Biological Molecules
Key Terms
Polymer: Chain of repeated sub-units
Macromolecule: Large, complex molecule
Monomer: Small sub-unit; basic
Anabolism: Process of building up molecules
Catabolism: Process of breaking down molecules
Metabolism: Sum of all reactions in the body
Hydrolysis: any chemical reaction in which a molecule of water breaks one or more chemical bonds.
Condensation Polymerisation: A reaction that produces a molecule plus at least 1 water molecule

Mono, Di, and Polysaccharides
General Formula: (CH2O)n
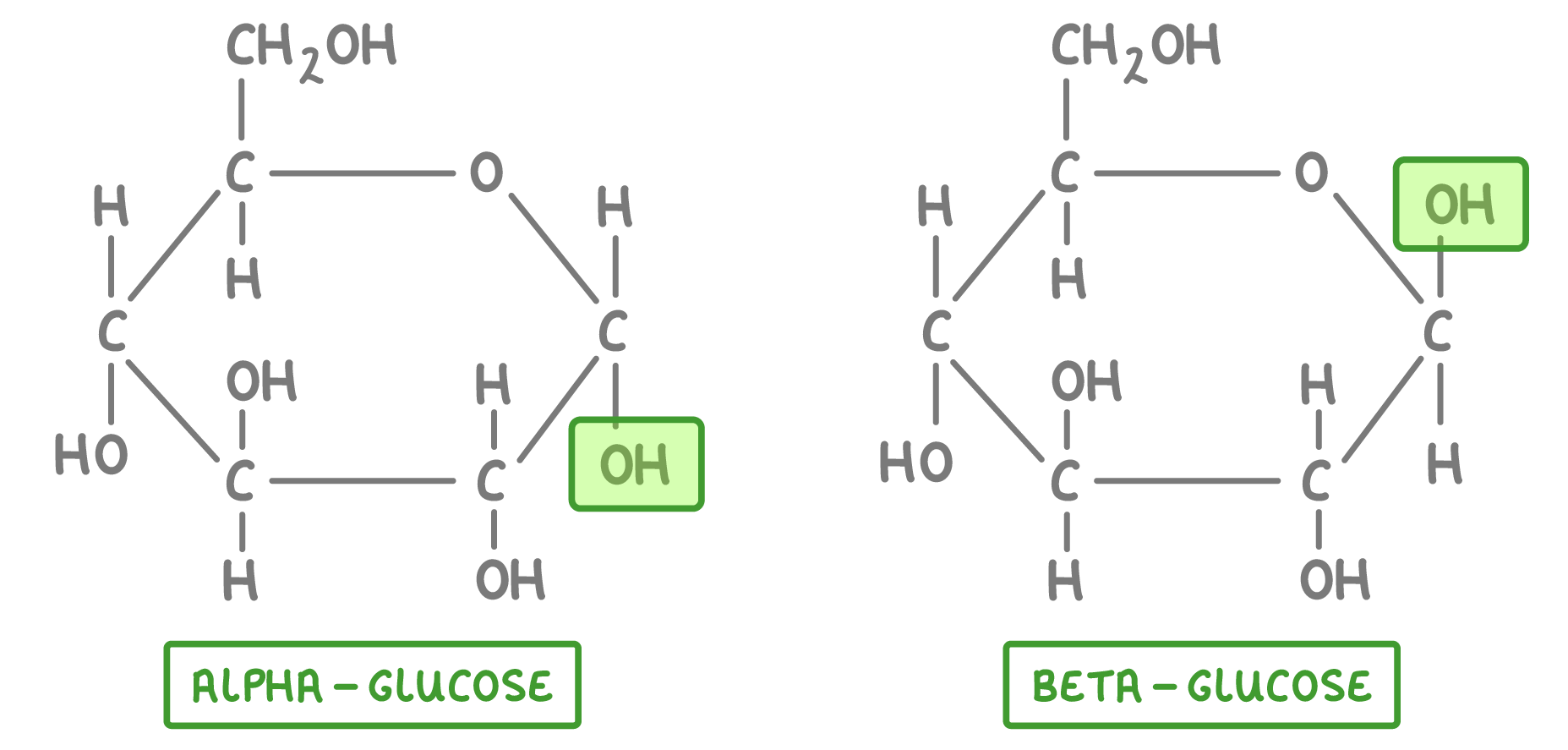

Monosaccharides
They are sweet, soluble
Benedict’s Reagent
Add 2 cm3 of food sample to be tested in a test tube; if sample is not a liquid, grind it up and put it in water
Add an equal volume of Benedict’s reagent to the solution
Heat mixture in a gently boiling water bath for 5 mins.
Solution turns orange-brown if sugar is present
Disaccharides

Maltose = 2 ALPHA glucose
Sucrose = 1 ALPHA glucose + 1 fructose
Lactose = 1 glucose + 1 galactose
Polysaccharide

