-sterile compounding
Compounding personnel: All personnel are responsible for knowing and performing their roles and responsibilities in accordance with the NAPRA non-sterile compounding standards as well as the provincial pharmacy authority
Training and skill assessment: Expertise must be equal to responsibilities of compounding personnel; special training for cleaning personnel
Day 2
NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations
General guidance on whether to compound a preparation
Are the active ingredients already available in a manufactured product?
Do you have a referenced formulation?
Do you have the beyond-use date (BUD) and relevant stability data?
Do you have a dedicated space for compounding that is clean and uncluttered?
Do you have the appropriate equipment and ingredients to make the compounded preparation
Are your pharmacy personnel competent to perform compounding of the preparation?
Can your pharmacy personnel compound the preparation without interruption?
Should you refer this compounded preparation to another pharmacy with appropriate facilities, equipment and expertise?
General guideline on Compounding and manufacturing activities
Is there a demonstrated patient–healthcare professional relationship?
Compounding - Yes
Manufacturing - No
Is there third-party reselling of the product outside of a patient–healthcare professional relationship
Compounding - No
Manufacturing - Yes
Is the activity regulated, and the facility possibly inspected, by the province/territory?
Compounding - Yes
Manufacturing – No
If the product is being compounded in anticipation of a prescription, is the amount consistent with the history of prescriptions received?
Compounding - Yes
Manufacturing - No
Is a large quantity of the product produced on a regular basis?
Compounding - No
Manufacturing – Yes
Is an identical product (e.g., dosage form, strength, formulation) commercially available?
Compounding - No
Manufacturing - Yes
Factors to consider in risk assessment
Complexity of compounding the preparation
Need for verification and uninterrupted workflow
Frequency of compounding high-risk or low-risk preparations
Risk of cross-contamination with other products
Hormones with anything else
concentration of ingredients in the product
quantity of ingredients being handled
physical characteristics of ingredients, such as liquid vs. solid vs powders, or water-soluble vs lipid-soluble
education and competency of compounding personnel
Availability of appropriate facilities and equipment
Classification of ingredients if identified by WHMIS
as presenting a health hazard or a drug classified by NIOSH as hazardous (see reference to NIOSH)
Type of hazardous drug (i.e., anti-neoplastic, non-antineoplastic, reproductive risk only) Exposure to compounding personnel for each preparation and accumulation of exposure over time
Risk of microbial contamination (liquids, creams and ointments may be particularly susceptible to microbial and other contamination)
NIOSH: National Institute of Occupational Safety and Health
https://www.cdc.gov/niosh/docket/review/docket233c/pdfs/DRAFT-NIOSH-Hazardous-Drugs-List-2020.pdf
NIOSH DEFINITION OF A HAZARDOUS DRUG
Approved for use in humans by the FDA-CDER; and
Not otherwise regulated by the U.S. Nuclear Regulatory Commission; and
Either:
Is accompanied by prescribing information in the “package insert” that specifies special handling information (Manufacturer Special Handling Information- MSHI) to protect workers handling the drug; or
Is identified as a carcinogenic hazard, developmental hazard, reproductive hazard, genotoxic hazard, or other health hazard by exhibiting one or more of the following toxicity criteria in humans, animal models, or in vitro systems:
carcinogenicity;
developmental toxicity (including teratogenicity);
reproductive toxicity;
genotoxicity;
organ toxicity at low doses; or
structure and toxicity profile that mimics existing drugs determined hazardous by exhibiting any one of the previous five toxicity types;
unless the drug also exhibits a molecular property that may limit the potential for adverse health effects in healthcare workers from exposure to the drug
Workplace Hazardous Materials Information system
https://www.canada.ca/en/healthcanada/services/environmental-workplace-health/occupational-health-safety/workplace-hazardous-materials-information-system/hazardous-substance-assessments.html
Risk Assessment
Compounding Personnel
If the pharmacy is small, one pharmacist may be responsible for fulfilling all roles and tasks; however, it is best practice to have another person verify calculations and measurements
Never do calculations by yourself
Pharmacy manager or pharmacy department head
The pharmacy manager or pharmacy department head: is responsible for the development, organization and supervision of all activities related to compounding of non-sterile preparations in the pharmacy.
These responsibilities may be assigned to a pharmacist or pharmacy technician, who will be designated as the non-sterile compounding supervisor
Nonsterile compounding supervisor
The non-sterile compounding supervisor: is a pharmacist or pharmacy technician who develops, organizes and oversees all activities related to compounding of non- sterile preparations in the pharmacy, as assigned by the pharmacy manager or pharmacy department head.
The non-sterile compounding supervisor may assign technical tasks related to compounding non-sterile preparations to non-regulated pharmacy personnel with the appropriate training and competencies. This assignment of tasks must be carried out using a formal delegation process or under supervision in accordance with the requirements of the provincial/territorial regulatory authority
Responsibilities
The non-sterile compounding supervisor ensures that the following requirements are met
Measures are in place (i.e., personnel training and assessment program) to ensure that personnel are competent to perform compounding, which includes training for any specific populations (e.g., pediatric, Geriatric, veterinary).
Personnel know and fully comply with policies and procedures.
The existing compounding process yields high-quality non-sterile preparations.
A risk assessment is performed to determine appropriate requirements for each compounded preparation.
Appropriate measures are taken to ensure the safety of personnel during each preparation.
Procedures for incident/accident reporting and follow-up, as well as recall procedures, are in place.
Policies and procedures covering all activities are developed, regularly reviewed and updated.
The facilities and equipment used to compound non-sterile preparations meet requirements and are maintained, calibrated or certified according to manufacturers’ specifications or standards, whichever are more stringent.
The available, recognized scientific literature is used to determine stability and to establish the BUD for each non-sterile preparation.
Master Formulation Records are developed, reviewed and updated.
An ongoing quality assurance program designed to ensure that preparation activities are performed in accordance with standards of practice, scientific standards, existing data and relevant information, is implemented, followed, evaluated and updated as required.
Current editions of mandatory and supplementary references, which should comply with provincial/territorial requirements, are available. Safety data sheets are available and updated regularly or are readily accessible in an electronic format.
All records of decisions, activities, or specifications required by the Model Standards are completed, and any changes are documented and traceable. The records are retained and readily available for audit and inspection purposes, as required by the provincial/ territorial pharmacy regulatory authority
Regulated Pharmacy Personnel
When more than one pharmacist or pharmacy technician is involved in compounding a non-sterile preparation, whether working in the same or different facilities/pharmacies, responsibilities toward the patient are shared between them. In such instances, all parties must comply with provincial/territorial requirements and standards regarding inter- and intra-professional collaboration.
