MICB 212 Immunology Notes
Levels of Defense Against Infection
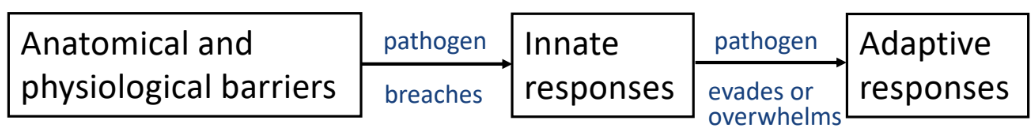
1. Anatomical and Physical Barriers (External Defense)
-intact skin provides a physical barrier
-mucosal surfaces of gut, respiratory urogenital and conjunctivae surfaces
-barrier pathogens encounter
intact skin
lysozyme in tears/saliva
stomach acid
mucus
-functions to keep pathogens from entering the body
-pathogens enter body via
wound/bites
inhalation/air breathed in
ingesting infected food
meat, dairy, etc
2. Innate Immune Response (Initial Internal Defense)
-quick and non-specific
-phagocytosis and activation of complement proteins
-limits spread of infection
-often resoles infection
-starts in minutes post-infection
peaks in 2-3 days
3. Adaptive Immune Response (Last Line of Defense)
-slow to respond but specific to particular pathogen
-involves antibody-mediated responses and cell-mediated responses
-reaches full activity in 7-10 days
Active Immunity
-immune system makes antibodies
-B cells, T cells activating, proliferating, differentiating
-develops a memory response
excepts T-independent B cell activation
-natural — no intervention by medical personnel (infection)
-artificial — intervention by medical personnel (vaccine)
Passive Immunity
-getting antibodies made by someone else transferred from one individual to another
-immune system doesn’t make antibodies
-no memory responses developed
-temporary — introduced antibodies degrade over time
-natural — antibodies crossing pregnant women’s placenta into baby — no intervention by medical personnel
breastfed antibodies
-artificial — convalescent plasma to SARS-Cov-2 antibodies after a snake bite to neutralize venom — intervention by medical personnel
used in life-threatening emergencies
Pathogen
-a microorganism that causes disease
Inflammation
-caused by physical or chemical insults or invasion by microorganisms
Infectious Inflammation
Acute Inflammation
short duration
initial response to infectious agent
causes very little tissue damage to host
Chronic Inflammation
occurs for months to years
persistence of infectious agent
due to microorganisms involving special pathogens that have extraordinary abilities to evade host’s immune response
causes tissue destruction
due to release of oxygen metabolites, nitric oxide (NO), proteases by inflammatory cells
Acute Inflammatory Response to Infection
-begins after damage to tissue
pathogen recognition by tissue-resident macrophages
bacteria enters wound and detected by tissue-resident macrophages
PRRs on macrophages bind to specific structures on bacteria → phagocytosis
TLRs recognize PAMPs → signaling cascade initiated
cytokine release and inflammatory response
signaling cascade activates transcription factors → production of pro-inflammatory (alarm) cytokines
TNF-α
IL-6
produces fever
IL-1
increases production of neutrophils in bone marrow
promotes inflammation → recruit immune cells to infection site
vascular changes induced by mast cells & macrophages
tissue-resident mast cells release histamine
vasodilation → increased blood flow → redness & heat
increased vascular permeability (cells, proteins, fluid leak into tissues) → swelling & pain
immune cells & proteins enter tissues
leukocyte recruitment & migration to infection site
IL-8 creates gradient that guides neutrophils, monocytes, dendritic cells to bacteria
adhesion molecules on blood vessel walls allow leukocytes to stick and squeeze through into infected tissue
neutrophils and monocytes arrive → mature to macrophages → phagocytic
phagocytosis of bacteria
neutrophils and macrophages engulf/phagocytose bacteria → phagosome forms
bacteria broken down and destroyed
clotting & tissue repair
clotting mechanisms activate wall off the site of infection and immobilize bacteria
tissue repair mechanisms begin to heal wound
pus formation
pus accumulates; consists of
dead and dying neutrophils and macrophages
live and dead skin cells
dead bacteria
plasma (fluid from blood)
Extracellular Bacterial Infections
-live outside of host cells
blood, tissue, mucosal surfaces
-releases toxins
-bacteria doesn’t invade cells of host
Appropriate Response
-antibody production most appropriate response
-antibodies would neutralize or opsonize pathogen to make phagocytosis more efficient
or activate complement to kill pathogen
Innate Immune Response
neutrophils & macrophages recognize and phagocytose bacteria
complement system (MAC) lyses bacterial membranes
inflammatory cytokines (IL-1, IL-7, TNF-α) recruit immune cells
Adaptive Immune Response
B cells produce antibodies that neutralize bacteria
help phagocytes clear infection
CD4 T helper cells bost neutrophil activity & enhance phagocytosis
Intracellular Bacterial Infections
-live inside of host cells
macrophages, epithelial cells
-evades immune detection by hiding in cells
-able to reproduce inside macrophages
-macrophage eventually dies and bacteria released from dead macrophage an invade other macrophages and further spread infection
-resists phagocytosis and survives inside macrophages
-antibodies not successful in eliminating infection
infection is inside cell and antibodies can’t enter
-evades antibiotics if host is taking them
antibiotic has to enter host cell and get to critical concentration
Appropriate Response
-cell-mediated immune response needed to combat infection
T cells and macrophages to kill pathogen compared to soluble proteins in blood
Super-Activated Macrophages
strong macrophages that can phagocytose and kill bacteria and viruses
super-activated by 2 signals
regular activation
macrophages detect bacteria or viruses → activate and try to destroy pathogens
phagocytose pathogens and release some toxic molecules but not at full power
binding of CD40L on T helper cell to CD40 on macrophage
super-activation by T cells
CD4 T helper cell detects macrophage needs more power
releases IFN-γ cytokine that super activates macrophages
IFN-γ + TNF-α = super-activate macrophage
more effective at fusing lysosomes with phagosomes
increase production of antimicrobial products potent enough to kill intracellular pathogens
reactive nitrogen oxide (NO)
oxygen radicals
proteases
→ reactive compounds may leak outside macrophage and damage healthy cells and tissues of host → inflammation
attracts neutrophils and macrophages that release bactericidal substances and phagocytose bacteria that have escaped from lysed cell
activates transcription of different genes
Tuberculosis (TB)
-infectious disease caused by Mycobacterium tuberculosis (MTB) bacteria
-MTB spread from one person to another via tiny droplets released into air
coughing & sneezing
-generally affects lungs
can affect other parts of body (kidney, spine, brain)
Latent Tuberculosis Infection
bacteria present in body
inactive site
doesn’t cause symptoms
Tuberculosis Infection
patient ill with symptoms
can spread bacteria to others
may occur in first few weeks after initial infection with TB bacteria or occur years later
if not treated properly → can be fatal
antibiotic therapy for ~6-24 months
Symptoms
coughing that lasts 3+ weeks
coughing up blood
chest pain, pain with breathing or coughing
unintentional weight loss
fatigue
fever
night sweats
chills
loss of appetite
tuberculosis of spine — back pain
tuberculosis in kidney — bloody urine
Progression of Infection
inhalation of bacteria
bacteria enters lungs
bacteria engulfed by macrophages in alveoli
some killed by macrophages
some survive & proliferate
macrophage may burst and bacteria spreads to infect more macrophages
dendritic cells in area engulfs bacteria and moves to lymph nodes to activate CD4 T cells
tells T cells to move to tissues to aid macrophages
CD4 T cells activated differentiate to effector T helper cells
effector T helper cells arrive at infection site
try to super-activate macrophages
formation of tubercule or granuloma
bacteria inside granuloma → inactive → latent TB
if immune system weakened → outer layer of granuloma may break → bacteria released & TB reactivated
Vaccination
BCG vaccines for TB disease
most asians have administered
scar develops
doesn’t protect children from pulmonary disease caused by TB bacteria
doesn’t prevent spread of disease or latent TB infection from progressing to active disease
prevents serious TB complications in children (TB meningitis)
~7-10 days to fully develop
generates memory T helper cells that activate macrophages and not an antibody or CTL response
secondary response to vaccine ~2 days to develop
Tuberculin Skin Test
test to identify if a person might be infected with MTB
small amount of tuberculin protein injected
positive tuberculin test
secondary reaction to a protein made by bacilli
indicates presence of effector & memory T helper cells
X-ray to confirm
person vaccinated with BCG may also test positive
reactivation of memory T helper cells — doesn’t indicate disease/infection
Location of Immune System
-distributed throughout the body
-cells of immune system found in
blood circulatory system
moves blood throughout body (including spleen)
lymphatic circulatory system
moves lymph fluid throughout lymph nodes
enables lymphocytes and proteins to move around body
lymph fluid similar to blood but lacks RBCs and platelets
connects lymph nodes together
lymphoid organs
primary lymphoid organs
secondary lymphoid organs
