Unit 1: Chemistry of Life
Chapter 2: The Chemical Context of Life
Overview: The Importance of Chemistry to Life
Biology, the study of life, is interdisciplinary
Basic concepts of chemistry apply to the study of life
Understanding the chemical characteristic of water and other substance is central to biology
Concept 2.1: Matter Consists of Chemical Elements and in Combinations called Compounds
Organisms are composed of matter
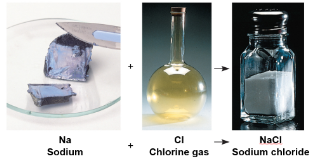
An element is a substance that cannot be broken down to other substances by chemical reactions
Example: oxygen, hydrogen, sodium
A compound is a substance that cannot be broken down to other substances by chemical reactions
Example: NaCL
Compound has emergent properties, characteristics different from those of its elements
The Elements of Life
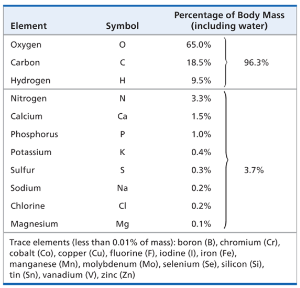
Of the 92 natural elements, about 20-25% are essential elements, needed by an organism to live a healthy life and reproduce
Trace elements are required in minute quantities
Example: Iodine (I) is required for normal activity of thyroid gland
Concept 2.2: An Element’s Properties Depend on the Structure of its Atoms
An atom is the smallest unit of matter that still retains the properties of an element
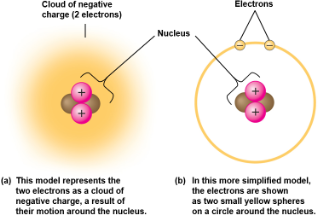
Atoms are composed of smaller subatomic particles
neutrons (no electrical charge)
protons (positive change)
electrons (negative charge)
Subatomic Particles
Neutrons and protons form the atomic nucleus (atomic mass)
Electrons form a “cloud” of negative charge around th enuclues
An element’s atomic number is the number of protons in the nucleus
An element’s mass number is the sum of protons plus neutrons in the nucleus
Atomic mass, the atom’s total mass, can be approximated by the mass number

Isotopes
All atoms of an element have the same number of protons but differ in number of electrons
Isotopes are two atomic forms of an element that differ in number of neutrons
Radioactive isotopes decay spontaneously, giving off particles and energy
Some applications of radioactive isotopes in biological research are:
dating fossils
tracing atoms through metabolic processes
diagnosing medical disorders
The Energy Levels of Electrons
Energy is the capacity to cause change
Potential energy is the energy that matter has because of its location or structure
The electrons of an atom have potential energy due to their distance from the nucleus
An electron’s energy level is correlated with its average distance from the nucleus
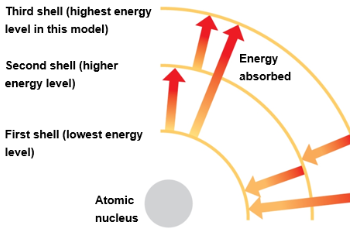
Electrons are found in different electron shells, each with a characteristic average distance from the nucleus
The energy level of each shell increases with distance from the nucleus
Electrons can move to higher or lower shells by absorbing or releasing energy, respectively
Electron Distribution and Chemical Properties
The chemical behavior of an atom is determined by the distribution of electrons in electron shells
The periodic table of elements shows the electron distribution for each element