Pharmacy students and interns may also compound non-sterile preparations, in accordance with their level of training and the scope of practice set out by the pharmacy regulatory authority.
perform and/or supervise compounding activities;
ensure compliance with policies and procedures related to the compounding of non-sterile preparations, including handling of hazardous drugs and materials where applicable;
enforce compliance with required rules relating to hygiene, cleanliness and safety;
ensure that all records related to ongoing activities are completed and that documentation clearly indicates who completed and who verified each activity;
ensure that all data required for monitoring and reproducing the preparation are recorded or digitized;
ensure that the equipment, instruments and space used are properly cleaned and maintained;
ensure application of and compliance with existing compounding procedures;
ensure that there is a compounding record for each compounded preparation, including any deviations from the Master Formulation Record;
ensure the accuracy of calculations and measurements;
ensure that appropriate equipment and instruments are used for each compounded preparation;
follow the compounding process defined in the Master Formulation Record;
perform verification during the various stages of compounding and verify the final preparation;
ensure that all required verification and quality control measures are performed to ensure the quality of each preparation;
ensure that preparations are packaged and labelled in accordance with provincial/territorial requirements and that a BUD is included on the label;
when a non-sterile preparation is prepared on behalf of another facility/pharmacy (where permitted by provincial/territorial legislation), provide any information required for storing and transporting such preparations (storage method, precautions, BUD, etc.) to the pharmacist or pharmacy technician at the facility/pharmacy where the preparation will be dispensed;
ensure that the final preparation is properly stored until delivery to the patient or to the pharmacist who ordered it (where compounding has been undertaken by another pharmacy, as permitted by provincial/territorial legislation);
when a preparation must be recalled, notify the patient and any pharmacist or pharmacy technician at any pharmacy/facility where the product was dispensed;
before dispensing or releasing a preparation to the patient, ensure that all standards of practice associated with dispensing the preparation have been met, including an assessment of therapeutic appropriateness, patient consultation and education, documentation and other patient care activities;
when a non-sterile preparation has been prepared on behalf of another facility/pharmacy (where permitted by provincial/territorial legislation), ensure that effective communication and collaboration occur between the healthcare professionals at both facilities to clarify who is responsible for which aspects of patient care and to ensure continuity of care.
Non-Regulated Pharmacy Personnel
TLDR Only under delegation by a pharmacist/manager may other pharmacy personnel compound nonsterile products with supervision and training
Non-regulated pharmacy personnel with appropriate training may compound non-sterile preparations or perform other technical tasks related to compounding non-sterile preparations only when assigned to do so by the non-sterile compounding supervisor and only after completion of a formal delegation of duties from a pharmacist or under appropriate supervision, in compliance with the requirements of the provincial/territorial pharmacy regulatory authority
The responsibilities of non-regulated pharmacy personnel assigned to compound non-sterile preparations or perform other technical tasks related to non-sterile compounding are determined at the discretion of the nonsterile compounding supervisor. The non-sterile compounding supervisor should assign only those tasks permitted by provincial/territorial legislation and for which the non-regulated pharmacy personnel has the appropriate training. Non-regulated pharmacy personnel must be supervised by a pharmacist or pharmacy technician according to established supervision protocols and appropriate quality measures.
Day 3
Training of Compounding Personnel
Training of compounding personnel
All personnel involved in compounding must possess expertise commensurate with their responsibilities. Therefore, before they undertake non-sterile compounding, they must have received the proper orientation, training and a skills assessment concerning their work and the type of compounding to be done.
A skills assessment program, which considers the type and complexity of operations performed, must be established for all personnel involved in non-sterile compounding. Compliance with operating procedures and application of non-sterile compounding techniques must be evaluated regularly and must be included in the skills assessment program for compounding personnel. The results of these evaluations and any corrective action taken should be noted in the employee’s file.
The following table of knowledge, skills and abilities is intended to assist in ensuring that personnel have the required competencies. This table should be used as appropriate, in accordance with regulations and policies in the particular pharmacy and jurisdiction.
Skills Assessment checklist for compounding
Below is an example of a checklist that personnel could use individually or with each other to assess their compounding skills. To avoid errors and maximize the therapeutic effect for the patient, compounding personnel should follow each compounding step and sign off at appropriate intervals.
Consider whether the compounded preparation prescribed is appropriate and safe for the patient, based on the therapeutic intention (pharmacist).
Determine whether a valid formula exists; if not, develop a Master Formula, in consultation with experts and/or reliable resources. Ensure that the Master Formula includes instructions for special handling considerations.
Calculate and verify the quantities of each ingredient required on the compounding record (pharmacist/pharmacist or pharmacist/pharmacy technician).
Ensure that personnel responsible for compounding are wearing the appropriate personal protective equipment (cap, mask, gloves) and a clean laboratory coat or disposable gown.
For preparations that contain hazardous products, ensure that personnel wears the appropriate personal protective equipment: cap, safety goggles, two pairs of gloves, an N95 mask and face protection, a gown and shoe covers, depending on the substance used.
Ensure that only one preparation is being compounded at a time.
Gather the ingredients and necessary equipment. Ensure that the equipment is ready for use (clean and in good repair).
Measure each ingredient using appropriate equipment in accordance with the compounding record.
You can use an independent check to confirm each ingredient and quantity with the compounding record before the preparation is compounded.
Ensure that compounding of the preparation is in line with the Master Formulation Record and the prescription, as well as with good practice and pharmacy science (compounding pharmacist/ pharmacy technician).
Verify that the labeling complies with the requirements of the provincial pharmacy regulatory authority
All active ingredients and the concentration of each ingredient are identified on the label.
The beyond-use date is marked on the label.
The storage information has been added.
Approve, through an independent check, the appearance of the final preparation (clarity, odor, color, consistency, pH, etc.) and sign the compounding record.
Ensure that the area and equipment are cleaned immediately after use
and dried according to manufacturer’s directions or standards
Please make sure that the products, ingredients and equipment are put away immediately after use for proper storage.
Knowledge required for cleaning personnel
Know all policies and procedures related to cleaning and decontaminating the equipment, furniture and facilities, notably those related to hygiene, personal protective equipment, and cleaning and disinfecting tasks
know and use personal protective equipment specifically for handling hazardous products
Know and use the emergency measures to be applied in case of accidental exposure, , accidents or spills
Policies and Procedures
Established policies and procedures provide detailed descriptions of all activities, including cleaning, regarding the pharmacy’s compounding of non-sterile preparations (see section 5.3.1 for a list of potential policies and procedures). The procedure template presented in section 5.3.2 can be used as a model for developing various procedures. The non-sterile supervisor must ensure the application of and compliance with these policies and procedures.
Established policies and procedures are promptly updated whenever there is a change in practice or in standards. At a minimum, policies and procedures are reviewed every 3 years to ensure currency. The review should be documented.