-lymphatic circulatory system and blood circulatory system are connected
fluids in tissues drain into lymphatic capillaries then into lymph nodes
lymphatic fluid returns to circulatory system via thoracic duct near heart
-spleen and lymph nodes act as filters
traps pathogens so immune system responses can develop
Lymph Fluid
contains WBCs and plasma
returned to blood circulatory system at thoracic duct
Primary Lymphoid Organs
-sites where lymphocytes develop and mature
bone marrow
thymus
-all HSC (blood & immune cells) complete developmentation & maturation process in bone marrow
except T cells → begins in bone marrow → completes in thymus
Secondary Lymphoid Organs
-sites where mature lymphocytes encounters pathogens/foreign molecules and begins adaptive immune system
spleen
lymph nodes
-specialized structures that allow lymphocytes to scan for antigens
spleen and lymph nodes
gut-associated lymphoid tissues (tonsils, adenoids, appendix, Peyer’s patches)
bronchial-associated lymphoid tissues (BALT)
other mucosal surfaces
Cells of Immune System
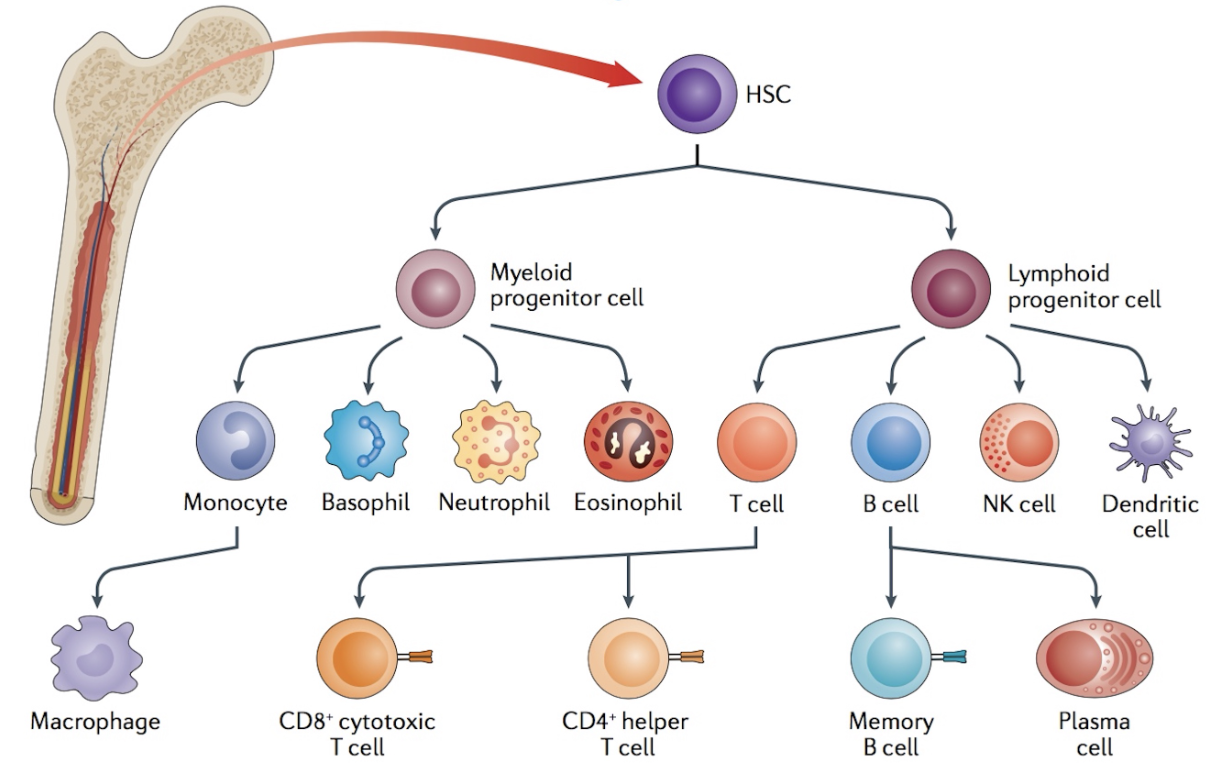
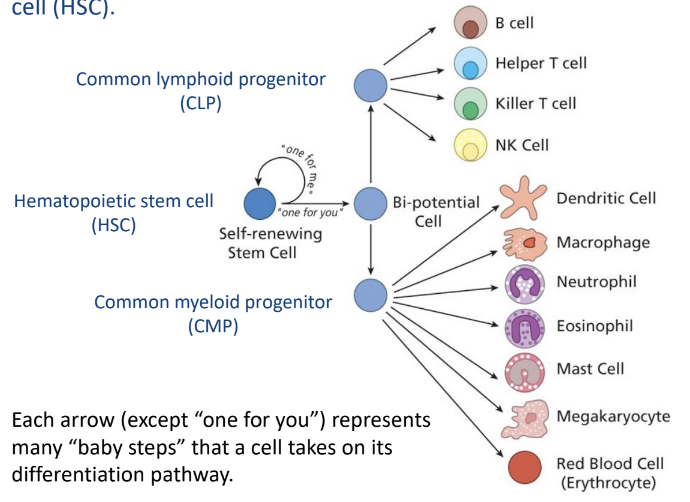
Hematopoietic Stem Cells
-all blood cells from hematopoietic stem cells (HSC)
common lymphoid progenitor cells (CLP)
lymphoid cell types
common myeloid progenitor cells (CMP)
myeloid cell types
-HSCs divide to replenish HSC pool and to provide progenitor cell types
-HSC and progenitor cells know what type of cells to develop into various chemical and environmental signals
soluble chemicals, receptor/ligand binding interactions
-HSCs are self-renewing
found in bone marrow, umbilical cords, in blood
-HSCs, CMPs, CLPs are sensitive to radiation
divide frequently
radiation damages the DNA
cells die when they divide
-bone marrow cells contains a mixture of cells — some HSCs but some cells that are in the process of maturing into lymphocytes, neutrophils
HSCs account for less than 1% of total bone marrow population
Blood
-3 major groups of cells in blood
erythrocytes (RBC)
platelets
leukocytes (WBC)
Erythrocytes (RBCs)
-carries oxygen to tissues
Leukocytes (WBCs)
-cells of immune system
-separated into
myeloid cells
granulocytes
neutrophils
basophils
eosinophils
monocytes
macrophages
lymphoid cells/lymphocytes
dendritic cells
Myeloid Cells
-participate in adaptive immune response
-includes
monocytes (mature into macrophages)
mast cells
granulocytes (neutrophils, basophils, eosinophils)
Mast Cells
live in tissues
found in skin, lungs, gut, around blood vessels
associated with allergic reactions/anaphylaxis
detects invaders → release signals by exploding and releasing tiny granules filled with histamine that triggers immune response/causes inflammation → blood vessels dilate and leak fluid to allow immune cells to rush to site of infection → release cytokines to attract macrophages, neutrophils, T cells to fight infection
fast immune response (first line of defense)
Neutrophils
phagocytose & kills bacteria
Macrophages
phagocytose and kill bacteria
alert immune system when presence of infection
tissue repairing
wound healing
not motile
serious pathogens can prevent macrophages from killing them after phagocytosis
macrophages recruit T helper cells to overcome time
presents antigen to T cells that had been activated before
Resident Macrophages
resides in tissues throughout the body
involved in early detection against invading pathogens
Lymphoid Cells (Lymphocytes)
-participate in adaptive immune response
-most are present in specialized lymphoid tissues
-develop and mature into primary lymphoid organs
-3 groups of lymphocytes
CTL (cytotoxic/killer T cells)
kill viral infected cells
prevents spread of viral infection
Th cells (T helper cells)
provide cytokines and other stimulatory signals to B cells, CTLs and macrophages
Treg
development occurs begins in bone marrow → completes in thymus
B Cells
secrete antibodies into body fluids
entire development occurs in bone marrow
presents antigen to T helper cells to receive T cell help
T cell help needed to get B cells to make high affinity, class-switched antibodies and develop memory B cells
NK (natural killer) Cells
Dendritic Cells
-phagocytose and kill bacteria/viruses
-migrates from site of infection to lymph node to activate adaptive immune response
-bridges innate and adaptive responses
-important cells for
activating T cells
initiating adaptive immune response
-derived from lymphoid cells or myeloid cells
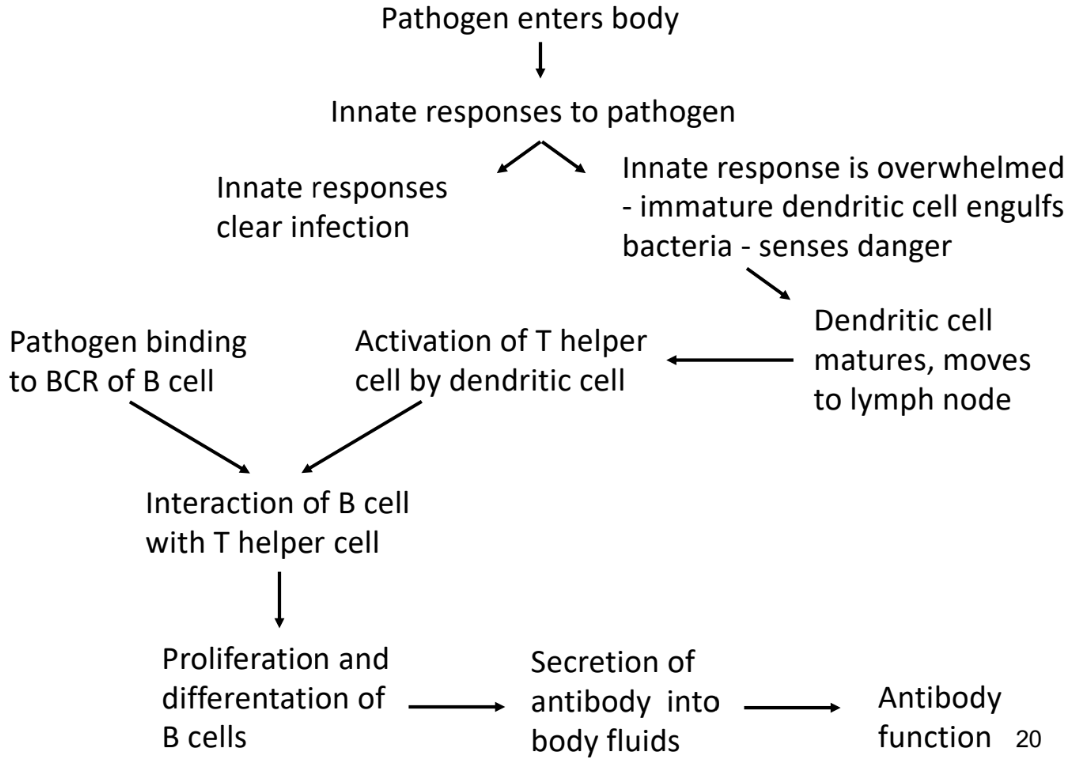
Innate Immune Response
-first line of defense internally
-acts immediately to remove pathogen without development of disease in host
fast, non-specific, triggered by components of pathogens
-includes both cellular component and protein component
-if innate immune response is unsuccessful or gets bypassed by pathogen → adaptive immune response is required
-innate immune response is able to distinguish dangerous things from non dangerous things
pathogens are recognized as foreign by PAMPs
recognized via pattern recognition receptors (PRRs)
-occurs in 2 phases
1. Immediate/Early Innate Response
preformed proteins already in blood/tissues and phagocytic cells already in tissues
ex: resident macrophages, complement in blood — when C3 breaks down to C3b → alternate pathway starts
starts within a few minutes after infection
1. activation of complement via alternative pathway
C3b — opsonin
C3a, C5a — enhances developing inflammatory response
membrane attack complex (MAC)
2. mast cells release histamine, dilation of blood vessels
brings more blood to injury site
more complement and cells, antibodies if preset
3. phagocytosis of bacteria by resident macrophages
C3b enhances phagocytosis
macrophage recognizes danger
4. production of alarm cytokines by resident macrophages → induced innate response
2. Induced Innate Response
recruitment of phagocytic cells from blood stream and into tissue (site of infection)
resident macrophages responsible for setting induced phase in motion (sending out alarm when pathogens are detected)
starts within a few hours after infection
resident macrophages recognizes bacteria and send out alarm to recruit phagocytic cells (neutrophils, monocytes, dendritic cells) to site of infection
change in blood vessel wall of vein so that neutrophils & monocytes can get to site of infection
phagocytic cells migrate towards bacteria in tissue → engulf & kill bacteria
leads to inflammatory response (complement activation)
Pathogen-Associated Molecular Patterns (PAMPs)
-pathogens (virus & bacteria) have PAMPs
-molecular structures that are
not found in multicellular hosts
present on numerous groups of pathogens
essential for pathogen’s survival, don’t mutate frequently
ex:
LPS; gram (-) bacteria
peptidoglycan; gram (-) & gram (+) bacteria
LTA; gram (+) bacteria
dsRNA; virus
single stranded DNA; virus
-recognized by PRRs in innate response and binds to phagocytic PRRs
results in
phagocytosis of pathogen
cytokine production by immune cell
Pattern Recognition Receptors (PRRs)
-receptors on neutrophil, macrophages, dendritic cells that bind PAMPs
-different PRRs have different functions
-some pathogens recognized outside of host cell via cell surface PRRs
-other pathogens recognized once inside host cell
recognized by PRRs in membranes of intracellular vesicles
Phagocytosis (Endocytotic) Receptors
-type of PRR
-on innate immune cells
-engulfed by cell when pathogen binds to this receptor
-used to bring particle inside phagocyte
Toll-Like Receptors (TLRs)
-type of PRR
-allow phagocyte to determine if particle is dangerous
-binding of PAMPs initiates intracellular signal
-upon binding of an infectious organism to toll
antifungal or antimicrobial peptides are released
-signal results in
release of cytokine
up-regulation of MHC class II proteins
up-regulation of B7 co-stimulatory molecule
-13 different TLRs in mammals
11 functional in humans
-every TLR recognizes a set of molecular patterns not found in host
-functions as homodimers or heterodimers
Complement
- > 20 plasma proteins in blood that work in a cascade process
-able to work alone or with other proteins (antibodies or soluble PRRs)
Function
-to attack extracellular pathogens
-form membrane attack complex on pathogen → causes death of pathogen
-some can work as opsonins to enhance phagocytosis of pathogen by phagocyte
-some can work to promote development of cellular inflammatory response
Complement Activation
-complement proteins are activated when antibodies made during a previous adaptive immune response binds to pathogen surface
-involves cleavage of protein to make 2 smaller proteins
-works as a cascade of reactions → one reaction is followed by another
product of one reaction catalyzes next reaction which catalyzes next reaction and so on
component A is cleaved
fragment of A acts by cleaving component B
fragment of B acts by cleaving component C
fragment of C acts by cleaving component D etc
-occurs almost immediately after infection
Alternative C Pathway
-spontaneous breakdown of component C3 into
C3a (attracts & activates)
C3b (binding)
-C3b is deposited on membrane of pathogen and recruits rest of components leading to formation of pore in membrane of pathogen
-in absence of infection → C3b broken down further → no harm to host
-in presence of infection → C3b binds to bacteria