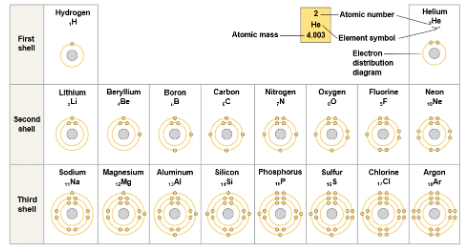
Chemical behavior of an atom depends mostly on the number of electrons in its outermost shell, or valence shell
Valence electrons are those that occupy the valence shell
The reactivity of an atom arises from the presence of one or more unpaired electrons in the valence shell
Atoms with completed valence shells are unreactive, or inert
Concept 2.3: The Formation and Function of Molecules Depend on Chemical Bonding Between Atoms
Atomis with incomplete valence shells can share or transfer valence electrons with certain other atoms
This usually results in atoms staying closer together, held by attractions called chemical bonds
Types of bond:
Covalent
Ionic
Hydrogen
Van der waals force
Covalent Bond
A covalent bond is the sharing of a pairi of valence electrons by 2 atoms
In a covalent bond, the shared electrons count as part of each atom’s valence shell
Two or more atoms held together by covalent bonds constitute a molecule
The molecular formula indicates that the molecule consists of what atoms
Electron sharing can be shown by an electron distribution diagram or a structural formula
The structural formula, the lines represent a pair of shared electrons or a single bond
A double bond, the sharing of two pairs of electrons, is indicated by a double line between atoms
A triple bond, the sharing of three pairs of electrons is indicated by a triple line between atoms
Each atom that can share valence electrons has bonding capacity, the number of bonds that the atom can form
Bonding capacity, or valence, usually corresponds to the number of electrons required to complete the atom
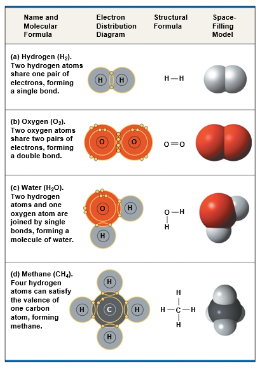
Pure elements are composed of molecules of one type of atom, such as H2 and O2
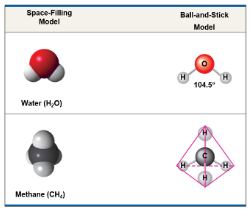
Molecules composed of a combination of two or more types of atoms such as H2O or CH4, are called compounds
Atoms in a molecule attract electrons to varying degrees
Electronegativity is an atom’s attraction for the electrons of a covalent bond
In a nonpolar covalent bond, the atoms share the electrons equally
Example: CO2
In a polar covalent bond, one atom is more electronegative and atoms do NOT share the electron equally
Example: H2O
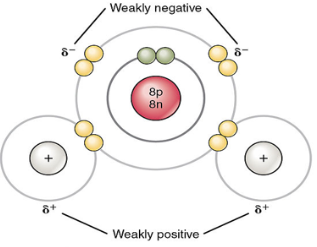
Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule
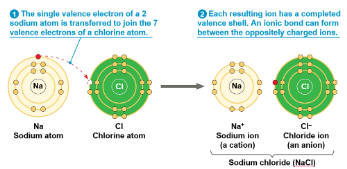
Ionic bonds
Atoms sometimes strip electrons from their less electronegative bonding partners
The two results oppositely charged atoms (or molecules) are called ions
A cation is a negatively charged ion
Example: Na+
An anion is a negatively charged ion
Example: Cl-
An ionic bond is an attraction between an anion and a cation
Example: NaCl
Compounds are formed by ionic bonds are called ionic compounds
Example: salts
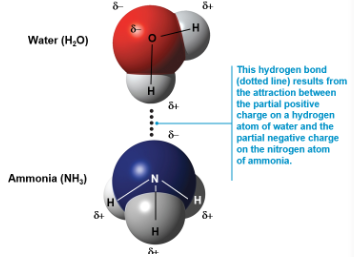
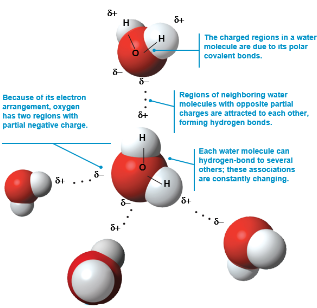
Hydrogen bonds
A hydrogen bond forms when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom nearby
In living cells, the electronegative partners are usually oxygen or nitrogen atoms
Van Der Waals Interactions