For handling or compounding hazardous drugs or materials, additional policies and procedures must be developed; including the safe receipt, storage, handling, compounding, labeling, transport and disposal of hazardous drugs and materials
When compounding is undertaken by another pharmacy, where permitted by provincial/ territorial legislation, the dispensing facility should include in its general procedures manual information about policies and procedures for acquiring compounded non-sterile preparations for patients (originating pharmacy, entry in the file, delivery, etc.).
Obligations of Personnel
attire and dress code
health conditions
expected behavior in compounding areas (no food, drinks, or anything unrelated to compounding)
Training and Assessment of Personnel
initial training and assessment program
program to asses maintenance of competency
Training and Assessment of cleaning and disinfecting personnel
Additional training in all aspects of handling and compounding complex or hazardous products
Delegation and Appropriate Supervision of Activities: Delegation of technical activities to persons other than pharmacists or pharmacy technicians
Facilities and Equipment
Access to a controlled area or room
Necessary facilities and equipment
Maintenance of facilities and equipment (e.g., certification of rooms and instruments, calibration, maintenance of pre-filters and high-efficiency particulate air filters, verification of pressure)
Cleaning activities for facilities and equipment
Compounded Non-Sterile Preparations
Determining beyond-use dates of products used in a preparation
Determining beyond-use dates of final preparations
Hand hygiene
Personal protective equipment in compounding areas and for compounding
Bringing equipment and products into the room
Deactivation, decontamination, and cleaning of the C-PEC (containment primary engineering control)
Receipt, unpacking and storage of hazardous products
Verification of the compounding process (including validation of calculations by a pharmacist) and of final preparations
labelling of final preparations
Packaging of final preparations
Storage of products used and final preparations
Transport and delivery of final preparations (to the patient, to patient care units or to the dispensing pharmacist)
Recording of preparations in the patient file
Hazardous waste management (e.g., at the pharmacy, returns from patients or patient care units, instructions to patients)
Action to be taken in case of accidental exposure of personnel to hazardous products (eyewash station)
Spills and spill management
Recall of products, ingredients, or compounded non-sterile preparations
Quality Assurance Program
Verification and maintenance of equipment, verification of appropriate storage of ingredients
Environmental control of facilities and primary engineering control (e.g., pressure verification, air and surface sampling plan)
Environmental monitoring of chemical contamination for hazardous products
Quality assurance of compounded sterile preparations (e.g. the existence of a protocol, compliance with prescription, documentation in logs)
Beyond Use Date
Beyond-use date and dating methods The BUD is the date after which a non-sterile compounded preparation should no longer be used. Non-sterile preparations are compounded for immediate use or shorshort-termrage; therefore, their BUDs are assigned on the basis of criteria different from those applied in assigning an expiry date to manufactured drug products
Extensive experience in non-sterile compounding and broad scientific knowledge is required to determine a BUD and interpret stability data concerning actual compounded formulation. BUD should be assigned conservatively . When assigning a BUD, compounders should consult the literature and documentation available on stability in general and on the specific stability of the active pharmaceutical ingredient (API).
When a manufactured drug is used as the API, information provided by the manufacturer may be used as a reference. The manufacturer’s expiry date for the drug should not be used as the BUD for the final preparation. As recommended in the following table, the BUD for non-aqueous formulations is not later than the time remaining until the earliest expiry date of any ingredient or 6 months, whichever is earlier.
The nature of the ingredient to be used, the compounding method, degradation mechanisms, compatibility, dosage form, the potential for microbial proliferation in the preparation, the container in which the preparation is packaged, the expected storage conditions, and the intended use and duration of therapy should all be considered, and the BUD assigned conservatively. The product should be observed at all stages of compounding for signs of instability and/or degradation
In the absence of any stability data for a drug or a specific non-sterile compounded preparation, The table on the next slide presents the maximum BUDs recommended for non-sterile compounded preparations that are packaged in airtight, light-resistant containers and stored at controlled room temperature, unless otherwise indicated. Drugs and chemicals known to be labile to decomposition will require shorter BUDs.

Where possible, susceptible preparations should contain suitable antimicrobial agents to protect against bacteria, yeast and mold contamination that may be introduced during or after the compounding process. When antimicrobial agents are contraindicated, susceptible compounded products should be stored at a controlled cold temperature, and patients educated about proper storage. Antimicrobial preservatives should not be used in place of good compounding practices.
Master Formulation Record
The Master Formulation Record for non-sterile preparation includes all necessary information to compound the preparation
To ensure preparation quality and safety, the master Formulation Record should include supporting rationale and references, and compounding personnel must be informed of the change
The development of a new Master Formulation Record is based on Scientific data, and includes appropriate references
Master Formulation Records should be kept together, in hard copy or electronic format, and be readily available.
The Master Formulation Record should include the following information as required to compound the preparation
Official or assigned name, strength and dosage form of the preparation
expected yield
calculations as needed to determine and verify quantities of ingredients and does of APIs for quantity produced
Description of all ingredients, along with their quantities, source, and lot numbers
Compatibility and stability data include references when available
references used to develop the formula and the consultation date, as appropriate
Equipment needed to compound the preparation (any special cleaning instructions)
special precautions to be observed by compounding personnel, including personal protective equipment (PPE)
source of origin of the formula
mixing instructions which may include
order of mixing
mixing temperatures or other environmental controls
duration of mixing
other factors pertinent to the replication of the preparation as compounded
sample labelling information, which should contain, in addition to legally required information
generic name and quantity or concentration of each active ingredient
assigned BUD
storage conditions
prescription or control number, whichever is applicable
type of container used in dispensing
packaging and storage requirements
description of the final preparation
quality control procedures and expected results
Training Indicate any specialized training that personnel must undergo before the specific compounding procedure is implemented.
References consulted:
Indicate the source of the specific compounding procedure.
Indicate any documentation supporting the stability of the final compounded non-sterile preparation.
Preparation data sheet history
Revised: (dd/mm/yyyy) Indicate the change made
Revised by: ________________ Version number changed: YES or NO
USP 795
Categories of Compounding
Simple
Making a preparation that has a United States Pharmacopeia (USP) compounding monograph or that appears in a peer-reviewed journal article that contains specific quantities of all components, compounding procedure and equipment, and stability data for that formulation with appropriate BUDs
or reconstituting or manipulating commercial products that may require adding one or more ingredients as directed by the manufacturer. Examples include Captopril Oral Solution, Indomethacin Topical Gel, and Potassium Bromide Oral Solution, Veterinary
Captopril Soln: http://www.uspbpep.com/usp31/v31261/usp31nf26s1_m1599.asp
Indomethacin Topical Gel: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m40422.html
Potassium Bromide Oral
Soln Veterinary: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m1307.html
Moderate
Making a preparation that requires special calculations or procedures (such as calibration of dosage unit mold cavities) to determine quantities of components per preparation or per individualized dosage units; or making a preparation for which stability data for that specific formulation are not available
Examples include Morphine Sulfate Suppositories, diphenhydramine hydrochloride troches, and mixing two or more manufactured cream products when the stability of the mixture is not known
Morphine Sulfate
Suppositories http://www.pharmacopeia.cn/v29240/usp29nf24s0_m54856.html
diphenhydramine hydrochloride troches https://pharmlabs.unc.edu/wpstorage/labs/formulation_records/diphenhydramine_troches_form.pdf
Complex
Making a preparation that requires special training, environment, facilities, equipment, and procedures to ensure appropriate therapeutic outcomes
Examples of possible complex preparation types include transdermal dosage forms, modified-release preparations, and some inserts and suppositories for systemic effects
Compounding Technology
The flowchart on the next slide summarizes the core steps specific to preparing the compounded medication. A systemic approach to quality assurance requires the designing and implementing of appropiate standardized procedures. Quality assurance requires verification through both qualitative and quantitative quality control measures.