-complement protein C3 (blood) → cleaves → C3a & C3b form → C3b binds to pathogen surface & recruits other complement proteins to form C3 convertase → additional C3b protein & C3 convertase forms → some C3b used as opsonin, some C3b used to form C5 convertase → C5 convertase cleaves component protein C5 → C5a & C5b forms → C5b used to form membrane attack complex
Activation of Inflammatory Response
-complement activation contributes to inflammatory response activation
-C3 convertase cleaves additional C3 → C3a & C3b → some C3b forms C5 convertase → C5 convertase cleaves C5 → C5a & C5b forms
C3a & C5a
affect blood vessel permeability when produced in large amounts
induces a generalized circulatory collapse → anaphylactic shock
small fragments that bind to
specific receptors on blood vessels
results in increased permeability of blood vessels
receptors on resident mast cells resident macrophages
release additional histamine & TNF-α
induces expression of adhesion molecules
allow leukocytes (neutrophils & monocytes) to attach to blood vessels
bind to recep
C5a is a power chemoattractant for neutrophils & monocytes
Opsonization & Enhancement of Phagocytosis
-opsonization is alteration of pathogen surface/particle surface so phagocytic cells can engulf efficiently
-C3b binds to pathogen surface can function as an opsonin
binds to receptor on surface of phagocytic cell
results in increase number of contact points between pathogen & phagocytic cell
-phagocytic cells have receptors that bind to C3b to pathogen surface
-coating pathogen with C3b enhances phagocytosis by neutrophils & macrophages
Formation of Membrane Attack Complex & Bacterial Cell Lysis
-when C5b is deposited on pathogen surface
assembly of late/terminal complement components into membrane attack complex (MAC) is initiated
punches holes in pathogen surface → pathogen cell lysis → death
Adaptive Immune Response
-2 main weapons
antibodies
produced by B cells
main defense against extracellular bacteria
protects against infection
T cells
-activated when innate immune response can’t eliminate infection
last line of defence
Primary Immune Response
-first exposure body has to pathogen
-takes longer to develop a defense
-5-7 days to develop
time lag due to differentiation and proliferation of naive T helper cells & B cells
recognition & activation
APCs detect pathogen, processes its antigens and presents them to naive T cells & naive B cells
few days for naive T cells and naive B cells to activate
clonal expansion & differentiation
activated B cells multiply and differentiate into plasma cells
produces antibodies (mostly IgM at first)
activated T cells expand and carry out immune functions
symptoms develop
memory B cells and memory T cells formed
remain in body for future protection
Secondary Immune Response
-re-exposure of pathogen in body
-2-3 days to develop
-faster and stronger than primary response
activation of memory cells
memory B cells & memory T cells recognize pathogen and activates immune response
strong antibody production
memory B cells → plasma cells
high affinity antibodies produced (mostly IgG)
antibodies produce much faster
fast elimination of pathogen
infection often cleared before symptoms develop
T Cells
-WBC (lymphocyte/leukocyte) that plays a role in adaptive immune response
-from HSC produced in bone marrow → matures in thymus
-responsible for recognizing specific antigens on surface of infected cells
recognizes and responds to antigens via T Cell receptor (TCR) complex
-co-receptors CD4 & CD8 defines function of T cell
CD4 T Cells (T Helper Cells)
recognizes peptides present on MHC class II proteins
help coordinate immune response by activating other immune cells
B cells, CD8 T cells
CD8 T Cells (Cytotoxic/Killer T Cells, CTLs)
recognizes peptides present on MHC class I proteins
directly attack and kills infected or cancerous cells
4 (CD4) x 2 (MHC II) = 8 (CD8) x (MHC I)
-every T cell has its own TCR and antigen specificity
-single mature T cells have ~30 000 identical copies of TCR on its cell surface
T Cell Receptor (TCR)
-structure on surface of T cells that recognize and bind to antigens presented by MHC molecules
allows T cells to recognize pathogens and trigger immune response
-membrane-bound protein made of 2 polypeptides (α & β chains) joined via disulfide bond
1 variable (V) region
Vα and Vβ chain regions combine to form antigen-binding site
one antigen-binding site per TCR molecule
1 constant (C) regon
-TCR antigen-binding subunit recognizes peptides bound to MHC proteins
doesn’t recognize native, intact antigens
-T cells respond to peptide antigens on MHC class I/II proteins
if host is healthy → peptide is from self protein
if host has infection → peptide is from pathogen protein
-CD3 is the signalling component of the T cell receptor complex
Regulatory T Cells (Treg)
-type of CD4 cell that help controls immune system and prevents autoimmune diseases
thymocytes that are positively selected with MHC class II
-self-reactive & secretes IL-10
calming, anti-inflammatory cytokine
-Treg deficiency → increased chances of autoimmune disease
-prevent autoimmunity
stops immune system from attacking body’s own tissues
-control inflammation
suppress overactive immune responses and limit tissue damage
-regulate other immune cells
keep T cells, B cells from attacking harmless molecules
-maintains immune balance
ensure immune system reacts properly
T Cell Development
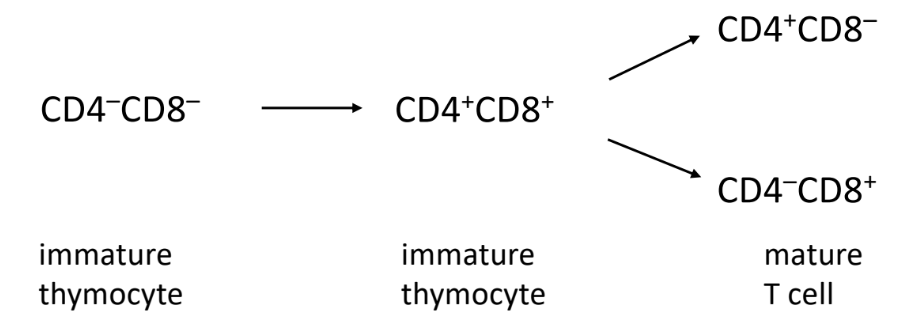
Thymocytes
immature T cells
develop in thymus before becoming fully functional T cells
T cell precursors (thymocytes) starts in bone marrow and travels to thymus
don’t have TCR
functionally distinct from T cell populations
developing thymocytes tracked by changes in expression of TCR, CD4, CD8
-T cells don’t know to be CD4 or CD8 T cell → undergoes thymocyte development
undergoes negative & positive selection
Double Negative Stage (Early Development)
-thymocytes don’t have CD4 and CD8 markers
-rearrange TCR genes to form unique TCR
Double Positive Stage (Intermediate Development)
-thymocytes express CD4 and CD8
-undergo positive selection to ensure TCR can recognize MHC molecules
T cells with TCR unable to recognize MHC molecules undergoes apoptosis (dies)
rendered useless
Single Positive Stage (Final Development)
-thymocytes that recognize MHC class I → CD8 T cells (CTL)
with high enough affinity to activate cell
-thymocytes that recognize MHC class II → CD4 T cells (T helper cell)
with high enough affinity to activate cell
-undergo negative selection to eliminate cells that might attack body
self-reactive T cells
identifies thymocytes that bind MHC and self-peptide too tightly
bind too tight → may be activated in lymph node/spleen → autoimmune disease
-surviving thymocytes mature into functional T cells and leave thymus
T Cell Activation
-T cell gets activated to fight infections
when antigen presenting cells present pathogens to naive T cells
Naive T Cell
T cells that haven’t been activated before
-activated only when it needs to be → T cell activation can be dangerous
requires 3 signals to activate
2 signals come from antigen-presenting cell
Signal 1 — Antigen Recognition (TCR-MHC Interaction)
-APC presents peptide on MHC class I/II molecule
CD8 T cell → MHC class I molecule
CD4 T cell → MHC class II molecule
-TCR on T cell binds to MHC antigen complex
Signal 2 — Co-stimulation
-makes sure immune system doesn’t attack by mistake
-prevents self-reactive T cells from attacking body’s own cells
-co-stimulatory receptor CD28 protein of T cell binds to B7 protein of APC
T cell gets activated
-signal 1 & 2 together allows T cell to start making IL-2 (cytokine)
-if no co-stimulation occurs → T cell becomes living but non-responsive (anergic)
Signal 3 — Cytokine Signalling
-cytokines directs T cell response and differentiation so T cells
know what type of response to generate
attack viruses/bacteria/parasite
multiply into
CD8 effector T cells
kills virus infected cells
CD8 memory T cells
reactivated if future infection with pathogen occurs
-T helper cells (CD4) make enough of their own IL-2 to proliferate but CTLs (CD8) don’t make enough
depend on IL-2 made by T helper cells
Dendritic Cells
-best antigen presenting cell (APC) for activation of naive T cell
presens MHC class molecules to naive T cell to activate it
-expresses
MHC class I
MHC class II
co-stimulatory molecule B7
-able to undergo endogenous, exogenous, cross-presentation pathways of antigen processing and processing
-bridge between innate and adaptive immune response
Dendritic Cell Functions
-phagocytose and break up pathogens
-transport pathogen remains to local lymph node
-presents peptides from pathogens to bind to MHC class molecules for activation of T cells
Dendritic Cells in Innate Immune Sytem
-has PRRs that detect PAMPs
-detect pathogen → engulfs & digests pathogen (phagocytosis) → break down into antigens
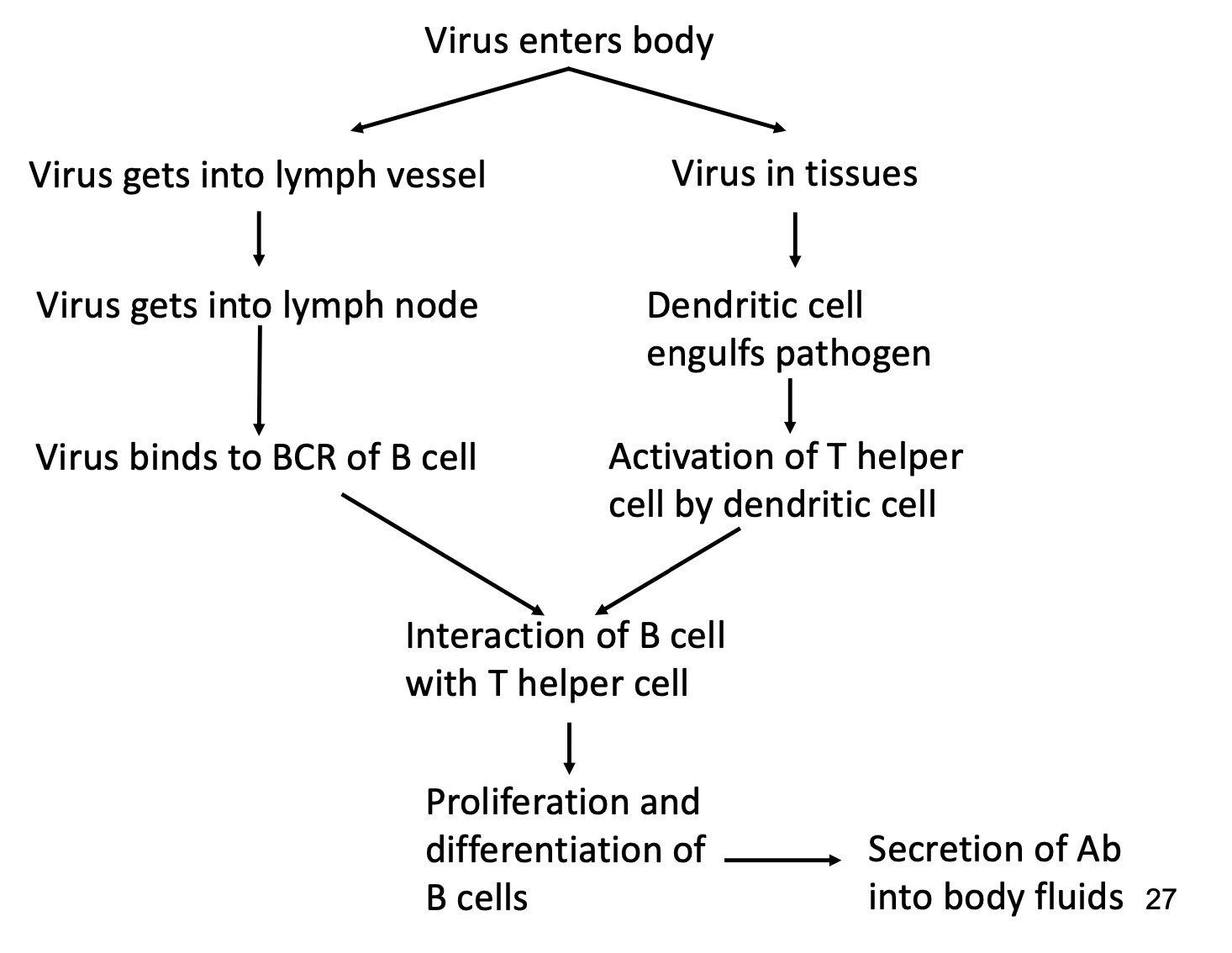
B Cell
-secrete antibodies into body fluids
-entire development occurs in bone marrow
-presents antigen to T helper cells to receive T cell help
T cell help needed to get B cells to make high affinity, class-switched antibodies and develop memory B cells
-becomes plasma cell after activation by Igα/Igβ signal
B Cell Development
-from common lymphoid progenitors in bone marrow