Electrons may be distributed asymmetrically in molecules or atoms
The resulting regions of positive or negative charge enable all atoms and molecules to stick to one another
These weak van der Waals interactions occur only when atoms and molecules are very close together
Collectively, such interactions can be strong, as between molecules of a gecko’s toe hairs and a wall surface
Molecular Shape and Function
A molecule’s size and shape are key to its function in a cell
Molecular shape determines how biological molecules recognize and respond to one another
Biological molecules may bind temporarily to each other through weak interactions if their shaped complementary
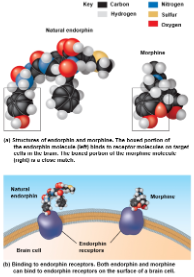
Molecules with similar shapes can have similar biological effects
Example: a molecular mimic
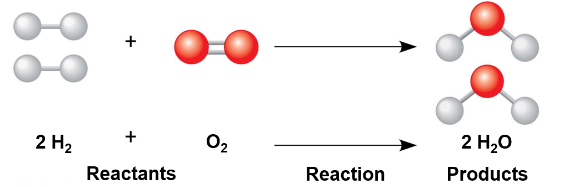
Concept 2.4: Chemical Reactions Make and Break Bonds
Chemical reactions are the making and breaking of chemical bonds
The starting molecules of a chemical reaction are called reactants
The final molecules of a chemical reaction are called products
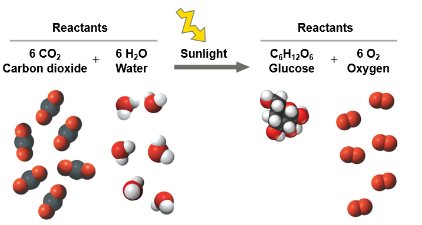
Photosynthesis is an important chemical reaction
Sunlight powers the conversion of carbon dioxide and water to glucose and oxygen
All chemical reactions are reversible: products of the forward reaction become the reactants for the reverse reaction
Chemical equilibrium is reached when the forward and reverse reaction rates are equal

Concept 2.5: Hydrogen Bonding Gives Water Properties That Help Make Life Possible on Earth
All organisms are made mostly of water and live in an environment dominated by water
Water molecules are polar molecules, with the oxygen region having a partial negative charge and the hydrogen region a partial positive charge
Two water molecules are held together by a hydrogen bond
Emergent Properties of Water Contribute to Earth’s Suitability for Life
Cohesive behavior
Ability to moderate temperature
High specific heat
High heat of vaporization
Evaporative cooling
Expanison upon freezing
Versatility as a solvent
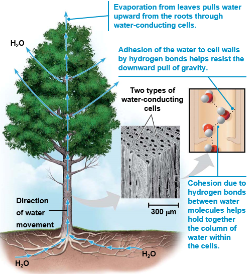
Cohesion of water molecules
Water molecules are linked by multiple hydrogen bonds
The molecules stay close together because of this and is called cohesion
Cohesion due to hydrogen bonding contributes to the transport of water and nutrients against gravity in plants
Adhesion, the clinging of one substance to another, also plays a role
Surface tension is a measure of how hard it is to break the surface of a liquid
Surface tension is related to cohesion
Moderation of Temperature of Water
Water absorbs heat from warmer air and releases stored heat to cooler air
Water can absorb or release a large amount of heat with only a slight change in its own temperature
The pecific heat of a substance is the amount of heat that must be absorbed or lost for 1g of that substance to change its temperature by 1°C
The specific heat of water is 1 cal/(g X degrees C)
Water resists changing its temperature because of its high specific heat