Weight/ Measure Ingredient |
|---|
Powder Preparation for Incorporation +/- blending, +/- levigation, +/- wetting, +/-sieving |
Solid-to-liquid incorporation geometric-sequential-direct-incremental addiction |
pH Testing → Transferring → Trimming |
Weigh and Measure Ingredients
Technology has improved
the vintage torsion balance scales
the electronic balance; higher accuracy, compulsory piece of equipment
Higher accuracy does not mean infallible.
Have their limitations; the lowest weight that may be measured accurately
The lowest weighable amounts may be the minimum accurately weighable quantity (MAWQ) or the least weighable quantity (LWQ).
MAWQ/LWQ is the smallest weight or mass that will produce no greater than a predetermined fraction of error
MAWQ/LWQ calculations rely on two main variables:
sensitivity requirement
maximum permissible error permitted during weighing Weigh and Measure Ingredients
To calculate the smallest quantity that can be weighed, on a balance of known sensitivity, to maintain a desired level of accuracy.
e.g., What is the least weighable quantity that will result in an error of 5% or less, knowing the balance has a sensitivity of 6mg?
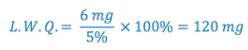
Oral Dosage Forms
Most common solid oral dosage forms: tablets, capsules, caplets
Less common for solid oral dosage forms are: S/L tabs, lozenges, pills, powders, granules, lollipops, and thin strips
Liquid Oral products are: suspension, solutions, colloidal dispersions, emulsions, gels and magmas
Oral liquids can be solutions; suspensions; colloidal dispersions(magmas and gels) or emulsions suspensions
Solutions are further subdivided into syrups; elixirs and water
Pharmaceutical Ingredients
Commercially prepared oral liquids contain many types of pharmaceutics ingredients:
Acidifying and alkalinizing agents
Antifungal and antibacterial preservatives
Antioxidant
Buffering and chelating agents
Emulsifying and suspending agents
Surfactants
Coloring and flavoring
Sweeteners
In addition to the solvent or liquid that forms the bulk of the preparation.
Solvents
Water is the most common solvent or liquid used in preparing oral liquids
Tap Water: partially purified. Not suitable for preparing pharmaceutical products.
Soft Water: rainwater is naturally occurring soft water.
Purified Water- will also be called Purified Water USP. It is water that has been treated by distillation or ion exchange.
Distilled Water: treated through distillation, tap water has been boiled, followed by a cooling of the water vapor and collection of the resulting condensate as distilled water. The 1st and last portions are discarded since they contain impurities.
Always used to reconstitute antibiotic powders
Alcohol is the 2nd most common solvent for pharmaceutical use
is a better solvent for organic compounds
acts as a preservative in concentrations over 20%
Not used in products for children, the elderly or diabetics
Alcohol: Varying proportions of alcohol and water combined are very effective solvents and vehicles for many products
Alcohol USP- 94.9 to 96% v/v ethanol
Diluted Alcohol NF- is 49% ethanol in water.
Oral preparations usually contain less than 50% ethanol since more concentrated solutions burn mucous membranes.
Concentrations of greater than 20% ethanol can produce pharmacological effects of drinking alcohol.
Denatured alcohol: denaturants are added to alcohol to make it unfit to use as a beverage. The denaturants in rubbing alcohol make it unpalatable but non-toxic
Glycerin
Colorless, viscous, hygroscopic, sweet-tasting liquid
Miscible with alcohol and water
It’s difficult to dissolve substances in but is a good solvent.
Adds body, smoothness and sweetness to liquid preparations
Acts as a preservative in higher concentrations and prevents water loss
Propylene Glycol
Viscous liquid with a slight acrid taste.
Miscible with water and alcohol.
Often used as a substitute for glycerin
Acts a preservative in higher concentrations.
Colours and Flavors
Important for patient acceptance of oral liquids.
Color has definite psychological effects; people feel the medicine is more potent if it is colored.
Addition of color can help to differentiate between products and enhance appearance.
Colors- most dyes today are synthetic derivatives of coal tar
Dyes are classified as:
FD&C: suitable for use in food, drugs and cosmetics
D&C: suitable for use in drugs and cosmetics
External D&C: suitable for externally applied drugs and cosmetics.
Some people are allergic to certain dyes and need to avoid products containing them.
Dyes are complex chemicals and frequently fade on exposure to light or change color with changes in pH.
Therefore the stability and compatibility of the coloring agent added is another factor that has to be considered.
Flavors: our taste buds are sensitive to at least five basic sensations
Sweetness: pleasure, produced by sugars and ripe fruit
Sourness: taste that detects acidity, lemons and vinegar
Saltiness: related to the amount of sodium chloride in food
Bitterness: sharp and perhaps unpleasant but sometimes desirable, raw chocolate, beer, grapefruit
Umami (savory): is an appetite taste, savory and meaty
fermented foods and aged foods
tomatoes, grains, and beans
Tastes are further influences by smell and temperature
Flavoring
has changed the traditional image of medicine as bad tasting
Flavoring agents are an important aspect of formulation for oral liquids.
The type of flavoring to be added depends both on the taste of the drug(bitter or salty) that needs to be masked and on the age group that is using the preparation.
Cocoa flavor is good for masking bitter taste
Citrus (lemon, lime or orange) for masking a sour taste.
Children have more taste buds than adults and are even more sensitive to taste.
Children tend to like sweet fruity flavors; oral antibiotics are usually fruit or banana flavored. Adults prefer mint flavors.
Solutions
A homogenous mixture of two or more ingredients.
Dissolving agent is the solvent
Substance being dissolved is the solute.
Extemporaneous Preparation of Solutions
Active ingredient in a solution can be either a liquid or solid
1st step is to calculate(from master formula or dr.’s prescription) the amount of each of the ingredients are required for the volume to be made.
If the active ingredient needed for the formula is only available in tablet form, and the active ingredient is water soluble, the tablets are crushed, dissolved in water and the excipients removed by filtration to clarify the solution.
Vehicles used for solutions primarily are purified or distilled water, ethanol, glycerin, syrups and blends of different ingredients.
