28. DMD Sepsis, Cardiovascular, GI and UT infections
Sepsis Overview
Definition
Bacteraemia: Presence of transient(short time) bacteria in the bloodstream (e.g., from brushing teeth); rapidly cleared.
Sepsis replaced the old definitions (including SIRS, severe sepsis, and septic shock).
Neutropenic Sepsis: Sepsis occurring in patients with a low neutrophil count of <1.0×10^9/L (immunocompromised/ immunosuppressed patients).
Sepsis and Septic Shock
Definitions and Diagnostic Criteria
Sepsis: Life-threatening organ dysfunction due to a dysregulated host response to infection.
Criteria: Infection + qSOFA score of at least 2.
Septic Shock: A severe form of sepsis with profound metabolic abnormalities, leading to higher mortality risk compared to sepsis alone.
Criteria: Hypotension requiring vasopressor support (MAP >65 mmHg) and lactic acid >2 mmol/L after fluid resuscitation.
Introduction of qSOFA as a new diagnostic tool with warning signs:
quick sequential organ failure assessment (13,100,22)
Altered mental status (GCS ≤ 13) - feeling lightheaded
Systolic blood pressure < 100 mm Hg - hypotension , shivering
Respiratory rate > 22 breaths/min - fast shallow breath
Advantages of qSOFA: Simple, bedside identifiable, no labs needed.
Presence of 2 or more parameters indicates a high risk of deterioration.
Pathophysiology of Sepsis
Overview
Sepsis results from the disruption of the immune response between inflammation and anti-inflammation
life-threatening condition caused by an uncontrolled immune response to an infection
Sequence of Events
Local infection or toxin enters body→ triggers an innate immune response
However, local defence fails → infection spread in the bloodstream
The immune system overreacts and releases too many inflammatory chemicals (cytokines like TNFα, IFNγ, IL-1, IL-6)
Excessive inflammatory response leads to organ injury (e.g., Acute Respiratory Distress Syndrome - ARDS, liver injury
The body's coagulation system goes out of balance.
This leads to too much clotting (thrombosis) and DIC (disseminated intravascular coagulation)—where small clots block blood flow and later cause excessive bleeding.
Initially, the immune system is overactive, but later, it becomes too weak (immunosuppression). dominant anti-inflammatory response
This happens due to apoptosis of immune cells, increase susceptibility to further infections.
Leads to tissue hypoxia and organ dysfunction → death
Diagnosis of Sepsis
Challenges
Clinical diagnosis is difficult; early intervention is crucial for survival.
Signs and symptoms can be subtle.
Microbiology
Samples should be taken before antibiotic treatment (minimum 2-3 blood cultures).
PCR: Identify infection sources.
Inflammatory markers: C-reactive protein & white cell counts.
Imaging
CT scans and ultrasounds may be utilized.
Management of Sepsis Patients
Antibiotics: Administer broad-spectrum antibiotics within the first hour of sepsis recognition (after specimens taken).
Haemodynamic Stabilization: Fluid resuscitation and vasopressors for hypotension.
Septic Response Modulation: E.g., steroid therapy.