Water’s high specific heat can be traced to hydrogen bonding
Heat is absorbed when hydrogen bonds break
Heat is released when hydrogen bonds form
The high specific heat of water keeps temperature fluctuations within limits that permit life
Evaportation (vaporization) is transofrmation of a substance from liquid to gas
Heat of vaporization is the heat a liquid must absorb for 1g to be converted to gas
As a liquid evaporates, its remaining surface cools, a process called evaporative cooling
Evaporative cooling of water helps stabilize temperatures in bodies of water and organisms
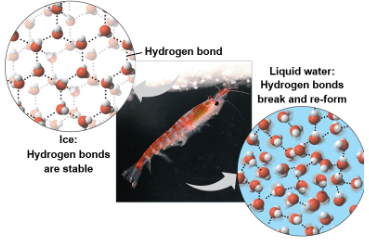
Expansion upon freezing
Ice floats in liquid water because hydrogen bonds in ice are more “ordered” making ice less dense
Water reaches its greatest density at 4°C
If ice sank, all bodies of water would evenutally freeze solid, making life impossible on Earth
Floating ice can insulate the water below, allowing life to exist under the frozen surface
Water is the solvent of life
A solution is a liquid that is a homogeneous mixture of substances
A solvent is the dissolving agent of a solution
A solute is the substance that is dissolved
An aqueous solution is one in which water is the solvent
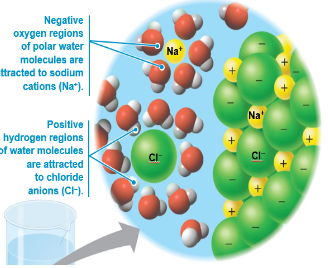
Water is a versatile solvent due to its polarity which allows it to form hydrogen bonds easily
When an ionic compound is dissolved in water, each ion is surrounded by a sphere of water molecules called a hydration shell
Hydrophilic and Hydrophobic Substances
A hydrophilic substance is one that has an affinity for water
Example: sugar molecules with polar covalent bonds
A hydrophobic substance is one that does not have an affinity for water
Example: oil molecules have nonpolar covalent bonds
Most chemical reactions in organisms involve solutes dissolved in water
Solute concentration in aqueous solutions
Molecular mass is the sum of all masses of all atoms in a molecule
Numbers of molecules are usually measured in moles
Avogadro’s number and the unit dalton were defined such that 6.02×10²3 daltons = 1 gram
Molarity (M) is the number of moles of solutes per liter of solution
Acids and Bases
A hydrogen ion (H+) is transferred from one water molecule to another leaving behind a hydroxide ion (OH-)
The proton (H+) binds to another water molecule forming a hydronium ion (H3O+)
By convemtion, H+ is used to represent the hydronium ion
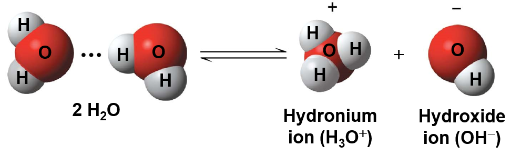
Though water dissociation is rare and reversible, it is important to the chemistry of life
H+ and OH- are very reactive
Solutes called acids and bases disrupt the balance of H+ and OH- in pure water
Acids increase H+ concentration in water
Bases decrease the concentration of H+
A strong acid like hydrochloric acid (HCl) dissociates completely into H+ and Cl- in water
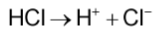
Ammonia, NH3, acts as a weak base when it attracts a hydrogen ion from the solution and forms ammonium, NH4+
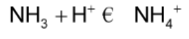
Sodium hydroxide, NaOH acts as a stong base by dissociating completely to form hydroxide ions