Solutions are prepared by dissolving water soluble substances in the aqueous portion of the solvent, and dissolving the less water soluble substances in the alcohol portion, then adding sufficient solvent (qs ad) to reach the final volume.
Flavors and sweeteners are added to overcome bitter taste or bad odor of the drugs.
Preservatives are added to prevent microbial growth.
Antioxidants are added to prevent rancidity or deterioration of the ingredients.
Buffers are added to maintain the pH in a certain range for stability of the compound
Syrups
Syrups are concentrated solutions of sugar in water.
Sucrose is most commonly used to prepare syrups
other sugars such as dextrose and viscous liquids like glycerin, sorbitol, and propylene glycol are frequently used in sugar-free formulations.
Sugar may be partially or completely replaced by sugar-substitutes and thickeners to simulate the sweetness and viscosity.
Artificial sweeteners such as sodium saccharin and aspartame are used with a thickener like methylcellulose to produce syrups for diabetics.
Simple Syrup BP 85% w/v strength
Extemporaneous preparation of Simple Syrup:
Add sucrose to boiling water and stir until dissolved, carefully
applying more heat if required.Excessive heat will cause caramelization and development of a yellow color.
The mixture is then cooled and made up to volume with purified water. Because this syrup is almost saturated, it does not require a preservative
Extemporaneous Preparation of Syrup
The active ingredient should be dissolved in a small amount of water before adding to the syrup.
If drugs are directly added to syrup before being dissolved, the viscosity and concentrated solution will slow down the dissolution of the solids and some drug may not completely
dissolve.
Elixirs
Elixirs are defined as clear, sweetened, hydro alcoholic liquids intended for oral use.
Elixirs are not as sweet or viscous as syrups and therefore are not as suitable for masking the taste of drugs.
The higher alcohol content of elixirs makes them better solvents for many drugs and more resistant to microbial growth.
The alcoholic bases have been phased out.
There are very few elixirs available, one is Tylenol with codeine Elixir and Benadryl Elixir.
Compounding Terms
active pharmaceutical ingredient (API): Any substance or mixture of substances intended to be used in the compounding of a drug preparation, thereby becoming the active ingredient in that preparation and furnishing pharmacological activity by direct effect in the diagosis, cure, mitigation, treatment, or prevention of disease or by affecting the structure and function of the body.
beyond-use date: The date after which a compounded preparation should not be used; determined from the date the preparation is compounded.
Blending: The process of mixing powders to be incorporated into the final drug product.
geometric dilution: The process in compounding in which the smallest quantity is added first, followed by the next largest amount, until all ingredients are incorporated
good compounding practices: the minimum standards for methods used in, and facilities or controls used for, compounding a drug to ensure that the drug has the identity and strength and meets the quality and purity characteristics it is represented to possess
Levitation: The incorporation of a drug powder in small quantities into an ointment
master formulation record: includes all
necessary information and appropriate
procedures to safely compound a specific
non-sterile preparation, whereas the
Compounding Record is generated every
time that preparation is compounded with
prescription- (or batch-) specific information
that must be verified before it is dispensed.Meniscus: the curve that exists on the
surface of a liquid when it is placed into a
container. This curve is either convex or
concaveStability: each active pharmaceutical ingredient (API) maintains its chemical integrity and potency. I.e. appearance, solubility, suspendibility and particle size are maintained
Trituration: grind to a fine powder.
Emollient: A substance that helps soothe, soften, and increase moisture levels, especially in the skin.
occlusive ointment: moisturizing ingredients that create a physical barrier on the skin to prevent trans-epidermal water loss and lock in hydration
Cream: opaque, soft solids or thick liquids for an external application consisting of medications dissolved or suspended in water-soluble or vanishing cream bases and can be either a water-in-oil (w/o) or an oil- in-water (o/w) type of emulsion
Diluent: An inert product either liquid or solid that is added to a preparation and that reduces the
concentration of the compoundExcipients: substances that are included in a
pharmaceutical dosage form not for their direct
therapeutic action, but to aid the manufacturing
process, to protect, support or enhance stability, or for bioavailability or patient acceptability.Solute: one or more active ingredients or excipients that are homogeneously dispersed at the molecular level (i.e., dissolved) in the solvent. The solvent can be a single liquid excipient or a combination of miscible liquids
Solvent: a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid.
Suspendibility: Ability of a fluid to suspend heavier particles
Vehicle: A substance used as a medium for administration of a pharmaceutical, used to dilute drugs to a suitable volume for proper dosing, to flavor a preparation for ease of administration, and to provide a stable and elegant preparation
electronic digital balance: a device used to measure mass or weight
graduated cylinder: a tall narrow container with a volume scale used especially for measuring liquids.
Calibrate- the technique of correcting or setting a measuring device by adjusting it to match a dependably known and unvarying measure.
nonvolumetric glassware: glassware are not requiring precise measurement of volume or those occurring after or prior to volume measurement
tare weight: the weight of the material
volumetric measurement: measurement of liquids
weighing boat: a box-like folded weighing paper that can be usually used for handling solids and powders
weighing paper: used when weighing solid, powdery substances on an analytical balance to prevent the substance from making contact with unwanted materials
Compounding Liquids
Why compound oral liquids
Many drug products not commercially available as oral liquids
Infant, pediatric, geriatric, psychiatric(?) patients may not be able to swallow oral solids
Large tablets?
Cheeking??
Enteral feeding
Wide diversity of strengths/concentrations
bioavailability
Concerns
Unstable drugs become more unstable
Poorly soluble drugs – make soluble or suspend?Taste/palatability
Consideration of physicochemical, pharmaceutical and patient factors
Source of active ingredient
Pure USP drug powder?
Prefabricated dosage forms? E.g. injectables, tablets, capsules (consider excipients)
Dosage Forms
Syrups: Concentrated aqueous prep of a sugar
(or substitute)Can serve as pleasant-tasting vehicles
for active drugsAppropriate when drug is water soluble
Viscosity will keep flavor in the mouth
longer but may decrease rate of
dissolutionIf appropriate, it is easier to dissolve AI
in small qty of water then ‘qs’ to volume with syrupTypes of simple syrup: acacia, cherry, ora-sweet SF, simple syrup
Elixirs: Clear, sweetened, hydro-alcoholic solutions
Suitable for drugs insoluble in
water aloneLess effective in masking taste than syrups
Different solvents include glycerin, sorbitol, propylene glycol and PEG 400
Suspensions
Suspensions
2 phase system consisting of finely divided solid dispersed in a solid, liquid or gas.