Source Investigation: Essential to locate and address the infection site. Then drain and remove the source
Infective Endocarditis (IE)
Overview
Infection of the heart’s endocardial surface, usually involving valves; can lead to significant morbidity and mortality. → can cause heart failure, stroke
Common among patients with pre-existing heart disease;
endogenously acquired.- bacteraemia from blood can result in IR
Patient Groups
Native valve IE (natural)
Prosthetic valve IE
IV drug users
Common Affected Sites
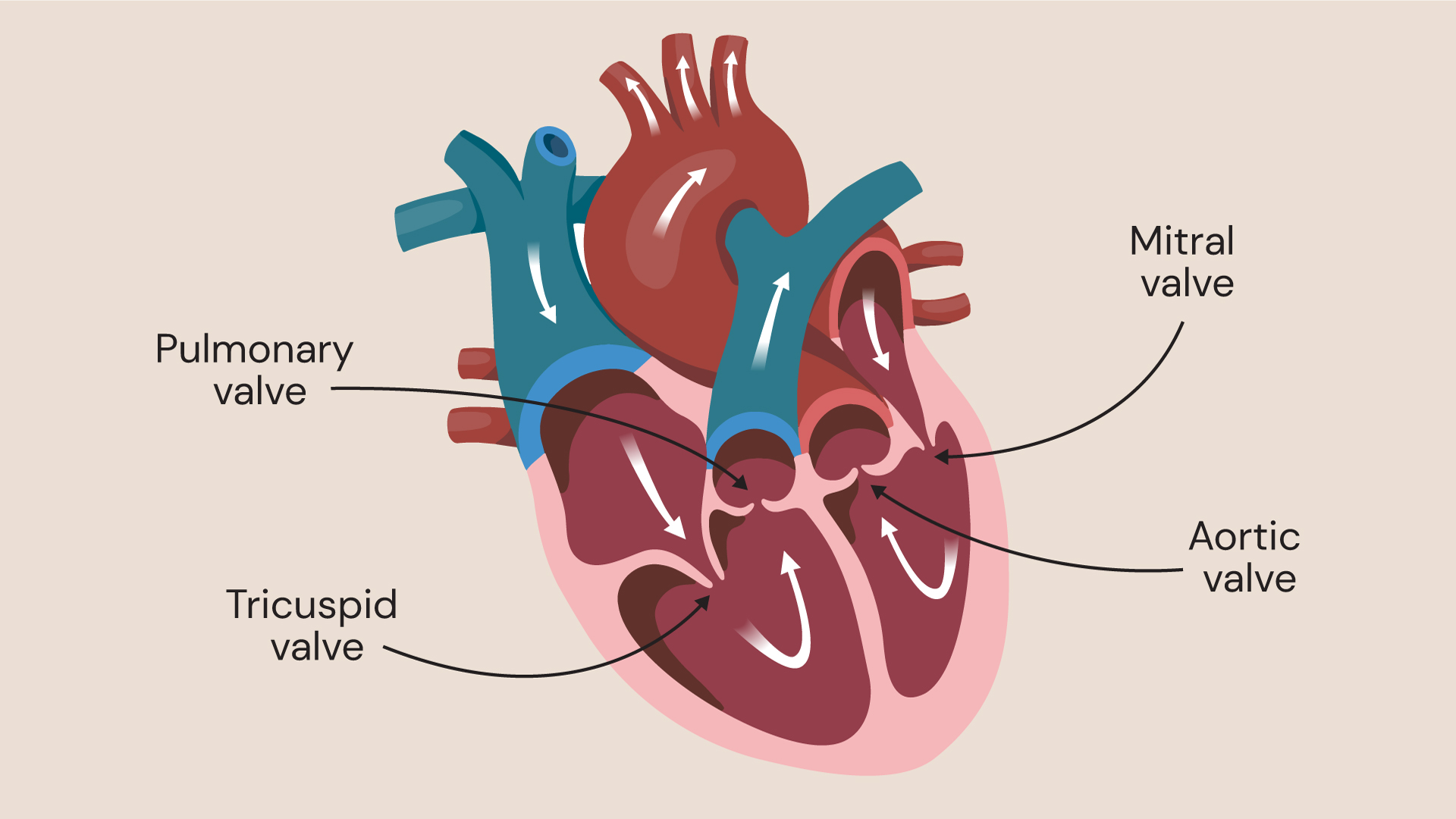
Mainly aortic and mitral valves; right-sided valves also involved, particularly in IV drug users.