remains in bone marrow for whole development process
-begins to express B cell receptor
-immature B cells with functional BCR are screened for self-reactivity in bone marrow
high affinity BCRs → deleted
B Cell Receptor (BCR)
-major developmental stages of developing cells can be tracked by changes in expression of B cell receptor (BCR)
-protein complex on B cell surface
-every B cell has a unique BCR
-allows B cells to recognize and bind to antigens
-helps B cells detect infections/invaders and start an immune response
when BCR binds to matching antigen → triggers signal inside B cell → antibody production
-provides long term immunity
helps immune system remember past infections
-BCR of mature naive B cell is
membrane bound immunoglobulin M (mlgM)
or
membrane bound immunoglobulin D (mlgD)
B Cell Receptor Structure
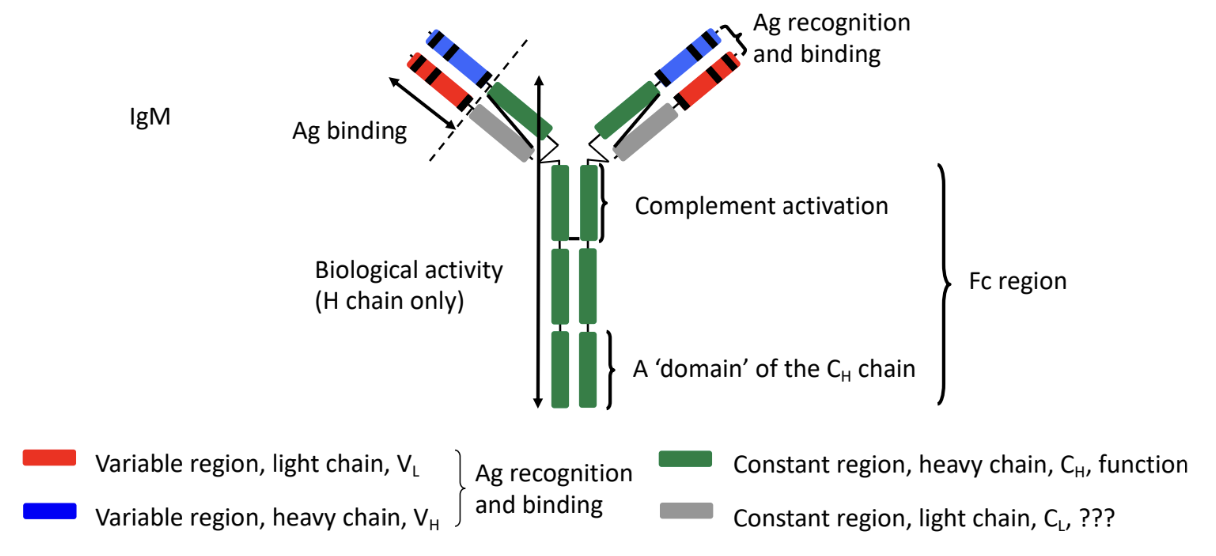
-quaternary protein structure
made of 4 polypeptides
-2 antigen binding sites per BCR
Antibody Component
binds with antigen
made of 2 heavy (H) and 2 light (L) chains → forms Y-shape
joined by disulfide bonds
each chain has a variable region (VL VH) located at tips of Y
determines antigen specificity
hypervariable region within variable region
makes direct contact with antigen
each chain has a constant region (CL CH) located at base of Y
Signaling Component (Igα/Igβ)
BCR alone can’t send signals to B cell
Igα/Igβ proteins transmits signal inside cell after antigen has bound
B Cell Receptor Process
Antigen recognition
B cell with correct BCR encounters antigen
BCR — Antigen binding
BCR binds directly with antigen
doesn’t require APC (like T cells)
Signal transmission
binding of antigen triggers Igα/Igβ → sends signals inside B cell
B cell Activation
activate and prepare for immune response
engulf antigen for further processing
communicate with helper T cells
differentiate into plasma cells → mass produce antibodies that match antigen
become memory B cells → long-lived cells that remember antigen for a faster response on the next encounter
B Cell Activation
-so B cells synthesizes and secretes antibodies
-requires multiple signals to differentiate antibody secreting cell
-responds to antigen in
T-independent manner
T-dependent manner
T-Independent Activation
-T helper cells not involved
-fast antibody response but low affinity IgM produced, no memory cells
few days
-short lived plasma B cells
-antigen has many epitopes
-repetitive, multivalent antigens that bind to multiple BCRs on a single B cell
-antigen binds directly to BCRs on B cell → Igα/Igβ signal inside cell → activates B cell → IgM antibodies produced
1 signal for activation
binding of antigen to 2 BCRs
-weaker immune response
no class switching to IgG, IgA, IgE
no memory B cell production → no long lasting immunity
Ex: Pneumococcal Vaccines
capsular material (polysaccharide)
13 strains of pneumococcus bacteria (> 90 strains)
promotes IgM antibody response → no memory cells
antibody neutralizes bacteria
for conjugated vaccines to activate T cells & B cells
covalently couple non-protein part (polysaccharide) to a protein molecule
dendritic cells can use protein part to generate peptides to activate T cells
one set of B cells recognizes protein part
one set of B cells recognizes non-protein part
T helper cells activated by peptide from protein part
helps both sets of B cells
add something to antigen to trick dendritic cells to think it’s dangerous
adjuvant → causes arm to be sore/hurt
stimulates danger → dendritic cells start immune system
T-Dependent Activation
-T helper cells involved
-slower, high affinity, class switched antibody response with memory cells
7-10 days
-long lived plasma B cells
-antigens have few epitopes
-T helper cells produce cytokines (signals) required for class switching and memory cell formation
-3 signals needed for activation
binding of antigens to 2 BCRs
cross-linking of BCRs
B cells identifies itself to T helper cell that it needs help binding to antigen
antigen-BCR complex into cell via endosome → displays peptide on MHC class II → moves to lymph node or spleen → TCR of T helper cell (CD4) recognizes peptide displayed on MHC class II → CD28 (T helper) binds to B7 (B cell)
*B cell acts as APC so T helper cell will help
binding of CD40 on B cell to CD40L on T helper cell
T helper cells provide CD40 ligand (CD40L) binding to CD40 on B cell via cytokines
cytokines secreted by T helper cell supports B cells to turn into
memory B cells
plasma cells
secreted antibodies
IgG, IgA, IgM, IgE antibodies that target specific pathogens
-T helper cells signals can promote class switching in B cell
can produce IgG, IgA , IgE from IgM
Antibodies (Abs)/Immunoglobulins (Igs)
-essentially the same as BCRs but BCR is attached to B cell while antibodies are secreted by activated B cells (plasma cells) into blood or tissues
-proteins made and secreted by B cells
-circulates in blood and other body fluids (mucus)
-antibodies bind to molecules that are foreign to individual
-kills bacteria (in presence of complement)
-opsonize pathogens (make phagocytosis more efficient)
-neutralize pathogens (prevent pathogens from binding/infecting cells)
Membrane Bound Immunoglobulins (mIg)
activates B cells
stays attached to B cell surface
part of BCR
amino acids are hydrophobic
IgM, IgD
Secreted Immunoglobulins (sIg)
destroys pathogens
free floating antibodies produced by B cell after it is activated and becomes a plasma cell
circulates in blood, lymph and tissues to attack pathogens
amino acids are hydrophilic
IgG, IgA, IgM, IgE
Antibody/Immunoglobulin Binding
-bind to epitopes (antigenic determinants)
distinct 3D shape
~4-5 amino acids
can be carbohydrates, lipids, synthetic chemicals etc
antigens have more than one kind of epitopes
-bivalent → 2 binding sites
Antibody/Immunoglobulin Classes
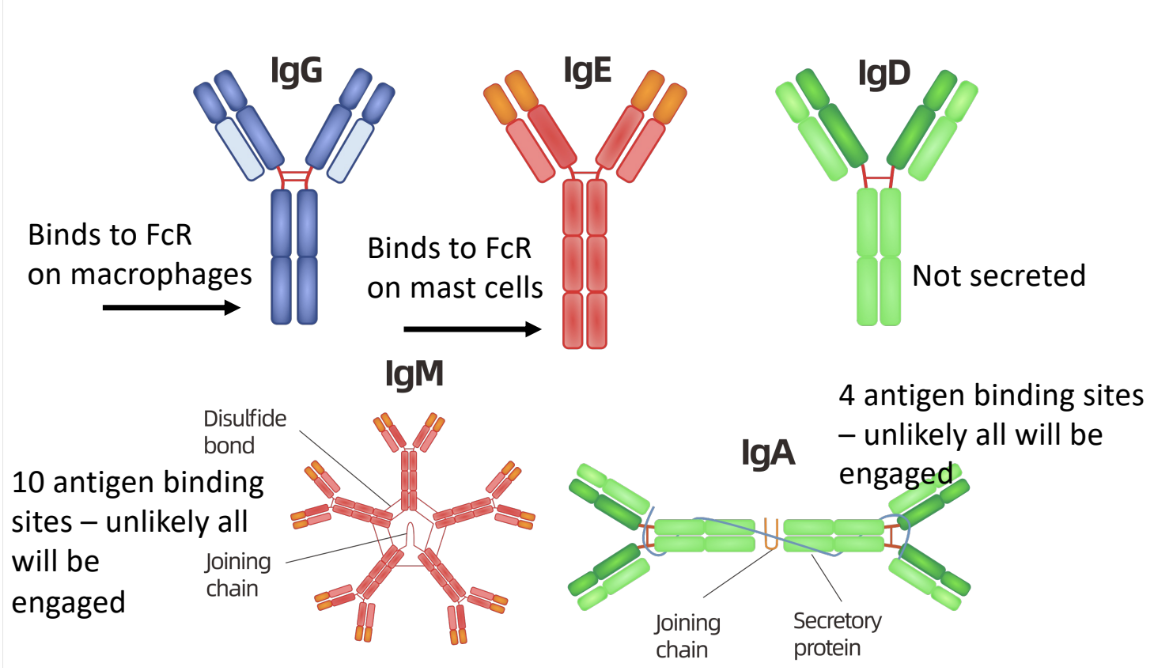
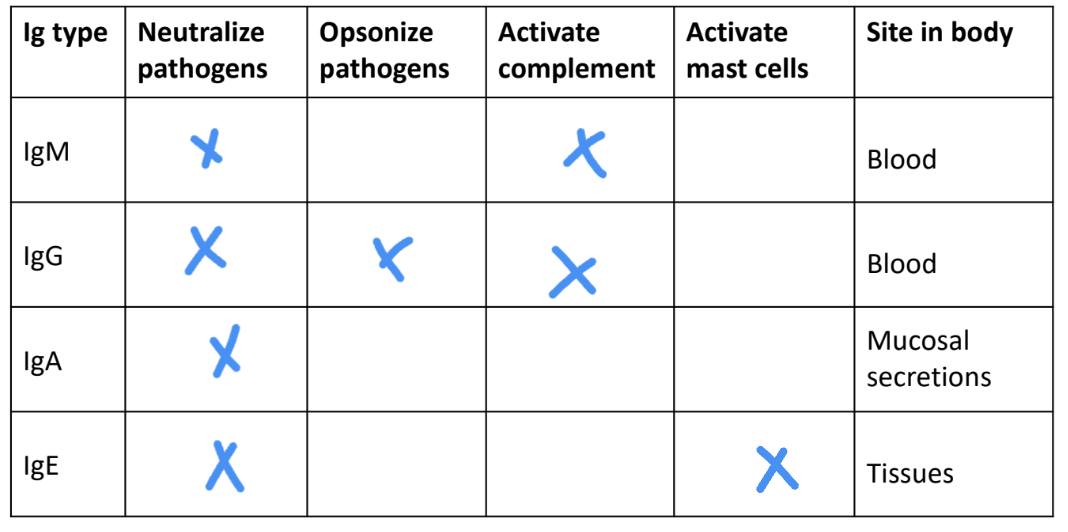
-IgM, IgG, IgA, IgE, IgD
-all have different functions in immune system
-can be
membrane bound (mIg) (IgD only)
secreted (sIg)
IgD
not secreted
only found on surface of naive B cells
IgM
secreted
low binding affinity
neutralizes pathogen
first antibodies produced in immune response
IgG
secreted
found in blood
neutralizes pathogen
kills bacteria in 2 ways
complement proteins kill bacteria coated with IgA
amino acid sequence in γ chain constant region binds to complement proteins
phagocytes ingest bacteria coated with IgG antibodies better than bacteria without IgG
amino acid sequence in γ chain constant region facilitates IgG binding with specific receptors on phagocytic cells
IgA
secreted
in serum → monomer
in bodily secretions → dimer
main antibody class in bodily secretions
binds to and neutralizes pathogens
prevents attachment of pathogens to host surfaces
IgE
secreted into serum
binds immediately to mast cells and basophils
not available in serum to neutralize pathogens
triggers allergic reactions
when IgE on mast cell/basophil binds an antigen
cell degranulates & releases large amounts of histamine
amino acid sequence in ε chain constant region binds to receptors
only antibody class that can bind to receptor (FCεRI)
main antibody class induced in response to infection by parasites
ex: intestinal worms → too big to be killed via phagocytosis
mast cell degranulation → diarrhea & vomiting to expel worms
Structure of Antibodies/Immunoglobulins
-Y shaped proteins made of 2 binding sites
-4 polypeptide chains
2 identical H chains
2 identical L chains
-variable region at tips of Y shaped antibody
binds specifically to antigen (hypervariable region)
hypervariable regions (HVH HVL)
makes direct contact with antigen
3 short stretches of amino acids
-constant region at base of Y shaped antibody
determines antibody type
interacts with immune cells
difference in amino acid sequence of C regions of heavy chains is significant
L Chain Types
kappa (κ) chain
lambda (λ) chain
immunoglobulin may have κ chain or λ chain L chain, but never both
H Chain Types
gamma (γ) chain
mu (μ) chain
delta (δ) chain
epsilon (ε) chain
alpha (α) chain
type of H chain defines immunoglobulin class or isotype
Function of Antibodies/Immunoglobulins
-different antibodies have different roles in immune system
determined by H chain of antibody
Neutralization
-antibodies neutralize pathogens by binding to them → prevents them from binding to surface
-reliez on variable region of antibody
Antibody/Immunoglobulin Class Switching
-best method for adaptive response to match antibody to antigen
-B cells can class switch more than once
switching is unidirectional
can’t switch back to original antibody after splicing → DNA is lost
-B cells always secrete IgM antibodies first (sometimes IgD)
fast first response but not good enough
switches to make a different class of antibody → IgM, IgA
-only CH regions change; VH/VL and CL regions don’t change
cutting out DNA of heavy chain genes (base of Y shape)
only constant region of heavy chain
-T helper cells induces class switching via different combinations of cytokines

antibody constant region is spliced out and gets replaced with a different constant region
ex: IgM made initially → switch to IgE BUT wants to switch to IgG afterwards
T cell sends signals from cytokines to B cells → IgM constant region spliced out → IgE constant region inserted to antibody
B cell now produces IgE → switch to IgG later on