Drug is not sufficiently soluble in ordinary solvents
Good suspension = uniform dispersion of
drug throughout vehicleStep 1 – obtain uniform, small particles of drug
Step 2 – should be thoroughly wetted prior to mixing w/vehicle
Resuspendability & ability to pour,
especially if refrigerated
Suspensions
Factors to consider
Properties of ingredients
Order of mixing
Incompatibilities
Stability and potency
Major physical and chemical considerations
concentration
solubility
pKa (strength of acid)
taste
stability
Preparation of oral Suspensions and syrups: Basic Concepts
what makes a good suspension
Small particle size so they do not settle
rapidlySediment must not form a hard cake
Capable of re-dispersion with a minimum
effortEasy to pour, pleasant to take, resistant
to microbial attack(Remington’s Pharmaceutical
Sciences)
Reasons to make a suspension
Prescriber may desire unique
concentrationProportion of the population, especially
very young, who have difficulty
swallowing tablets or capsulesMore recently developed drugs
hydrophobic in nature which do not lend
themselves to solutionsReligious concerns: e.g. Islam does not
allow ingestion of pork products,
including gelatin capsules
Issues to consider in order to obtain an “acceptable suspension”
settling: need a defined final concentration so we can’t change the density of the active ingredient BUT we can increase the density of the vehicle by adding sucrose, sorbitol, glucose, glycerin or other soluble non-toxic materials
Viscosity best controlled by adding
thickening or suspending agents –
methylcellulose, xanthan gum,
tragacanth, acacia
wetting
Hydrophylic – most easily wetted by
waterHydrophobic – repel water – generally wetted by non-polar liquids. (alcohol, glycerin, contents of a docusate capsule)
Only minimal amount of wetting agent compatible with producing an adequate dispersion should be used – excessive amounts can produce foaming or unacceptable taste
Basic. instruments = mortar and pestle
Common mistake = too much
suspending liquid in wetting stepHigh shear is critical, accomplished
thru localized high viscosity system
(i.e. thick paste)After thoroughly wetting, suspension can be diluted with further portions of the vehicle
KEEP IT THICK!
caking
Development of deflocculated sediment which is not suspensible
Strategies:
Controlled flocculation – a flocculated suspension is the result of the formation of loose aggregates. Because of surface charges the flocs repel each other and intimate contact is
avoided, eliminating caking conditionsElectrolytes, polymers and/or
surfactantsCombination of colloids
Alternative to Lengthy Compounding Procedures
Ora-Plus is a suspending vehicle designed to meet the widest range of potential uses. Several different agents in optimal ratios are used to produce suspension with excellent synergistic
properties.Significant thixotropic characteristics – it is capable of suspending significant amounts of material while maintaining acceptable pour properties.
Certain gels or fluids that are thick, or viscous, under static conditions will flow (become thin, less viscous) over time when shaken, agitated, sheared or otherwise stressed (time dependent viscosity)
Measuring Liquids
Both graduated cylinders and conical graduates are commonly used to measure liquids. Cylinders are more accurate and should be used preferentially over conical graduates. Even though conical graduates are easier to mix in and clean, the error associated with their use is larger than when a
cylinder is used. When observing the graduations on the side of the graduate, an error of 1 mm in reading is relatively constant in a cylinder but the error increases as a bigger volume is measured in a conical graduate. For example, in a 100 mL
graduated cylinder, the error is about 0.5 mL but in a conical graduate it could be 1.8 mL. In pharmacy compounding, conical graduates having a capacity of less than 25 mL should not be usedIt is best to select a graduate to use that has a capacity equal to or just exceeding the volume to be measured. Generally, it is best to not measure a quantity less than about 20-25% of the total volume of the graduate.
When measuring small volumes, it is best to use a pipet or even a micropipette. Pipets are available in different sizes and the same general rule applies for selecting which one
to use. It is best to determine whether or not the pipet is a TC (to contain) or a TD (to deliver). A TC pipet will need to be "blown out" to deliver the entire quantity; A TD pipet
will allow the correct quantity to drain from the pipet. Pipets can be either volumetric where they contain a set volume when filled to a mark, or can be graduated to deliver different quantities. Special pipet washers can be obtained to make sure they are properly cleaned and finally rinsed with distilled water. Pipet bulbs should be used to assist in filling the pipet.If measuring volumes less than a milliliter, micropipettes can be used. There are many types available, including fixed and variable. They generally have disposable tips to prevent cross-contamination. One can generally cover a range of from about 10 microliters to 1 milliliter with two variable micropipettes. Smaller volumes are also available. Micro-pipettes are especially valuable for
measuring small quantities or aliquots of potent drugs and for measuring flavoring agents, etc.Droppers must be individually calibrated for the liquid being dispensed. A dropper meeting USP standards will deliver 20 drops of water at 15 C. It may also deliver up to 50 drops of alcohol; consequently, droppers must be calibrated for each liquid being
measured.
Topical Preparations
Functions of topical Preparations
prophylactic effect of keeping the skin in optimal condition
therapeutic effect to correct disease conditions of the skin or
systemic effect
Topical Drug
Topically applied drugs are intended to penetrate into only the top layer of skin and not be absorbed further into the blood stream.
Topical drug therapy, involves direct application of the drug to the affected area of skin.
Skin disorders are clearly visible so the dosage form selected for the drug depends on some extent upon the appearance of the condition, the structure and functions of the skin and some of the pathetical conditions affecting the skin
Common Types of Skin Conditions
Common skin conditions include: photoaging, acne, eczema, psoriasis, rosacea and hyperpigmentation.
Photoaging: Occurs from UVA or UVB radiation. Ultraviolet radiation causes accelerated aging process in collagen, increasing the rate at which collagen breaks down. This effect produces a cascade of events that lead to wrinkles
Skin damage includes: dryness, fine and course wrinkles, roughness, leathery texture, hyperpigmentation and skin cancer.
Products used:
Tretinoin products-Vitamin A Acid Cream or gel
Tazarotene- Tazorac (Rx)
Acne: Condition of acute inflammation of the skin that causes an eruption of blackheads, whiteheads, and red spots usually called pimples.
Initial treatments of acne include:
Salicylic acid,
Sulfur,
Benzoyl peroxide
These treatments are used to peel the outer layer of skin and permit drainage of the sebum.
Rx products that are commercially available are:
Clinda T
Clindoxyl Gel
Compounded products can be made with erythromycin.
Eczema: Generally occurs in the creases of the body, at the elbows and backs of the knees but can also occur on ear lobes and elsewhere in the body.
Patches of itchy, dry skin that fade and reappear.
Treatments:
Topical corticosteroids
Ratio-Ectosone- betamethasone,
hydrocortisone
Psoriasis: Characterized by scaly, red patches of skin, covered with dry, silvery scales resulting from too rapid epithelial cell growth.
Treatment involves:
Interrupting the cycle that causes increased production of skin cells, reducing the inflammation and plaque formation,
Remove scales and smooths the skin.