Glycogen
It is found in animal muscle and liver cells
Monomer: Alpha glucose in a 1-4 glycosidic bond; highly branched with alpha glucose in a 1-6 glycosidic bond
Structure: Less dense and more soluble than starch; More branches, less glucose in these bonds than amylopectin
Function: Broken down rapidly due to higher metabolic requirement of animals compared to plants. Storage molecule for respiration
Starch
Found in plants
Monomer: Alpha glucose
Structure: Amylose
Unbranched chain of alpha glucose in a 1-4 glycosidic bond compound helical structure
Structure: Amylopectin
Chain of alpha glucose in a 1-4 glycosidic bond with more branched chains of alpha glucose in a 1-6 glycosidic bond.
Function: Storage, respiration and a source of carbon for other molecules
Cellulose
Found in plants
Composed of beta glucose units linked by 1-4 glycosidic bonds, providing structural support in plant cell walls.
Structure: A linear chain formation that allows for strong hydrogen bonding between adjacent chains, contributing to its rigidity and strength. Microfibrils between chains. Chains are anti-parallel to each other and have cross linkages
Function: The primary function of cellulose is to provide structural integrity to plants, allowing them to maintain shape and resist external pressures when taking in excess water.
Tests for Carbs
Benedict's Test: Used to detect reducing sugars by changing color when heated with a reducing sugar solution. Can only react with a sugar if the sugar has a carbonyl group (C=O) and at least one hydroxyl group (–OH) in its structure, which is characteristic of reducing sugars.
If Benedict’s Test has been carried out and no reducing sugars are present, a modified test can be done.
Tests for non-reducing sugars is performed by first hydrolysing the sugar with dilute hydrochloric acid to break it down into its monosaccharide components. After hydrolysis, the solution is neutralized with sodium hydrogen carbonate before reapplying Benedict's Test to check for the presence of reducing sugars.
This two-step process is essential because non-reducing sugars, such as sucrose, do not react with Benedict’s reagent until they are broken down into their reducing monosaccharides.
Iodine Test: A test for starch, where iodine solution turns blue-black in the presence of starch.
Fehling's Test: Another test for reducing sugars that involves a color change when mixed with Fehling's solution.
Lipids
Monomers are fatty acids and glycerol, which combine to form triglycerides and phospholipids, essential components of cell membranes.
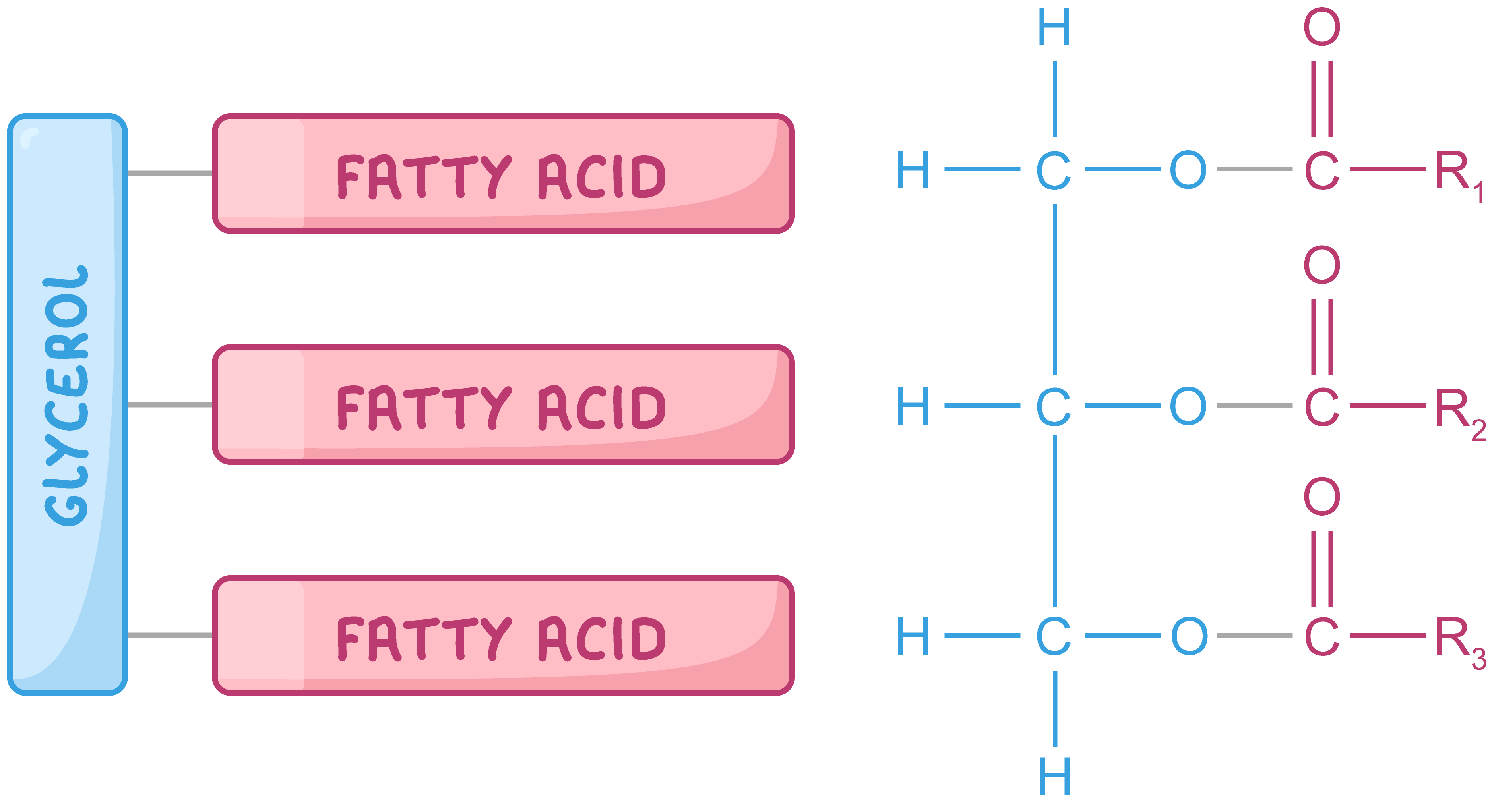
GLYCEROL + FATTY ACID = TRIGLYCERIDE + 3 WATER MOLECULES
Creates an ester bond.
Saturated fats are denser (no C=C DOUBLE BONDS) - SO they tend to be solid at room temperature, contributing to the structural integrity of cell membranes.
Unsaturated fats are less dense (at least 1 C=C double bond), so they are liquid at room temperature.
Phospholipids
They are a major component of cell membranes, consisting of two fatty acids and a phosphate group attached to a glycerol backbone, which allows them to form bilayers that provide a barrier between the interior of cells and their external environment.
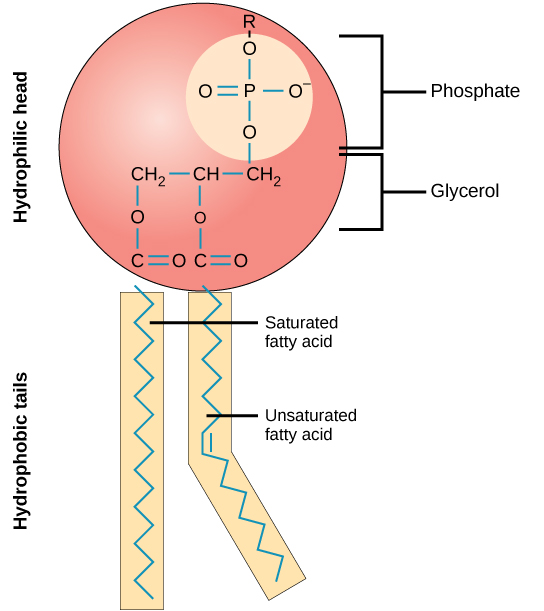
Hydrophobic Tails have no charge so they do not want to interact with water
Hydrophilic Heads have a slight negative charge, so are attracted to the water molecule as water is polar.
Proteins
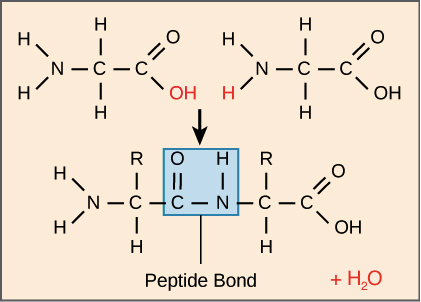
When 2 amino acids join together they produce water + dipeptide and the bond between them is called a peptide bond.