T cell sends signals from cytokines to B cells → IgE constant region spliced out → IgG constant region inserted
B cell now produces IgG
Antibodies as Tools in Research and Medicine
-clinical uses, research laboratories, diagnostic services
-antibodies can be produced by
immunizing animals (rabbits, mice, goats)
cells (hybridomas) that grow in tissue culture
-antibodies can be purified and used for detecting and quantifying antigens that they recognize
ex:
detection of pathogens (bacteria, viruses, toxins) in. patient samples
detection of antibodies in blood that indicate exposure to antigen (covid test)
detection and measurement of hormone levels (thyroid hormones, pregnancy tests)
analysis of blood cells and immune cells (blood and tissue typing, enumeration of cell types)
therapeutic medication for medical conditions (cancer, psoriasis, Crohn’s disease)
-amino acid sequence of variable regions of antibodies different for constant regions of dogs & humans
species difference
Polyclonal Antiserum
-mix of different antibodies in serum (anti-serum)
-animals can be immunized with antigen (virus, snake venom, etc) and antibodies can be isolated from animal’s serum
contains mixture of antibodies that recognize different epitopes on antigen that was injected
antigens have many epitopes that activate B cells
each B cell recognizes a different epitope
antibodies with different specificities and affinities
-blood serum that contains polyclonal antibodies specific to particular antigen
-after immunizing animal with specific antigen
created by immunizing an animal with specific antigen
-constant regions of heavy chains of antibodies from different species are immunogenics
antibodies from another species can be antigens → antibodies against antibodies are made
ex: inject dog anti-rabies antibodies into human → patient’s immune system produces human anti-dog antibody antibodies
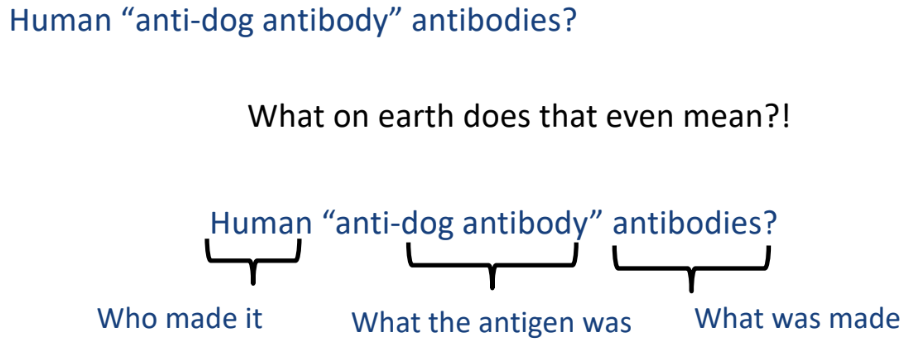
Polyclonal Serum
-blood serum containing mixture of antibodies against multiple antigens or epitopes
broad mix of antibodies, not specific to one antigen
-normal blood serum of organism after exposure to infections
Monoclonal Antibodies
-antibodies produced by descendants of one clone of B cells
maintained in cell culture (in vitro)
repeatedly immunize mouse with antigen to get secondary immune response
B cells specific for epitopes on antigen get activated → plasma cells secreting antibodies
remove mouse’s spleen → antibody secreting plasma cells immortalized by fusing them with myeloma cell that grows in culture
myeloma — plasma cell tumour that can’t make antibodies
B cells fuse to myeloma cells via polyethylene glycol → hybridoma
hybridoma — characteristics of B cell (make antibody) & myeloma (immortality)
grow and divide indefinitely in tissue culture dishes
mixture of immortal hybridomas make antibodies against different epitopes
clones of hybridoma making antibody made
single cell placed in each well of 96 well tissue culture plate
cells in each well divide and form clones of identical cells
hybridoma clones making antibodies against desired epitope are selected
small amount of culture medium from each well
using ELISA assay to see if antibodies secreted into medium by hybridoma cells bind to epitope of interest
Antigens
-markers found on surface of viruses, bacteria, blood that immune system recognizes
-can be foreign or self
-if immune system recognizes antigen as foreign → triggers immune response
-if antigen from body → immune system ignores
sometimes makes mistakes → autoimmune diseases
Antigen Processing
-degradation of protein into peptide fragments
Antigen Presentation
-displaying peptides derived from pathogens or other protein on dendritic cell surface that T cells can see
-MHC proteins on cell surface provides physical structure to display antigenic peptides to T cells
-binding and display of antigen as peptide fragment bound to MHC proteins on surface of a cell
MHC Class I
displays peptides derived from proteins in cytoplasm of cell
proteins coming from inside of cell
proteins synthesized by ribosomes
MHC Class II
displays peptide derived from soluble proteins taken up by cell via endocytosis/phagocytosis into an endosome/phagosome
proteins coming from outside of cell
proteins include
normal blood proteins
toxins
bacteria
virus particles
Antigen Presenting Cells (APC)
-immune cell that activates immune system (tells immune systems about infections)
shows pieces of pathogens to immune cells
capture & engulf (phagocytosis)
processing antigens
pathogens broken down into peptides (antigens)
displaying antigens
APC places antigen on MHC molecules (class I/class II)
MHC class I presents antigens to cytotoxic T cells (CD8 T cells) → destroys infected cells
MHC class II presents antigens to helper T cells (CD4 T cells) → coordinates immune response
activation of T cells
T cells recognize antigen
activates to kill infected cells
help other immune cells fight infection
Types of Antigen Presenting Cells
dendritic cells
macrophages
B cells
Major Histocompatibility Complex (MHC) Proteins
-region of chromosome containing genes that encode proteins that have a role in immune response
-proteins that have grooves so peptides can bind
-allow dendritic cells to communicate to T cells that an infection is occurring so adaptive immune response can be initiated
-MHC is polygenic
several genes that encode proteins of similar function
-MHC is polymorphic
different alleles in human population
-HLA = human leukocyte antigen
codominantly expressed
cells transcribe both alleles and make protein products of both genes
MHC Class I Proteins
-expressed on all nucleated cells
-transmembrane α chain non-covalently bonded with β2-microglobulin
peptide bonding groove on α chain
~8-10 amino acids long
-3 different MHC class I proteins
HLA-A
HLA-B
HLA-C
-50% of MHC class I expressed on each cell from mom, other 50% from dad
-cells have ability to make 6 different variants of MHC class I proteins
3 (1 HLA-A, 1 HLA-B, 1 HLA-C) from mom
3 (1 HLA-A, 1 HLA-B, 1 HLA-C) from dad
MHC Class II Proteins
-expressed only on antigen-presenting cells (dendritic cells, macrophages, thymic epithelial cells)
-2 transmembrane polypeptide chains
α & β chains
peptide bonding groove between α & β chains
at least 13 amino acids ong
-3 different MHC class II proteins
HLA-DP
HLA-DR
HLA-DQ
-all proteins only expressed on antigen presenting cells
MHC Polymorphism
-different alleles in human population
different alleles for each MHC gene
-allows cells to present as many types of peptides as possible
-protects against wide range of pathogens
-recognition by immune system wouldn’t work properly without MHC polymorphism
Disadvantages to MHC Polymorphism
-hard to find matching donor for someone that needs an organ/bone marrow transplant
same versions of MHC molecules
MHC Diversity
-goal of MHC protein is to be able to bind and present peptide derived from any protein
especially foreign proteins from a pathogen
-if unable to present peptides from a pathogen → adaptive immune response not activated
-single MHC proteins can bind many but not all peptides
Pathways for Processing and Presentation of Protein into Peptide Fragments
MHC Class I Peptide Loading (Endogenous Pathway)
-MHC class I displays peptides from inside of the cell to cytotoxic T cells (CD8 T cells)
proteins coming from inside cell
helps immune system detect infected or cancerous cells
-occurs 24/7
proteins inside cytoplasm are broken down into peptides via proteasomes
peptides moved to lumen of endoplasmic reticulum (ER)
TAP (transporter protein) moves peptides from cytoplasm to ER
MHC class I molecule made in ER
newly made MHC I associates with β2-microglobulin
peptide binds (loaded) to MHC class I molecule
only peptides of right length and quality are able to bind
MHC class I becomes stable and ready to leave ER after binding
MHC class I peptide complex moves from ER to Golgi apparatus
MHC class I peptide complex gets packed up in Golgi
MHC class I peptide complex sent to cell surface via vesicles
displayed on cell surface for ~24 hours before internalized and replaced
CD8 T cells inspect MHC class I peptide complex
peptide is normal (self-protein) → T cell ignores it
peptide is from a virus/cancer → T cell recognizes cell as threat and kills it
MHC Class II Peptide Loading (Exogenous Pathway)
-MHC class II displays peptides from outside of cell to helper T cells (CD4 T cells)
external proteins are taken into cell
cell takes in bacteria, viruses, blood from surroundings via endocytosis
cell becomes phagosome
proteins are broken down into peptides via phagosome — lysosome fusing
proteins are broken down into peptides via lysosomal proteases
MHC class II molecule made in ER
invariant chain (li) blocks peptide binding groove
prevents molecule from binding to wrong peptide
MHC class II-invariant chain complex travels from ER to phagosome via vesicle
enzymes cut invariant chain
leaves small piece (CLIP - class II associated invariant chain peptide) in binding groove
peptide exchange
CLIP replaced by peptide from degraded pathogen (antigenic peptide)
MHC class II peptide complex sent to cell surface
displayed for immune system inspection for ~48 hours before internalized and replaced
CD4 T cells inspect MHC class II peptide complex