Three main types of treatment
Topical corticosteroid creams and ointments,
Creams used along with oral medications,
Light treatment
Psoriasis Treatments
Topical corticosteroids- anti-inflammatory drugs that most frequently prescribed for treatment of mild cases. Long term use of corticosteroids can cause skin thinning and resistance to the treatment’s benefit.
Salicylic acid from 2 to 10% used in corticosteroid bases
Coal Tar (LCD) used in corticosteroid bases.
Rosacea: Inflammatory condition that causes redness or blushing of the face. Other symptoms include visible blood vessels on the nose or cheeks.
Without treatment rosacea can permanently disfigure the face.
Metronidazole (MetroCream or MetroGel) is commonly used.
Hyperpigmentation: Changes in pigmentation that are commonly called age spots or liver spots.
A condition where there is darkening of areas of the skin.
Products that help hyperpigmentation contain hydroquinone.
Other Skin Conditions
Bacterial Infections: treated with topical antibiotics such as and OTC Polysporin or prescription products like Bactroban or Fucidin.
Fungal infections: topical antifungal products are clotrimazole(Canesten), miconazole (Monistat), ketoconazole (Ketoderm), terbinafine(Lamisil).
Skin Infestations: such as scabies or lice. Scabies can be treated with permethrin Nix Cream. Lice can be treated with Nix, R&C shampoo and conditioner, Kwellda P. None of these products seem effective as lice are becoming resistant to the medications. Tea Tree Oil or mineral oil may be a better method of lice control.
Burns
Extensive burns are life-threatening and need hospital care.
Flamazine-silver sulfadiazine is used for treatment.
Flamazine comes in a tube or jar but has a very limited shelf-life after opening. Tube-1 week, jar- 24 hours.
Diaper Rash: Skin of the buttocks becomes inflamed after contact with urine and feces. The skin then becomes susceptible to bacterial and fungal infections.
Diaper rash creams usually contain zinc oxide, which acts a protectant.
Prescription only products can contain anti-inflammatory steroids, antifungals and antibiotics in combination with zinc oxide. There are OTC products available.
Allergic Reactions
Group of symptoms such as inflamed, itchy, weeping papules or dry, scaly, itchy patches. Also could be contact dermatitis. Allergic reactions to food and drugs may cause hives and rashes.
Treated with oral meds like hydroxyzine (Atarax), Benadryl, Aerius.
Treated with topical creams, ointments such as betamethasone, clobetasol, Calamine lotion.
Drug Absorption from Topical Preparations
Many drugs are well absorbed from the skin into the blood stream. Nitroglycerin for example in patches or ointment or Transderm V (motion sickness) is also well absorbed.
The following factors influence the degree of drug absorbed:
Concentration of the drug- greater the concentration of the drug in the vehicle, the great the amount absorbed into the skin.
Remember a vehicle is a non-medicated dosage form to which drugs can be added.
Area of application- the larger the area the drug is spread over, the greater the amount of total drug absorption.
Time- the longer the drug is left on the skin, the greater the amount eventually absorbed.
Hydration of the stratum corneum- most drugs are better absorbed if the stratum corneum is well hydrated.
Nature of the drug and ointment base or vehicle used- complex relationship between the polarity of the drug, its affinity for the vehicle and its interaction with the skin tissues. Compounding pharmacies rely on reference materials to find the most suitable drug-vehicle combination for extemporaneous preparations if the dr doesn’t specify a base.
The following factors influence the degree of drug absorbed:
Condition of the skin- is the skin damaged from injury, burns or scratching. Drugs would be more easily absorbed into the blood stream since the skin isn’t acting as a barrier anymore. The excessive absorption is not desirable and toxicity could happen.
Inunction: absorption of drugs is increased if the product is rubbed on the skin for sometime rather than just applied.
Age of the skin- infants have thinner skins more permeable to absorption. The skin of elderly people also have different characteristics.
Thickness of the epidermal layer and rate of blood flow- absorption of the palms of the hands and soles of the feet is much slower than other parts of the body.
Ointments
Semi-solid preparations for external application
Consistency that they can be easily applied by inunction(rubbing)
The ointment base which is a non-medicated vehicle usually is the major portion of the preparation and influences drug absorption and patient acceptance. It can also provide protective properties to the product.
The large portion of ingredients for ointment bases are synthetic and petroleum-based compounds.
The USP groups ointment bases into four general types:
Hydrocarbon or oleaginous bases
Absorption bases
Water-removable bases
Water-soluble bases
Hydrocarbon or Oleaginous Bases-Ointments
Bases are prepared from petrolatum(Vaseline) or liquid petrolatum (mineral oil) with wax or other stiffening agents added. Petrolatum is frequently used as a base without modification.
Comes in a white or decolorized form that is used when white or translucent ointments are desired and in its regular yellowish color.
Hydrocarbon bases contain no water and allow incorporation with difficulty of only very small amounts of aqueous solutions.
Stay on skin for long periods of time, do not allow escape of moisture from the skin and are greasy and hard to wash off with soap and water.
Common components of these bases:
Petrolatum- also called yellow soft paraffin, petroleum jelly, yellow petrolatum and Vaseline.
Yellow to amber in color
Melts between 38⁰C and 60⁰ C
Used in preparation of eye ointments
White petrolatum- also called white soft paraffin, white petroleum jelly, White Vaseline.
Decolorized petrolatum
Used as a base in formulas that contain white powders
Not used in the preparation of eye ointments.
Mineral Oil- also called liquid paraffin and heavy liquid petrolatum.
Mixture of liquid hydrocarbons obtained from petroleum
Used to soften hydrocarbon bases and to levigate powders before addition to a hydrocarbon base.
Also used internally in plain or emulsion form as an emollient laxative.
Paraffin- also called hard paraffin and paraffin wax
Purified mixture of solid hydrocarbons obtained from petroleum that congeals between 48⁰ C and 60⁰ C.
Used to stiffen hydrocarbon ointment bases.
White and yellow wax- bleached and unpurified wax obtained from the honeycomb of bees.
Has a faint odor of honey and melts between 62⁰C and 65⁰C.
Used in some cases to stiffen hydrocarbon bases as well as other types of bases and pastes.
It increases the amount of aqueous solution that can be incorporated in hydrocarbon ointment bases.
Plastibase- a soft colorless jelly-like substance
Made from a combination of mineral oils, hydrocarbon waxes, and polyethylene
Melts at 90⁰C to 91⁰C.
Retains its consistency over a wide temperature range.
Absorption Bases-Ointments
Absorption refers to the water-absorbing properties of the bases and not the action on the skin.
Two types of absorption bases:
Oil-in-water o/w (anhydrous)
Water-in-oil w/o
Both types readily permit incorporation of water.
Common components of these bases:
Anhydrous lanolin- also called wool fat and refined wool fat.
Purified anhydrous solution obtained from the wool of sheep.
Yellowish brown, sticky semi-solid with a characteristic odour.
Melts between 36⁰ C and 42⁰ C.