Key Functions
Carrier proteins: Used to transport smaller molecules
Defence proteins: Attack foreign invaders, e.g. antibodies
Structural proteins: for structure and support
Hormones: Chemical messengers e.g. a receptor molecule is one in which other molecules fit.
Enzymes: Biological catalysts that speed up biological reactions
Level of Protein Structure
Primary Structure:
This is the linear sequence of amino acids in a polypeptide chain. The primary structure determines the protein's unique characteristics and is held together by peptide bonds between amino acids.
Secondary Structure:
This level involves the local folding or coiling of the polypeptide chain into structures such as alpha-helixes and beta-pleated sheets. These structures are stabilized by hydrogen bonds between the backbone atoms (amine and carbonyl) of the amino acids.
Tertiary Structure:
The tertiary structure is the overall three-dimensional structure of a single polypeptide chain. This level of structure is formed by the interactions between the side chains of the amino acids, including hydrophobic interactions, ionic bonds, hydrogen bonds, and disulphide bridges, leading to a folded shape.
Quaternary Structure:
This level consists of the arrangement and interaction of two or more polypeptide chains (subunits) to form a single functional protein complex. The interactions between the subunits can include hydrophobic interactions, hydrogen bonds, and ionic bonds.
A different amino acid sequence means different bond positions. E.g. bonds forming between different R groups (disulphide, hydrophobic, ionic)
Biuret Test
Add biuret solution to the aqueous food solution, then if a purple colour is formed, it contains protein. If not, it stays blue
Protein Production, Transport and Release
RNA transcripts DNA
RNA heads to the RER where ribosomes translates the RNA into protein
Protein is then transported by vesicles to the Golgi body where it can gain a lipid/carbohydrate/ion/another proteins
Protein is then removed from the cell via exocytosis