scan MHC class II molecules on antigen presenting cells (macrophages, dendritic cells, B cells)
peptide is from a pathogen → CD4 T cell activates → coordinate immune response → release cytokines
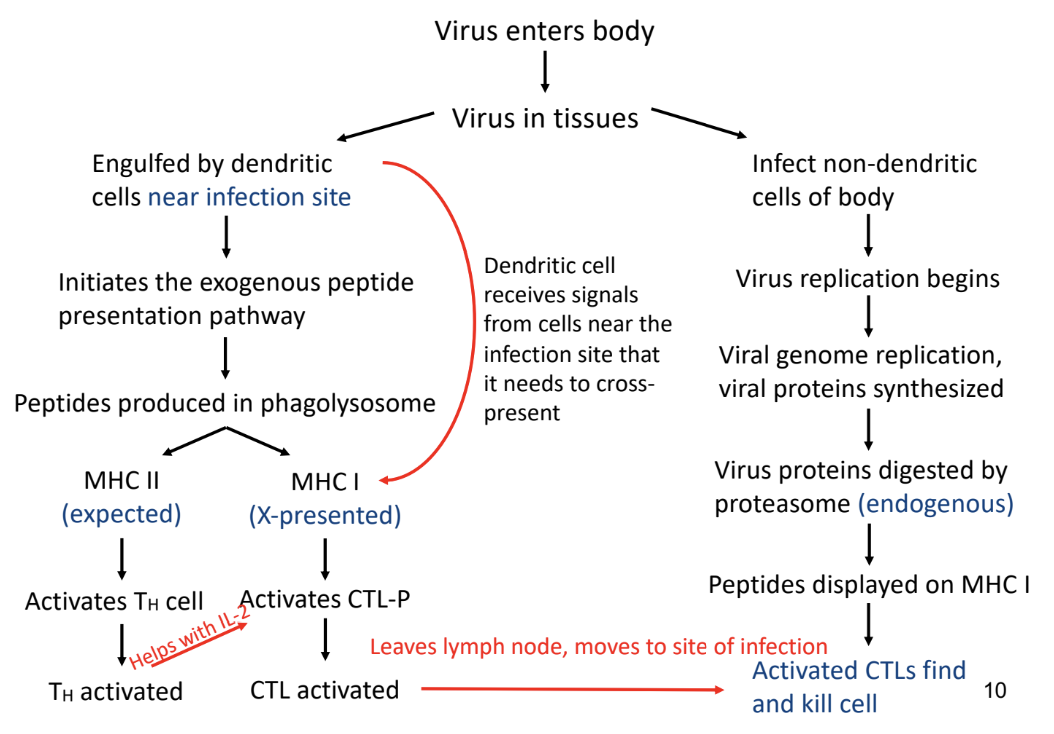
MHC Class I & MHC Class II Peptide Loading (Cross-Presentation Pathway)
-mix of exogenous and endogenous pathways aided by dendritic cells
-different subsets of dendritic cells used
from common myeloid precursors
from common lymph precursors
-presents peptides on
MHC class I via endogenous pathway
MHC class II via exogenous pathway
-extracellular antigens (virus/blood/bacteria proteins → peptides) presented on MHC class I molecule to CD8 T cells
Cytosolic Pathway (Endogenous Pathway)
-proteins from extracellular antigens escape into cytoplasm → broken down into peptides via proteasome in cytoplasm → peptides travels to ER via TAP → peptides binds to MHC class I
dendritic cell engulfs extracellular antigens
cell takes in bacteria, viruses, blood from surroundings via endocytosis
cell becomes phagosome
some proteins escape from phagosome into cytoplasm
proteins are broken down into peptides via proteasome in cytoplasm
peptides in cytoplasm transported to ER via TAP
peptides bind to MHC class I molecule in ER
MHC class I peptide complex sent to cell surface
CD8 T cell inspect MHC class I peptide complex
Vacuolar Pathway (Exogenous Pathway)
-proteins from extracellular antigens broken down into peptides via lysosomal proteases in phagosome → MHC class I travels to phagosome → peptides bind to MHC class I
dendritic cell engulfs extracellular antigens
cell takes in bacteria, viruses, blood from surroundings via endocytosis
cell becomes phagosome
proteins broken down into peptides via lysosomal proteases in phagosome
MHC class I molecules made in ER travels to phagosome
peptide binds to MHC class I in phagosome
MHC class I peptide complex sent to cell surface
CD8 T cells inspect MHC class I peptide complex
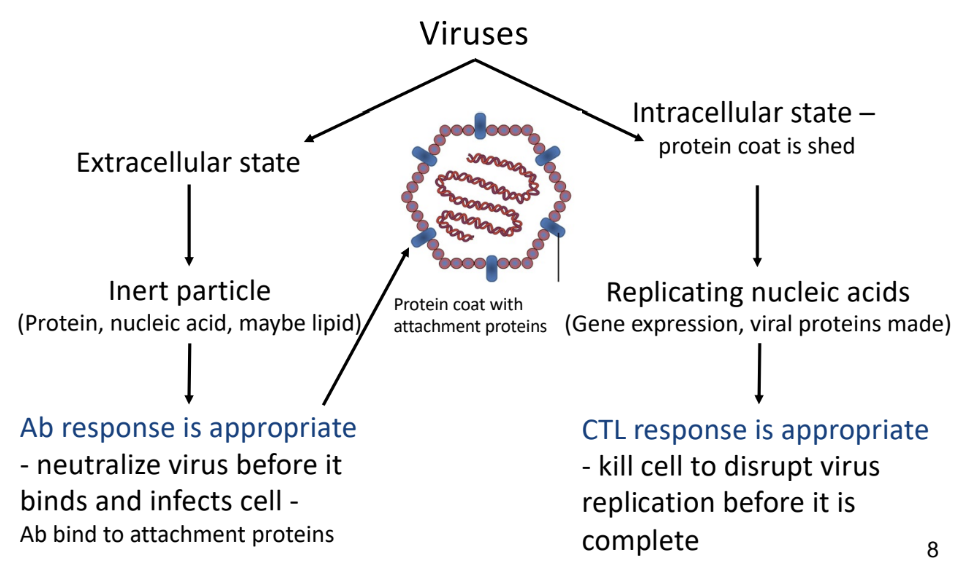
Virus
-must infect cells in order to replicate
viruses are not living/cells
can’t synthesize their own ATP or amino acids or nucleotides or proteins
-binds to a structure on surface of cell
-takes over host cell’s metabolic resources and uses cell’s machinery in order to replicate genomes and assemble new virus particles
Innate Immunity Response
-less effective than innate responses to bacterial pathogens
-TLRs and PRRs to recognize virus-specific molecules
double-stranded RNA
double-stranded DNA
uncapped single-stranded RNA
-PRRs initiate signal transduction cascade that results in expression of Type I interferon (IFN-α/β) genes and secretion of IFN-α/β
-IFN-α/β cytokines
bind to a common receptor found on neighbouring uninfected cells
triggers a signalling cascade that induces anti-viral response
-uninfected cell shuts off ability to synthesize protein
virus infected cell → virus can’t replicate since protein synthesis is essential in virus replication cycle
Adaptive Immunity Response
-involves antibodies and cytotoxic T cell responses
prevents infection of more cells by virus
-antibody production against viruses can be induced via
natural infection with virus
use of vaccine
-antibodies secreted by B cells bind to and neutralize viruses
prevents them from infecting host cells
Intracellular Virus
-protein coat is shed
-CTL response
sacrifice infected cell so that virus replication cycle can stop and not other cells can be infected
-APC picks up viral peptides and loads them onto MHC class I molecule
MHC class II peptide complex is delivered to naive CD8 T cell in lymph node
-CD8 T cell makes CTLs that spread through body to find infected cells
-CTL-P activated to CTL
CTL-precursor (naive T cell) in lymph nodes or spleen
T helper cells provide extra IL-2 for CTL proliferation
dendritic cells to activate T helper cells and CTL-P
-CTLS recognize infected cells by binding to MHC-class I viral peptide
CTL releases toxic molecules
-memory CTLs remain for future infections
some CTLs turn into memory T cells
Extracellular Virus
-antibody response
neutralize virus so it can’t bind and infect cells
-B cells to synthesize & secrete antibodies
-T helper cell to provide signal to B cells to fully activate them
-dendritic cells to activate T helper cells
Autoimmunity
-immune system attacks body’s own healthy cells
-when immune system responds against normal tissues/organs
results in damage to or impaired function of tissue
-clonal deletion failure causes autoimmune diseases
clonal deletion ensures that the T cell & B cells that recognize own cells are eliminated during development in thymus/bone marrow
-antibody-mediated autoimmune disease
making antibodies against self-components causes autoimmune diseases
block function of tissue/organ
activation of complement by antibodies → death of tissue/organ
-cell-mediated autoimmune disease
activated T cells
damage to tissues/organs via cytokine secretion
activate macrophages that destroy tissues/organs
-T cells activated against normal components of an individual
normal components reacting with BCR or TCR → auto-antigens or self-antigens
-self-reactive B cells & self-reactive T cells
-everyone has self-reactive antibodies & self-reactive T cells
go through negative selection but process isn’t perfect
Autoimmune Diseases
Autoimmune Hemolytic Anemia
-RBCs removed from circulation at end of normal life span (~120 days)
-hemolysis
premature destruction of RBCs
shortened RBC life span (< 120 days)
caused by antibodies against RBCs
-anemia results when bone marrow production can’t compensate for shortened RBC survival
Myasthenia Gravis
-severe muscle weakness
-nerve impulses don’t reach muscle
weakness and fatigue in affected muscles
-antibodies binding to acetylcholine receptor on muscle cell
blocks receptor and prevents muscle cells from responding to neurotransmitters (acetylcholine) released by nerve cells
acetylcholine released by nerve cells unable to bind to receptor because antibodies are already bound to it
-T-dependent B cell response
patient has memory B cells and long-lived plasma cells
repeated relapses and remissions
Type I Diabetes
-insulin dependent diabetes
-self-reactive T helper cells against antigen on pancreatic islet cells
-T helper cells cause inflammatory response similar to DTH response
-activated macrophages infiltrate tissue containing self-antigen
-insulin-producing cells in pancreas are destroyed as result of inflammatory response
Multiple Sclerosis
-immune system mistakenly attacks protective nerve of brain & spinal cord
-protective coating around nerve fibers attacked
damages myelin sheath → inflammation
-without myelin → nerve signals slow down or get disrupted
muscle weakness, vision problems, trouble with movement/coordination
Celiac Disease
-genetic autoimmune disease that results from breakdown of tolerance of foods containing gluten
gluten — protein in grains; gives dough its elastic feature
-in healthy systems → soluble proteins from food don’t stimulate an immune system
-oral tolerance of food breaks down → loss of villi in epithelial cells
dendritic cells are activated → CD4 T cells activated → secrete cytokines that damage epithelium of GI tract and activate CD8 T cells → antibodies
Treatment of Autoimmune Diseases
-non-specific immunosuppression with steroid hormones
suppresses immune system
-avoidance of trigger (gluten in celiac diseases)
-some conditions — removal of spleen may improve patient’s conditions
Cytokine Release
-cytokines are important for recruiting and activation of cells but they can cause other symptoms
fever, muscle aches, headaches, fatigue, nausea
symptoms result from immune response to a pathogen
Cytokine Release Syndrome
too much cytokine is released
potentially fatal
Medical Complications from Immune Responses
-sometimes it’s better not to have an immune response
allergies
delayed type hypersensitivity (DTH)
transplant rejection
contact dermatitis
serum sickness
autoimmune diseases
-body’s response involves the same events that occured in localized inflammatory response
but on a systemic scale
results in septic shock
low BP & organ failure
Septicemia
bacteria entering bloodstream and multiplying
very serious
difficult to treat
antibiotics unhelpful
Snake Venom
-some snake venom are toxic and potentially lethal
neurotoxins, cytotoxins, cardiotoxins, hemotoxins
rapid neutralization of venom is required → anti-venoms
preformed antibodies to venom administered as passive immunization
Anti-Venom
mostly protein; adjuvant is added so dendritic cells recognize it as dangerous
often produced in horses (large blood volume)
milk snake → get venom → add adjuvant to venom → inject small amount to horse (small enough not to harm animal) → few months later antibodies are extracted via blood draw → formulation of snake antivenom
binds to and neutralizes venom → resolves emergency
stops further damage
doesn’t reverse damage already done
effective at preventing death from bites if enough is administered in time
people given large amounts of anti-venom
leftover anti-venom → production of human anti-horse antibody antibodies
results in rashes
Poison Ivy/Poison Oak/Poison Sumac
-produce urushiol (type of oil)
penetrates skin & covalently binds to self-proteins
can penetrate cell lipid bilayers and bind to intracellular proteins