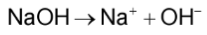
The hydroxide ions then combine with hydrogen ions to form water
A solution with equal concentration of H+ and OH- ions is said to be neutral
Weak acids act reversibly and accept hydrogen ions
Carbonic acid H2CO3 acts as a weak acid

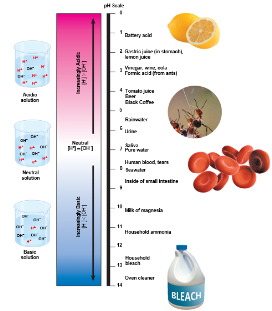
The pH Scale
Acidic solutions have pH values less than 7
Basic solutions have pH values greater than 7
Most biological fluids have pH values in the range of 6 to 8
A solution’s pH is measured on a logarithmic scale
The change of one pH unit reflects a 10-fold change
Buffers
The internal pH of most living cells MUST remain close to a pH 7
Buffers are substances that minimize changes in concentration of H+ and OH- in a solution
Most buffer solutions contain a weak acid and its corresponding base
Carbonic acid is a buffer that contributes to pH stability in human blood
Chapter 3: Carbon and the Molecular Diversity of Life
Carbon Compounds
Living organisms are made up of chemicals based on the element carbon
Carbon is unparalleled in its ability to form large, complex molecules
A compound containing carbon is an organic compound
Example: C6H12O6
Critically important molecules of all living things fall into four main classes
Carbohydrates
Lipids
Proteins
Nucleic Acids
Concept 3.1: Carbon Atoms Can Form Diverse Molecules
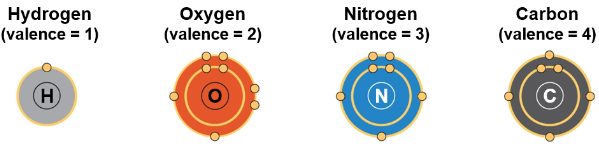
Carbon can bond with 4 other atoms, this is the source of carbon’s versatility
Four valence electrons enables carbon to form four covalent bonds
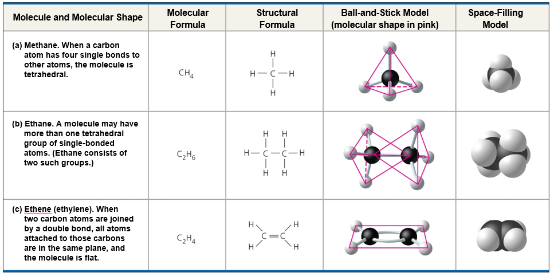
Formation of Bonds with Carbon
The number of covalent bonds an atom can form is its valence
The electron configuration of carbon gives it covalent compatibility with many different elements
Carbon atoms can partner with atoms other than hydrogen
Example: carbon dioxide, CO2

A carbon atom can also form covalent bonds to other carbon atoms, linking them into chains
Carbon chains form the skeleton of most organic molecules
Carbon chains vary in length and shape
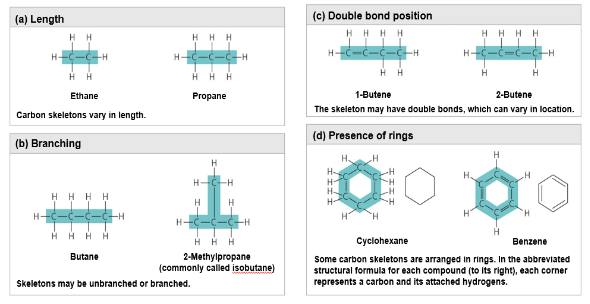
Hydrocarbons
Hydrocarbons are organic molecules consisting of only carbon and hydrogen
Example: methane, CH4
Many organic molecules, such as fats, have hydrocarbon components
Hydrocarbons can undergo reactions that release a large amount of energy
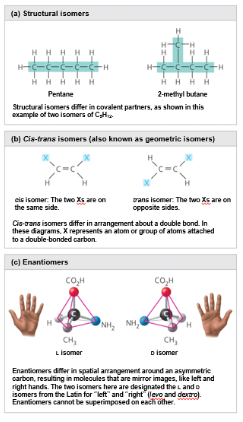
Isomers
Isomers are compounds that have the same number of atoms of the same elements but have different structures and properties
Example: C6H12O6
Structural isomers differ in the covalent arrangement of their atoms
The number of possible isomers increases as carbon skeletons increase in size
Single bonds allow the atoms they join to rotate freely about the bond axis
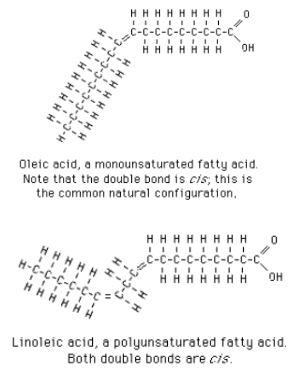
In cis-trans isomers, carbons have covalent bonds to the same atoms, but the atoms differ in their spatial arrangement due to inflexibility of double bonds
The subtle differences in shape between cis-trans isomers can greatly affect the activities of organic molecules