Risk Factors for IE
Presence of artificial heart valves or heart defects
scarring causing by heart valve damage → bacteria can grow
Previous endocarditis episodes → tissue damage
Injecting illicit intravenous drugs
Pathophysiology of IE
1. How It Starts: Turbulent Blood Flow.
people with risk factors (like heart valve disease, prosthetic valves, or congenital heart defects) → the blood flow becomes turbulent
This damages the endocardium, creating a rough surface where bacteria can stick.
2. Formation of a “Sticky” Site
heart’s lining is damaged → the body tries to “patch it up” → depositing platelets and fibrin
This can lead to a non-bacterial thrombus (small clot-like structure) or vegetation (a larger mass).
3. Bacteria Enter the Bloodstream (Bacteraemia)
Bacteriaemia (from sources like dental procedures, IV drug use, or infections elsewhere in the body), they get carried to the heart.
→ attach to damage site and colonise → leading to an infection.
There are two main mechanisms for bacterial adherence:
Mechanism 1 (Clot Formation & Cytokine Activation)
The damaged valve allows direct blood contact with underlying tissues, leading to clot formation.
Bacteria bind to this clot, triggering immune cells (monocytes) to release cytokines, promoting infection growth.
→ enlarge infected vegetation
Mechanism 2 (Fibronectin Binding by Bacteria)
Local inflammation makes heart valve cells express fibronectin-binding proteins.
Staphylococcus aureus and other bacteria use surface proteins to bind fibronectin, increasing attachment and colonization.
4. Formation of Infected Vegetations
Bacteria become trapped within layers of fibrin and platelets, forming infected vegetations.
These vegetations shield bacteria from immune cells, allowing the infection to grow unchecked.
Over time, this can damage heart valves, cause embolism (fragments breaking off and traveling to organs), and lead to heart failure or stroke.
Clinical Presentation of IE
Predominantly presents with fever (90%); heart murmur in 85% of cases.
Symptoms may include chills, poor appetite, weight loss, and peripheral signs (e.g., Osler’s nodes, Janeway lesions).
Atypical presentations are common in elderly/immunocompromised patients.
Microbiology of IE
Predominantly Gram-positive bacteria (Streptococci 60-80%, Staphylococci 20-35%) with some fungal infections.
Culture negative cases may involve HACEK organisms or Q fever.
Diagnosis of IE
Combination of laboratory investigations, imaging, and clinical examination is essential.
Blood cultures must be collected prior to antibiotics, using aseptic techniques.
Utilizes Duke Criteria for diagnosis (major and minor criteria).
Management of IE
Antibiotic Treatment: Tailored to pathogen identification.
Surgical Intervention: valve replacement, remove risk of emboli
Continuous monitoring for relapse and dental evaluations.
Prophylaxis for IE
Antibiotic prophylaxis before dental and non dental procedure
Recommendations focus on maintaining good oral health and managing risks of bacteraemia from common activities.
Gastrointestinal Disease
Overview
gastroenteritis - inflammation of stomacha and intestine - nausea, vomiting, diarrhoea, abdonminal discomfort
diarrhea - increase fluid and electrolyte loss - frequent and fluid like faecal discharge
dysentery - large intestine inflammation - blood and pus in faeces - painful
enterocolitis - inflammation of small and large intestine mucosa
Infectious Spread in GI Disease
Faecal-oral route - human or animal faeces contain pathogenic organisms or toxin
patient consume contaminate food, fluid, fingers → ingest pathogen and toxin
pathogen multiply and toxin produce → localised in GIT
OR pathogen invade other organs, toxic spread → systemic infection
At the end pathogens are excreted in faeces
Pathogens Associated with GI Diseases
Common bacteria (e.g., Salmonella, E. coli) and viruses (e.g., Norovirus, Rotavirus). toxin producer - S aureus
Urinary Tract Infections (UTIs)
Overview
Most common bacterial infections, especially in women.
E. coli is the predominant pathogen.
urinary catheters → may cause sepsis
Lower UTI → endogenous
Upper UTI - pyelonephritis - fever → my cause renal damage