can be active for ~1 year
sensitization on first time exposure
delayed type reaction if previously exposed
First Exposure
dendritic cells pick up bound self-proteins and present to MHC class II molecules to activate T helper cells (CD4)
proteins can be processed by proteasome and presented to MHC class I molecules to activate cytotoxic T cells (CTLs/CD8)
Second Exposure
if re-exposure → memory T cells & memory CTLs are reactivated
macrophages will pick up urushiol bound to self-proteins and present to memory T cells
macrophages and T helper cells secrete cytokines to activate B cells → antibodies
Contact Dermatitis
inflammation of skin
if allergic to something that causes dermatitis as a response upon skin contact→ allergic contact dermatitis
delayed type hypersensitivity (DTH)
rash develops ~2-3 days upon re-exposure
hypersensitivity because urushiol in absence of immune attack is harmless
Allergies
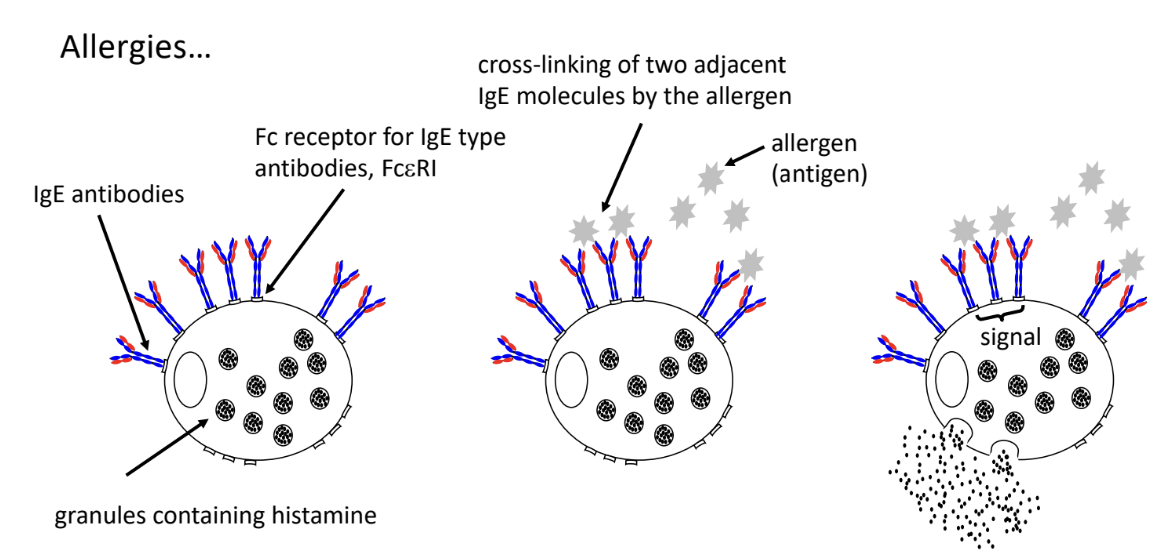
-person has primary antibody response to antigen causing the allergy
T helper cells somehow convinces B sells to secrete IgE antibodies
-IgE antibodies bound to FcR of mast cells
burst of mast cells triggers release of histamine → causes allergy symptoms within minutes of exposure to antigen
-mast cell gets primed
primary & secondary response, class switching of antibodies to IgE
-cross-linking of IgE
allergen needs to enter tissue to bind to IgE
IgE bound by same antigen (cross-linking)
triggers signal that causes explosion of mast cells (release histamine into tissus) → dilation of blood vessels
Respiratory System
excess mucous secretion → sneezing & coughing
Skin
hives/welts
Gut
diarrhea/vomiting
Hay Fever
localized/seasonal allergy
allergic rhinitis
allergic reaction to airborne particles inhaled through nose or mouth
pollen → trapped in upper airway leading to degranulation of mast cells
symptoms localized to upper respiratory tract
Mosquito Bites — Skeeter Syndrome
localized allergy
2 types of responses
small round (2-10 mm) & surrounding edema that peaks 20-30 minutes after bite
pruritic papules
IgE mediated allergic response to proteins in mosquito saliva
Penicillin Allergy
systemic allergy
reactive B lactam ring reacts with amino groups on host proteins to form conjugates
naive CD4 T cells recognize penicillin modified peptides and present to MHC class II molecules
B cells get activated in T-dependent manner
memory B cells express IgE BCRs and plasma cells secrete IgE antibodies
IgE antibodies bind to FcR of mast cell → degranulation of mast cell → release of histamine
Anaphylactic Shock
severe and life threatening allergic reaction
within minutes of exposure to allergen
peanuts, penicillin, bee venom may induce anaphylactic shock
severe over reaction of immune system
symptoms
swelling, wheezing, shortness of breath, difficulty swallowing, weak pulse, drop in BP
Allergy Treatments
Localized Allergic Reaction
anti-histamines
block histamine receptor on smooth muscle cells and endothelium that binds histamine
doesn’t prevent release of histamine; just blocks histamine from binding to cells
Systemic
epinephrine (epipen)
reverses symptoms of anaphylactic shock
relaxes airways, constricts blood vessels
lie person on floor if epipen unavailable
Allergy Shots
desensitization
deliberate exposure to small amounts of allergen
since new B cells constantly made
new B cells could be encouraged to switch to IgA or IgG type antibodies and out-compete IgE for binding to allergen
ex: grass pollen injected under skin trigger B cells to switch to IgG/IgA
Hypersensitivity
-substance not dangerous but immune response to substance is dangerous
-inappropriate immune response to substance that isn’t dangerous
-can be
CD4 T cell mediated
CD8 T cell mediated
B cell mediated (antibody)
Ex: Tuberculin Test
tuberculin isn’t harmful → test to see if person has tuberculosis
immune response to it is harmful
Immune Deficiency
-immune system is too weak or missing key components
unable to fight infections properly
frequent/severed illnesses
-can be genetic (primary) or acquired (secondary)
Primary Immunodeficiency
-person born with immunodeficiency
Severe Combined Immunodeficiency (SCID)
-mutation in HSC that impacts development of all mature lymphocytes
-lack of T cells
life isn’t sustainable without T cells (medical intervention required)
-some cases where B cells present but not fully functional
life is sustainable (continued medical assistance)
-T-B-
no T cells
no B cells
most severe form of SCID
no adaptive immune response
no antibodies
-T-B+
no T cells
has B cells
not functioning properly because some plasma cells are T-dependent to make antibodies
no functional antibodies
Treatment
HSC transplant/bone marrow transplant
within first 2 years of life
in future → CRISPR-Cas system may be an option
gene editing that can be used on living cells
HSCs could be removed and isolated from patient
bubble boy syndrome → extreme isolation to protect against infections
SCID-Like/Bare Lymphocyte Syndrome Type II (BLS II)
-absence of functional CD4 T cells but different reasons than SCID
immune cells don’t express MHC class II molecules → CD4 T cells not activated
MHC class I expression is normal → CD8 T cells activated
-normal amounts of B cells, neutrophils, monocytes/macrophages, dendritic cells
-weakened antibody production
plasma cells that rely on T cells for secretion of antibodies and class switching
Treatment
HSC transplant within first 2 years of life
not a complete cure
new macrophages, B cells, dendritic cells able to express MHC class II but epithelial cells don’t
patients do better → gain ~20% of CD4 T cell population
Neutropenia
-too little neutrophils produced
weak immune system → high chance of infection
-neutrophils essential in controlling infections from extracellular bacteria
Treatment
HSC transplant
daily injection of recombinant human granulocyte colony-stimulating factor (rhG-CSF)
Secondary Immunodeficiency
-acquired immune deficiency
not inherited or congenital
-result of infection or injury to immune system
AIDS
-acquired immunodeficiency syndrome
-untreated HIV infection can lead to AIDS
-T helper cell (CD4) count is < 20% of normal level
-people with AIDS usually die to opportunistic infections
can’t mount an adequate immune response against infections
Treatment
anti-retroviral medications
medications that control virus load in patient → maintenance of CD4 T cell population
Graft Rejection and Transplantation
-removal of organ, tissue or hematopoietic stem cell from a donor and placed into body of recipient
-replacement of solid organs/tissues or bone marrow
due to congenital defects or infectious disease that has damaged the organ
Transplant Rejection
-after transplant, graft initially survives and appears healthy
due to slow activation of T cells
-CD8, CD4 T cells in charge of destroying grafted organs/tissues
antibodies play a minor role in graft rejection
-when organ, tissue, or bone marrow is transplanted (unless from identical twin)
there will be foreign proteins
major mismatch → differences in MHC (tissue type)
minor mismatch → differences outside MHC
-transplantation rejection caused by strong immune response to non-self MHC
Allo-Antigen
MHC on transplanted tissue
Self-Antigen
MHC on recipient’s cells
Indirect Allorecognition
-APC from recipient take up and process MHC proteins from transplanted organ/tissue
-recipient T cells recognize presented peptide as foreign and mount a response
-proteins released from cells of grafted issue (surgery process may damage cells)
DAMPS — damaged associated molecular patterns
activate signaling PRRs (TLRs)
Direct Allorecognition
-T cells become activated against graft via donor-derived APC
-transplanted into recipient with donated organ
-once in recipient’s body → donor-derived APC able to move out of transplanted organ and interact with recipient’s T cells
-recipient T cells recognize MHC proteins on donor derived APCs as foreign
react and triggers immune response
-acute graft rejection
Solid Organ/Tissue Transplant
-transplanting
organ
heart
kidney
lungs
liver
pancreas
tissue
bones
tendons
cornea
skin
heart valves
-concerns
donor and recipient may have different MHC class I molecules on their cells
immune responses are directed against foreign MHC molecules
transplanted organs/tissues from donor may have different proteins than those of recipient
Host vs Graft Disease
-recipient’s immune system attacks transplanted organ/tissue
-immune system sees transplanted organ/tissue as foreign and attacks it
-T cells of recipient activate and attack graft due to differences in MHC class I molecules
inflammation & organ failure
-transplanted organ may need to be removed → second transplant
-improve chances of successful transplant
donors HLA matched as closely as possible to recipient
identical twin is the best donor → all genes identical
close relative is next best choice → high chance of sharing MHC alleles
ex: kidney rejection after kidney transplant
Bone Marrow or HSC Transplant
-transplanting an immune system
-concerns
donor and recipient may have different MHC class molecules on their cells
immune responses are directed against foreign MHC molecules
Graft vs Host Disease
-donor’s immune cells attack recipient’s body
transplanted system attacks body it goes into