Enatiomers are isomers that are mirror images of one another
They differ in shape due to the presence of an asymmetric carbon
Enantiomers are left-handed and right-handed versions of the same molecule
Usually only one isomer is biologically active
Chemical Groups Most Important to Life
Chemical groups can replace one or more of the hydrogens bonded to the carbon skeleton of a hydrocarbon
Functional groups are the chemical groups that affect molecular function
Each functional group participates in chemical reactions a certain way
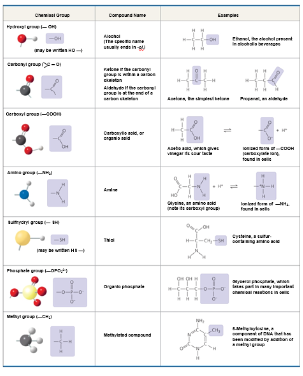
Seven Functional Groups Most Important to Life
Hydroxyl
Carbonyl
Carboxyl
Amino
Sulfhydryl
Phosphate
Methyl
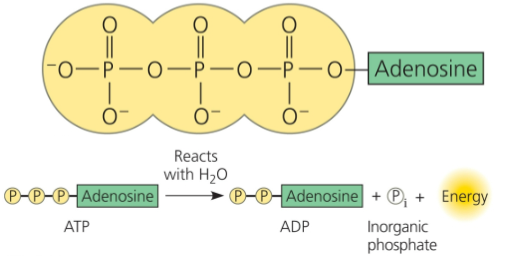
ATP: Source of Energy for Cellular Processes
Adenosine triphosphate (ATP) is an organic phosphate molecule that provides energy to cells
ATP consists of an organic molecule called adenosine attached to a string of 3 phosphate groups
ATP stores the potential to react with water, releasing energy
Concept 3.2: Macromolecules are Polymers, Built from Monomers
A polymer is a long molecule consisting of many similar building blocks
These small building block molecules are called monomers
Some molecules that serve as monomers also have functions of their own
The Synthesis and Breakdown of Polymers
Cells make and break down polymers by the same mechanisms
A dehydration reaction occurs when 2 monomers bond together through the loss of a water molecule
Polymers break apart into monomers by hydrolysis through the addition of water molecules
These processes are facilitated by enzymes, which speed up chemical reactions
Each cell has thousands of different macromolecules
Macromolecules vary among cells of an organism, carry more within a species and vary even more between species
An immense variety of polymers can be built from a small set of monomers
Macromolecules
Macromolecules are large organic molecules
Carbohydrates
Classified by the number of simple sugars
Carbon (C), Hydrogen (H), and Oxygen (O)
There are three types:
Monosaccharides
Disaccharides
Polysaccharides
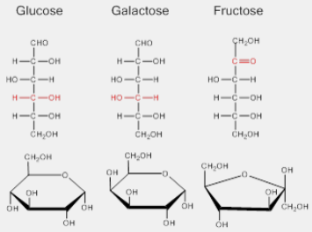
Monosaccharides
C-H-O ratio is always CH2O
Major nutrients for cells
Store ENERGY in chemical bonds
Carbon skeletons are raw materials for other organic molecules
Act as monomers for di- and pollysaccharides
-OH is attached to all but one carbon
That carbon is a carbonyl (C=O)
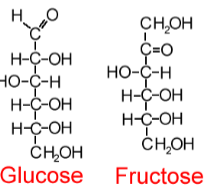
The carbon skeleton contains 3 to 7 carbons
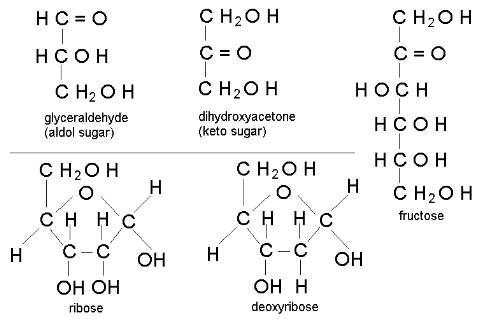
In aqueous solutions, many monosaccharides form rings (favored in chemical equilibrium)
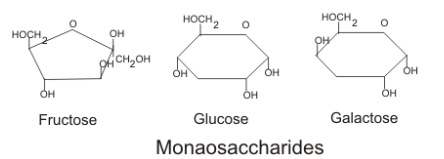
Disaccharides
2 monosaccharides joined through condensation (dehydration synthesis) in a bond known as a “glycosidic linkage”
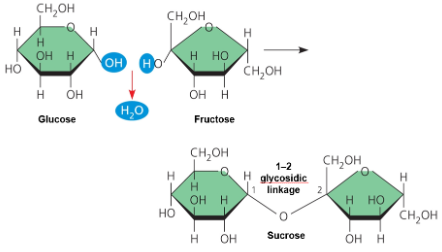
Disaccharides → → → Monomers
Maltose glucose + glucose
Lactose glucose + galactose
Sucrose glucose + fructose
Polysaccharides
Polymers of a few hundred to a few thousand monosaccharides
2 types:
Storage polysaccharides
Structural polysaccharides