Insoluble in water but mixes with about twice its weight of water without separation.
Wool fat improves the absorption of substances from a base and helps the base to retain uniform consistency over a wide temperature range.
Some people are allergic to this animal product and its use is limited because of allergies.
Lanolin: also called hydrous wool fat.
Wool fat with 25 to 30% water added.
Pale, yellow, sticky ointment which will absorb additional water although not as much as anhydrous wool fat.
Common components:
Cholesterol, Wool Alcohols- to avoid allergic reactions either pure cholesterol or the sterol fraction from the wool fat can be used instead of lanolin for their emulsifying properties.
Cetyl alcohol and stearly alcohol- white waxy substances are added to absorption bases for their emulsifying action and the smoothness and texture they provide.
Stearic acid- hard, white, glossy solid or powder added to ointment bases to act as an emulsifier and thickener. It reacts with basic substances to form a soap.
Examples of Absorption Bases
Hydrophilic Petrolatum, USP
Contains cholesterol, stearly alcohol, white wax and white petrolatum.
It is anhydrous and will absorb large amounts of water or aqueous solutions of drug to form a w/o (water in oil) emulsion.
Aquaphor is a commercially prepared absorption base
Cold Cream, USP
Water-in-oil cream (w/o) containing a bleached beeswax, mineral oil, sodium borate(borax) and cetyl esters wax.
Water-removable Bases- Creams
Water-washable bases
Cream bases that are o/w(oil-in-water) emulsions and they are water washable.
Referred to as creams by pharmaceutical manufacturers to distinguish them from ointments.
Readily mix with additional water or an aqueous solution.
When large quantities of water are added, the consistency will become very soft.
Examples of water-removable bases-creams
Hydrophilic ointment, USP
Oil-in-water emulsion which contains sodium lauryl sulfate as the emulsifying agent, white petrolatum and stearyl alcohol represents the oleaginous(oily) phase of the emulsion and propylene glycol and water represents the aqueous phase. Methyl and propyl paraben are often used as preservatives.
Glaxal base-
commercially prepared
Water-removable base used for extemporaneous compounding.
Water-Soluble Bases
Contain only water-soluble compounds.
Often referred to as greaseless.
They soften with the addition of water so aqueous solutions are not effectively incorporated into the base.
Most common ingredients in these preparations are polyethylene glycols of various molecular weights.
Other substances that are added to the polyethylene glycols:
Stearyl alcohol- added for stiffness and to allow incorporation of more water into the base.
Some of these creams are subject to mold growth and need to have preservatives added to the formulation.
Extemporaneous Preparation of Ointments/ Creams
There are two general methods for preparing ointments
Mechanical incorporation
Fusion
The method used depends on the physical properties of the components of the base and the nature of the drug to be added.
Mechanical incorporation is the method most frequently used by pharmacist for extemporaneous compounding.
Terms associated with mechanical incorporation:
Trituration,
Levigating,
Geometric dilution,
Eutectic mixture.
Trituration: Process of grinding a drug in a mortar and pestle, to reduce its particle size to obtain a fine powder. In order to product a product that is not gritty, the drug must be reduced to a very fine powder.
A mortar or pestle may be used or the ointment may be prepared using an ointment pad or ointment slab.
Mechanical Incorporation
Levigation: Involves adding a small portion of a levigating agent, a liquid in which the solid material is insoluble, to a finely powdered drug(s) to form a paste. The paste is then triturated in order to reduce the particle size. The levigated paste is then added to the ointment base and the mixture is made smooth and uniform.
Examples of levigating agents:
Mineral oil
Glycerin
Are often used depending on the drug and base involved.
Urea crystals are dissolved in hot water, menthol in alcohol or the crystalline structure of menthol is broken down with mineral oil before being incorporated into the ointment base.
The levigation process is performed on an ointment pad/slab with a spatula, using a “figure eight1’ motion to incorporate and levigate the materials.
Geometric Dilution: When small amounts of a drug are to be incorporated into a base, the base is added to the levigated powder mass by “geometric dilution”
If large quantities of powder are to be incorporated, the powders are first reduced to fine uniform mixture in a mortar before weighing, and then the powders one side of an ointment slab and the base on the other. The powder and base are mixed together a little at a time into a uniform mixture and then more of each added and mixed in.
Eutectic Mixtures: When two crystalline substances are mixed together they melt into a liquid which can easily be incorporated into a base.
Fusion: uses heat to liquefy solid waxy ingredients to form a base to which the active ingredient or ingredients are added.
For an ointment base:
Solids such as a mixture of beeswax; paraffin wax; cetyl alcohol; stearyl alcohol; and high molecular weight polyethylene glycols; are weighed out and separated in order of their descending melting points.
They are then heated in a vessel in order of their melting points, one at a time, until liquified. When all are liquefied they are removed from the heat and stirred constantly until cooled.
Melting points of chemicals can be found in the Merck Index.
Beeswax and hard paraffin, have the highest melting point as they are dense and solid. They would be melted first and then the flaky substances added and finally the semi solid chemicals incorporated are heated together.
For a cream base:
Water-in-oil emulsion bases are made by this method.
The non-polar or oleaginous solids and semi-solids are melted together, slowly to a minimum temperature required.
The aqueous phase, containing all the water-soluble ingredients, is heated to the same temperature.
The aqueous solution is then added to the melted oleaginous mixture, with constant stirring, removed from the heat and the mixture stirred constantly until congealed.
Constant stirring is necessary to ensure the formation of a stable emulsion and prevent the separation into phases by the immiscible warm liquids.
Adding the active ingredient to the base:
Insoluble powder maybe levigated with a portion of the melted base before addition to the remaining base by incorporation.
In some cases, the fine powder may be added to the warm fluid base and stirred well until the mixture congeals.
Heat labile and volatile ingredients should be added when the mixture has cooled down.
Crystalline powders such as salicylic acid, need to be triturated with alcohol to break down the crystals, so the end product will be smooth.
Packaging and Storage of Ointments and Creams
Ointments are usually dispensed in plastic jars or tubes.
Ointment pots are carefully filled, with the ointment and cream forced down the sides of the jar to eliminate air bubbles. Tapping the ointment pot carefully on a padded surface to bring the air bubbles up and settle the ointment or cream maybe be helpful.
The top of the ointment or cream should be neatly finished, with a slight indentation in the middle to keep the ointment from touching the lid.
Holding the jar on the counter top in one hand, hold a small clean spatula in the other.
Resting the edge of the spatula on the rim of the jar, slightly indent the spatula into the center of the ointment or cream.
Slowly rotate the jar, leaving the surface of the mixture slightly indented.
Place the lid on the jar.
Clean the outside of the ointment pot particularly near the top of the lip with alcohol. Let the alcohol dry before affixing the label.
Ointment should be stored at temperature below 30⁰C to prevent excessive softening of the base.