-donor’s immune cells recognize recipient’s body as foreign and starts attacking it
-immunosuppressant may help with problem
ex: bone marrow transplant rejection leading to skin, gut, liver damage
Autograft
-organ, tissue, HSC cells transplanted within same person’s body
ex: veins from leg in a heart bypass surgery
Allograft
-organ, tissue, HSC cells harvested from one individual and placed into body of a different person
-alleles of MHC proteins on donor graft not normally present in recipient’s body (non-self antigens)
-recipient may have T cells that bind strongly to foreign MHC proteins on transplanted person
Increase Successful Surgeries
-better surgical procedure & healthier organs = less damage to graft
-matching MHC molecules
donors that are related to patient
-immunosuppressive drugs to stop T cells from proliferation
anti IL-2 to block T cell proliferation
stop T cell activation (cyclosporin A)
disadvantage → person’s susceptibility to infection increases
ELISA
-enzyme-linked immunosorbent assay
-useful for measuring molecules in solution
detection of soluble antibodies in solution
detection of any other type of soluble molecules
Detection of Soluble Antibodies in Solution
-useful for measuring hormones or any type of molecule in solution
-need 2 antibodies
bind to different epitopes of antigen
1 of 2 antibodies tagged with enzyme
-determine if a soluble antigen is present in sample
-antigen x is coated to well of microtitre plate
unbound antigen washed away
-sample added to well
if antibody present → binds to antigen (primary antibody)
excess antibody washed away
-secondary antibody (with enzyme bonded to it) is added
if antibody bound to antigen → antibody will bind to it
-colourless substrate added and converted to coloured product by enzyme on secondary antibody
concentration can be measured
proportional to amount of secondary antibody present (proportional to amount of primary antibody)
Detection of any Other Type of Soluble Molecules
-detection of presence of antigens
-antibody specific for the antigen is coated to well of microtitre plate (primary antibody)
excess antibody is washed away
-sample added to well → if soluble antigen is present → binds to primary antibody
excess antigen is washed away
-secondary antibody (with enzyme covalently bonded to it) is added
recognizes different epitope
doesn’t bind primary antibody
excess antibody washed away
-colourless substrate added and converted to coloured product by enzyme on secondary antibody
concentration of product measured
proportional to amount of antigen captured by first antibody
Lateral Flow Devices — ELISA Variation
-used to detect pathogens (virus, bacteria)
-has a control line to confirm test is working properly
along with one or more target/test lines
-time to complete: 5-10 minutes (not including sample preparation)
-uses immunoassay technology using nitrocellulose membrane coloured nanoparticles and antibodies to produce results
-when sample added → flows along test device passing through conjugate pad into nitrocellulose membrane then onto absorbent pad
-conjugate pad storing conjugated labels and antibodies receive sample
target present → immobilized conjugated antibodies and labels bind to target and continue to migrate along test
-as sample moves along device → binding reagents situated on nitrocellulose membrane will bind to target at test line → coloured line forms
Fluorescence Microscopy
-small fluorescent dyes (fluorochromes) covalently attached to antibody
antibody becomes useful molecular probes
-different dyes excited by different light wavelengths
allows to stain sample with several antibodies at once
-fluorescent labeled antibodies used to detect
antibodies covalently linked to fluorescent molecules
expression of protein
change in expression
distribution (location) of a cell
movement of protein in cell
-cell needs to be fixed to microscopic slide
-if target on cell surface → labeled antibodies can be incubated with samples directly
-if target inside cell → cells treated with weak detergent solution to permeabilize cells
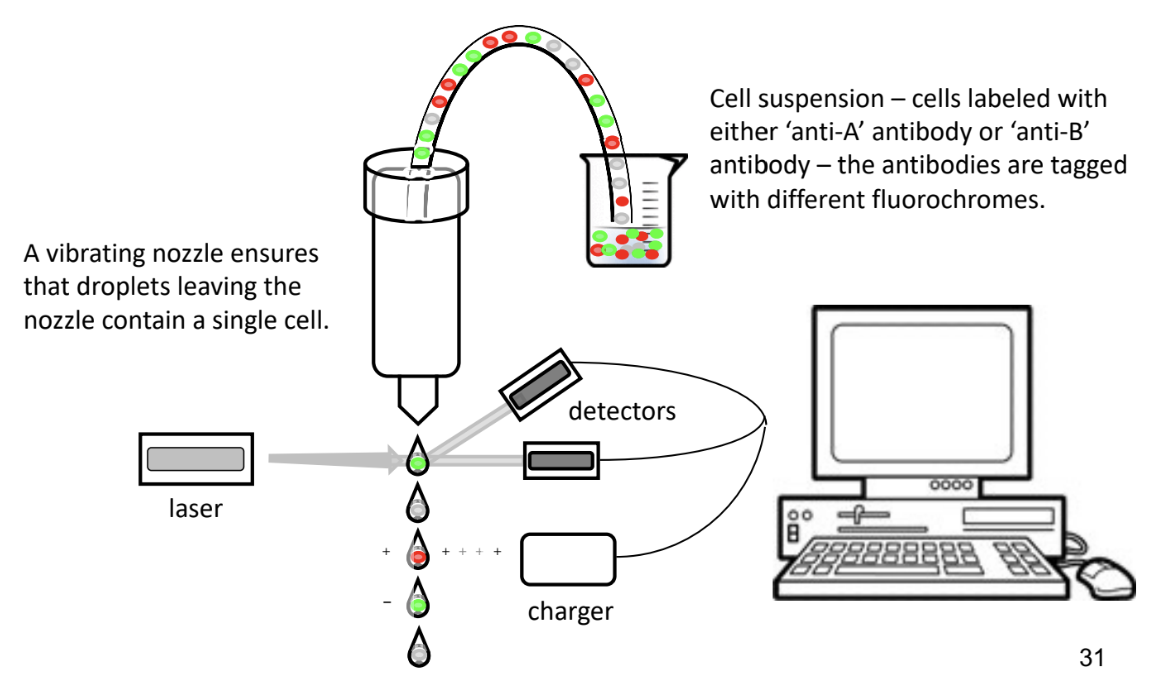
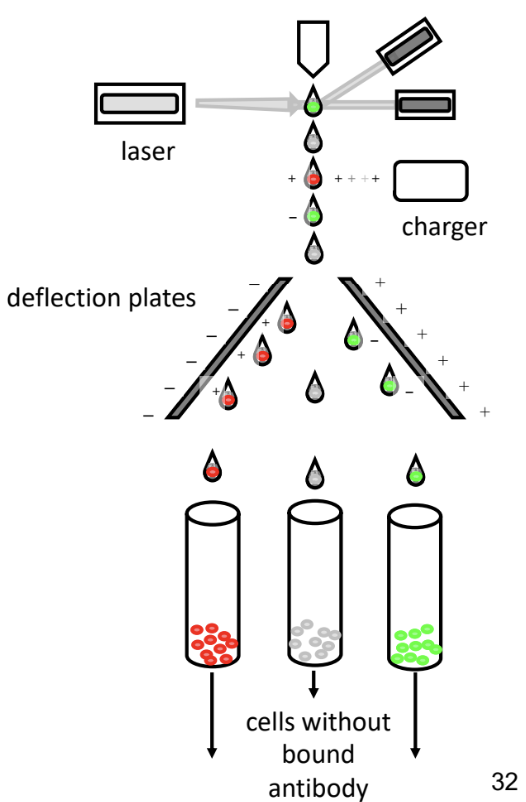
Fluorescent Activated Cell Sorting (FACS)
-machine that counts and sorts cells
antibodies with different fluorescent molecules attached or cells without any fluorescence are sorted and category counted
sort cells based on expression of particular protein
on or inside cell surface
count cells and see if protein is expressed on cell
-cells sorted with laser that detects colour and counts them
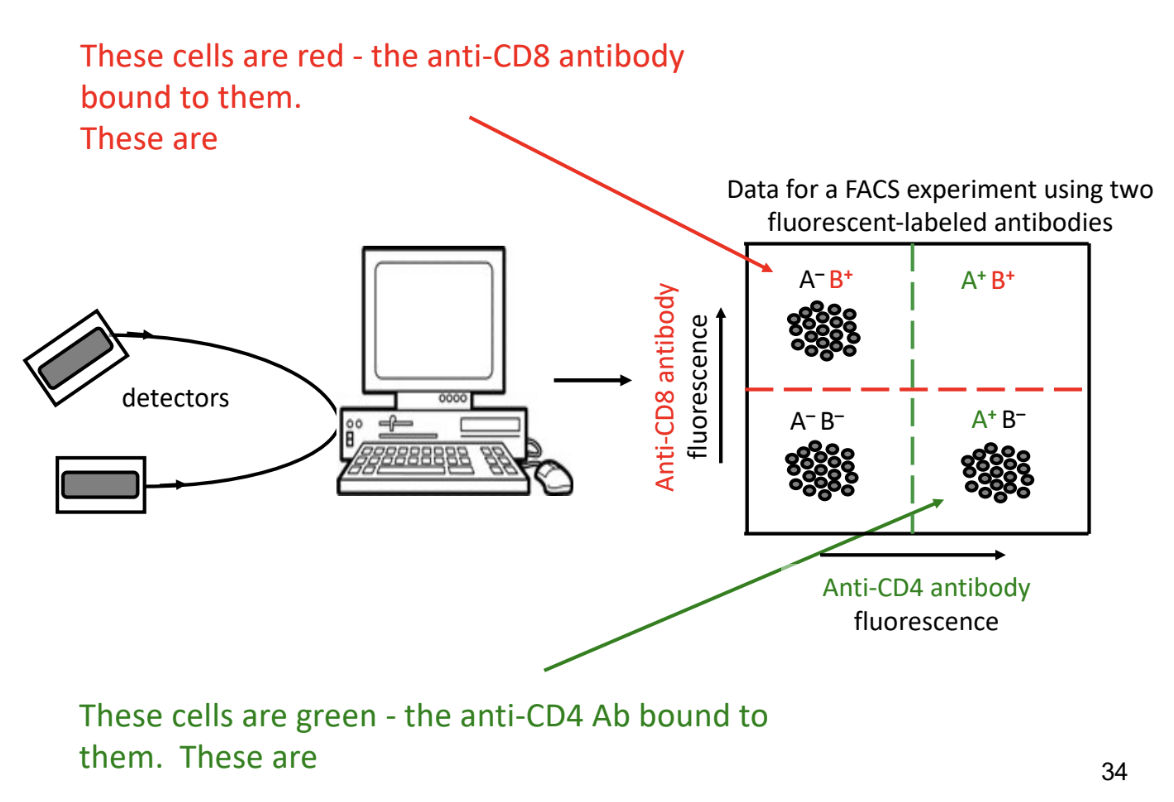
-after sorting & counting cells
computer generates 4 quadrant cell
experiment thresholds indicated by green and red dashed line
-if amount of fluorescence detected exceeds threshold → cell considered positive
-if amount of fluorescence detected doesn’t exceed threshold → cell considered negative
-A-B-
B cells and dendritic cells
-A+B-
T helper cells
-A-B+
cytotoxic T cells
-no cells in A+B+
cells aren’t stained with either antibody
non-T cells (eg. B cells, lymph node stromal (structural) cells)
ABO Blood Groups
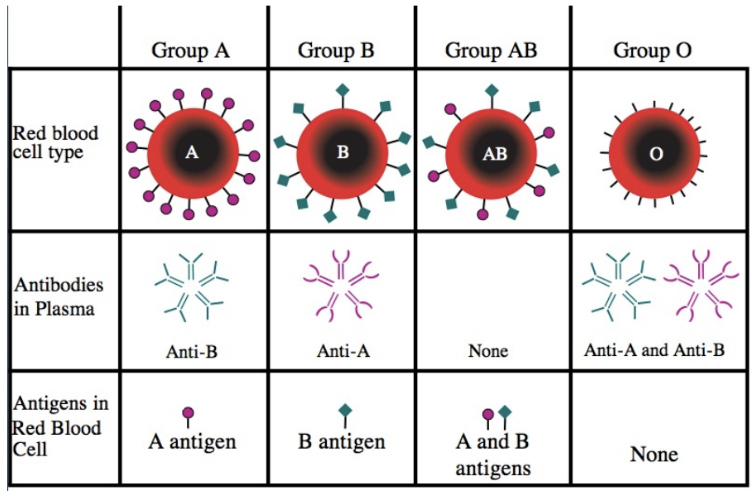
A Blood Group
-A antigens on surface of RBCs
-anti-A cells removed by negative selection
-anti-B antibodies in plasma
-can donate to A, AB
-can receive from A, O
Type A Blood (Type A Rh-)
-RBCs have A type polysaccharide and B cells that make anti-A antibodies are negatively selected
-type A RBCs should never be given to
type O blood (anti-A & anti-B antibodies)
type B blood (anti-A antibodies)
-B cells that make anti-B antibodies aren’t negatively selected
plasma contains anti-B antibodies
person with type O blood can receive plasma (already have anti-B antibodies)
person with type B blood can’t receive plasma
B Blood Group
-B antigens on surface of RBCs
-anti-A antibodies in plasma
-can donate to B, AB
-can receive from B, O
AB Blood Group
-A & B antigens on surface of RBCs
-no antibodies in plasma
-can donate to AB only
can receive from A, B, AB, O
O Type Blood
-no A or B antigens on surface of RBCs
-anti-A & anti-B antibodies in plasma
-can donate to A, B, AB, O
-can receive from O only
Type O Blood (Type O Rh-)
-universal donor
only for RBCs, not whole blood
-RBCs lack A and B type polysaccharide
-someone with type A or B blood should never receive plasma from person with type O blood type
type O plasma only safe for type O blood type
Rh Blood Groups
-protein found on surface of RBCs
-D antigen/Rh factor
Rh+ → have D antigen
Rh- → don’t have D antigen
-Rh+ can receive Rh+ or Rh- blood
-Rh- can only receive Rh- blood
-IgM antibodies produced by B cells that respond in T-independent manner
Blood Donation
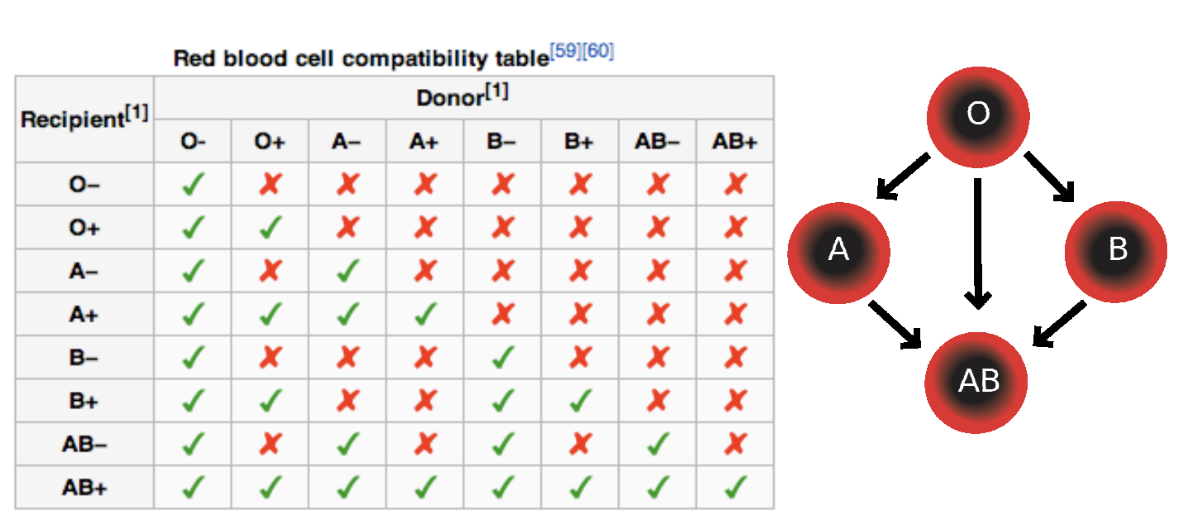
-whole blood donations are spun in centrifuges to separate it into transferable components
red blood cells
platelets
plasma
may be processed into components (cryoprecipitate)
controls risk of bleeding by helping blood to clot
allows for flexibility when it comes to matching donors and recipients
-blood type of donor and recipient must be known
-patient with iron deficiency or anemia may receive RBCs to increase hemoglobin & iron levels → improving oxygen in body
-patients unable to make enough platelets due to illness or chemotherapy may receive platelet transfusions to stay healthy
-plasma transfusions used for patients with liver failure, severe infections, serious burns
-whole blood can be used for transfusions
matching of donor and recipient has to be precise
Scenarios
Person with O Rh- blood receives a transfusion of B Rh- blood
antibodies that recognize the B antigen bind to transfused cells
IgM can activate the complement
transfused RBCs are lysed
contents of RBCs leak out
unpleasant symptoms
complicate person’s medical treatment
Person with O Rh- blood receives transfusion of O Rh+ blood
person is sensitized to Rh antigen & will produce IgG antibodies
depending on how much transfused blood was received
person may make anti-Rh IgG antibodies ~10 days later
these antibodies activate complement
if there are any O Rh+ blood cells present → content of RBCs leak out
unpleasant symptoms
person is okay as long as they don’t receive O Rh+ blood again
Mother with O Rh- blood carries fetus with O Rh+ blood
mother is sensitized to Rh antigen after childbirth
mother makes anti-Rh IgG antibodies ~10 days later
mother & baby is okay
Problem: future pregnancies, if 2nd fetus also has O Rh+ blood → pregnancy complications
IgG antibodies can cross placenta and enter fetus’ circulation
problem occurs because mother has memory B cells, long-lived plasma cells producing anti-Rh IgG type antibodies
severe anemia in fetus → prenatals important
Mother with O Rh- blood carries a fetus with B Rh- blood
mother & baby is okay
IgM antibodies are too big to cross placenta → don’t enter fetus’ circulation
Person with Rh- blood accidentally receives a transfusion Rh+ blood
make anti-Rh antibodies of IgG class
these antibodies are important medically to treat expecting mothers with Rh- blood carrying a fetus with Rh+ blood
mothers are passively immunized with some anti-Rh+ antibodies during late stage of pregnancy and after childbirth
prevents mother’s immune system from being sensitized to Rh antigen and no anti-Rh antibodies are made
protects fetus in subsequent pregnancies