In storage polysaccharides, cells hydrolyze the molecules into sompler sugars as needed
A. Starch: glucose polymer stores as granules in plants
Most animals have enzymes that can hydrolyze plant starch, making glucose available as a nutrient
“Amylose” is the simplest (unbranched) form
“Amylopectin” is branched
B. Glycogen: glucose polymer in animals
More highly branched than amylopectin
Stored in muscle and liver of vertebrates
In structural polysaccharides, provide support and form the physical frameworks of cells and tissues
A. Cellulose: linear unbranched polymer of glucose
Major structural component of plant cell walls
Cannot be digested by most organisms
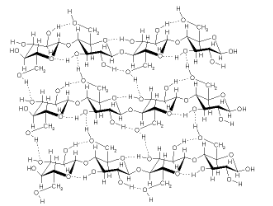
B. Chitin: amino sugar polymer (contains nitrogen)
Forms anthropod exoskeletons
In cell walls of some fungi
Lipids
Lipids are diverse hydrophobic (water-fearing) compounds composed largely of carbon and hydrogen
Insoluble in water, but will dissolve in nonpolar solvents
Lipids DO NOT form true polymers
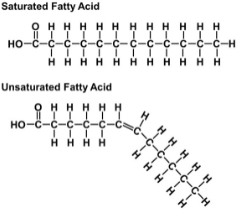
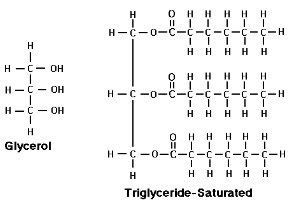
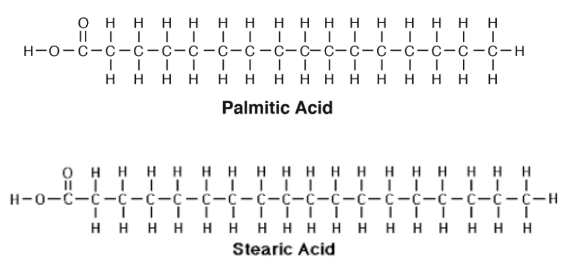
Fats: components are glycerol + fatty acids
Types of Fats

Uses of fats
Energy storage
Cushion vital organs in mammals
Insulation against heat loss in mammals
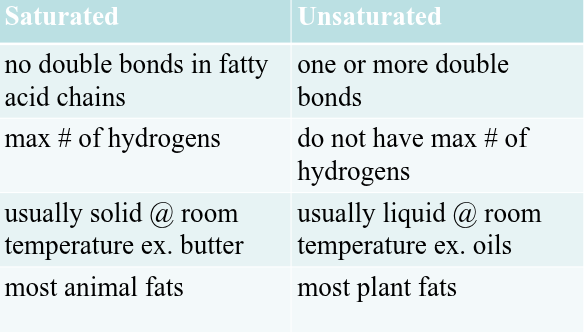
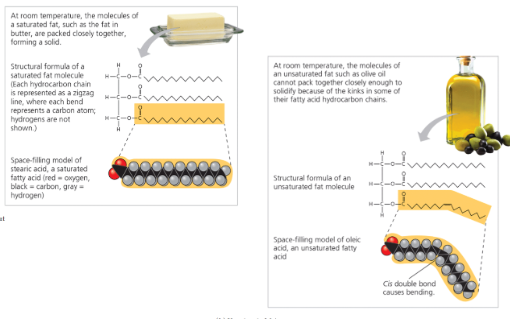
Saturated Fatty Acids
Unsaturated Fatty Acids
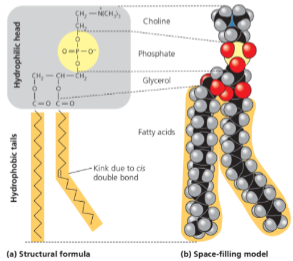
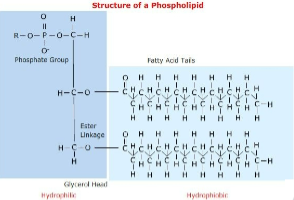
Phospholipids have glycerol, 2 fatty acids, and one phosphate group
Usually a small chemical group attached to phosphate
Amphilphilic molecule - hydrocarbon tails are hydrophobic and polar head is hydrophilic
Major constituents of cell membrane
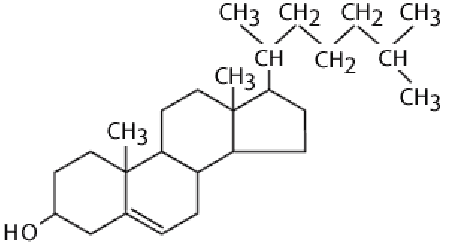
Steroids
Lipids which have 4 fused caron rings
Various finctional groups
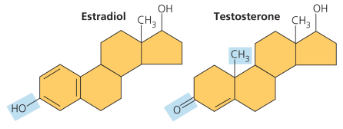
Cholesterol is a precursor to man hy othersteoirds (including sex hormones) and is a common component of animal cell membranes
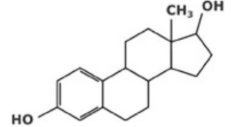
Female sex hormone, estrogen (C18H24O2)
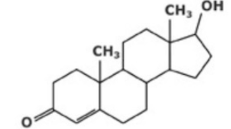
Male sex hormone, testosterone (C19H28O2)
Waxes
Animal wax examples
Beeswax
Earwax
Plant wax ecample
Castor wax
Babyberry wax
Proteins
One or more polypeptide chains folded and coiled in specific conformations (shapes)
Proteins account for more than 50% of the dry mass of most cells
All about the SHAPE = function
Uses for proteins:
Structural support
* Example: keratin and collagen
Storage (of amino acids)
Example: casein (milk protein) and plants in their seeds
Transport
Example: membrane pump
Signaling
Example: ligand-gated ion channel
Cellular response ot chemical stimuli
Example: insulin
Movement
Example: actin and myosin (muscle contraction)
Defense
Example: antibodies
Catalysts of biochemical reactions
Example: enzymes
There are 20 amino acid monomers
Generalized amino acid:
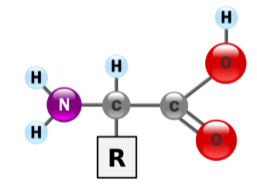
“R” side chains may be
Nonpolar (hydrophobic)
Polar (hydrophilic)
Uncharged polar
Charged polar
Acidic side groups
Basic side groups
Peptide bonds:
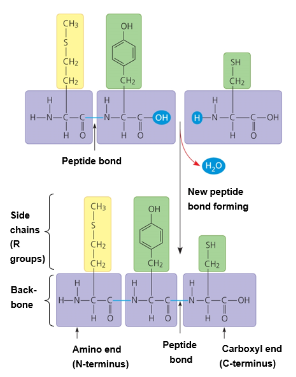
A protein’s function depends on its unique conformation (the 3-D structure)
Native conformation - the functional shape of protein under normal biological conditions
Enables protein to recognize and bind specifically to another molecule
Due to specific linear sequence of amino acids
The shape is produced when newly formed polypeptide chain coils and folds spontaneously
Stabilized by chemicaol bonds (like S-S bonds) and weak interactions between neighboring regions of folded protein
Primary (1°) Structure
Unique sequence of amino acids
Determined by genes
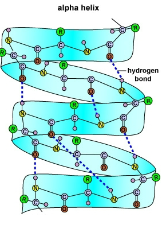
Secondary (2°) Structure
Regular, repeated coiling and folding of protein’s polypeptide backbone
Stabilized by hydrogen bonds between peptide linkages
The 2 major types are the \alpha helix and the \beta pleated sheet
The \alpha helix is a helical coil and found in fibrous proteins
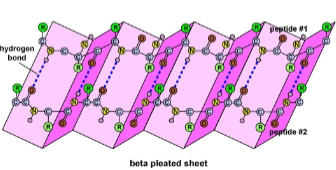
The \beta pleated sheet is a sheet of antiparallel chains folded accordion pleats and they make up dense core of many globular proteins
Tertiary (3°) Structure
Irregular contortions of a protein due to bonding between side chains (R groups)
Disulfide bridges (covalent bond) may further reinforce shape (form where 2 cystine monomers are close together)
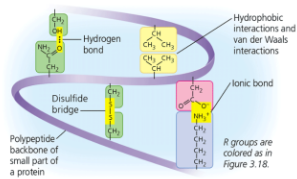
In a hydrophobic interaction, amino acifds with hydrophobic (nonpolar) side chains usually end up in the core of thei protein
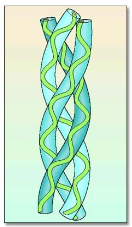
Quaternary (4°) Structure
Comes from interaction among several polypeptide (subunits) in a single protein
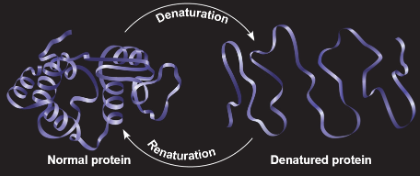
Denaturation - a process which changes a protein’s native conformation
Can occur from exposure to:
Organic solvents
Chemical agents
Excessive heat
pH changes
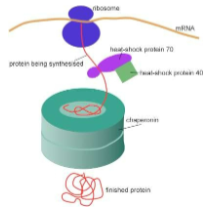
Chaperone proteins help in the proper folding of new protein molecules
Temporarily brace a folding protein
Nucleic Acids
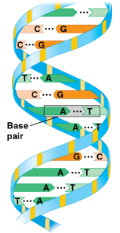
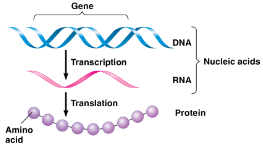
DNA (deoxryribonucleic acid)
Double helix
Contains code to program all cell activity
Directions for its own replication
Genes contain directions for protein synthesis
RNA (ribonucleic acid)
Single polynucleotide chain
Functions in protein synthesis
The monomer = nucleotide (3 parts)
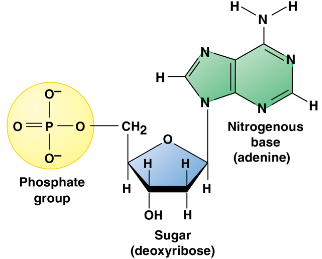
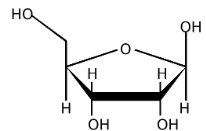
5-carbon sugar
DNA has deoxyribose
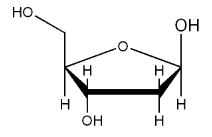
RNA has ribose
Phosphate group
Attached to the #5 carbon of the sugar
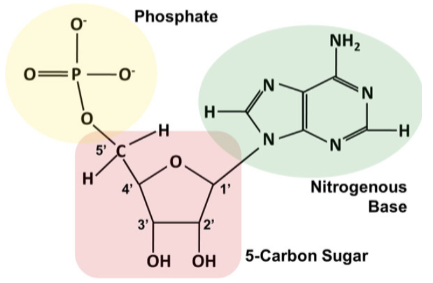
Nitrogenous base
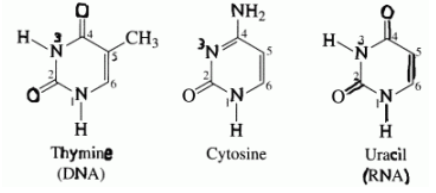
a) pyrimidines (6-membered ring)
cytosine
thymine
uracil (RNA replces thymine)
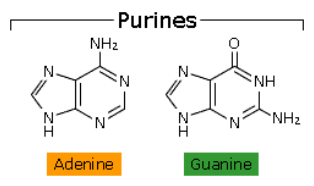
b) purines (5-membered ring fused to 6-membered ring)
adenine
guanine
Structure of DNA
Adjacent nucleotides are joined by a phosphodiester linkage, formed in a dehydration reacton
These links create a backbone of sugar-phosphate