Ch02 Hydrogen-like Atomic Orbitals — Quick Notes
Overview
Hydrogen-like atomic orbitals described by the Schrödinger equation; energy levels depend on the principal quantum number n.
Bohr model relation (precursor): discrete energy levels and discrete radii; spectral series names include Lyman, Balmer, Paschen, Pfund, etc.
Energy for hydrogen-like levels:
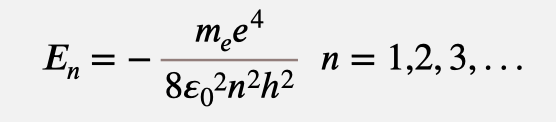
Wavefunctions are indexed by quantum numbers $n, \ell, m$ (and spin $ms$): \psi{n\ell m ms}(\mathbf{r}) = R{n\ell}(r)\,Y\ell^{m}(\theta,\phi)\,\chi{m_s}, with radial and angular parts.
Schrödinger Equation and Wave Functions
The Schrödinger equation describes orbitals as spatial probability densities $|\psi|^2$ (always nonnegative).
Solutions separate into radial part $R{n\ell}(r)$ and angular part $Y{\ell}^{m}(\theta,\phi)$ in spherical coordinates.
Orbitals are labeled by four quantum numbers: $n, \ell, m, m_s$.
Quantum Numbers
Principal quantum number: n = 1,2,3,\ldots defines energy level.
Angular momentum quantum number: \ell = 0,1,2,\ldots,(n-1) defines orbital shape.
Magnetic quantum number: m = -\ell, -\ell+1, \ldots, +\ell defines orientation.
Spin quantum number: m_s = +\tfrac{1}{2}, -\tfrac{1}{2} (spin of electron; not an orbital quantum number).
Degenerate set: for a given $n$, there are $n^2$ orbitals in total.
For each $n$, there exists an $s$ orbital ($\ell=0$).
Orbital Types (by $\ell$)
s orbital: \ell=0,\; m=0; 1s, 2s, 3s, …; spherical electron density.
p orbitals: \ell=1,\; m=-1,0,+1; 2p, 3p, 4p, …; oriented along axes (px, py, pz).
d orbitals: \ell=2,\; m=-2,-1,0,+1,+2; 3d, 4d, 5d, …; five distinct shapes.
f orbitals: \ell=3,\; m=-3,-2,-1,0,+1,+2,+3; seven distinct shapes.
Node Structure and Radial Probability
Radial nodes: n_r = n - \ell - 1\,.
Total nodes: \text{nodes} = n - 1 for any orbital.
For fixed $n$, increasing $\ell$ changes the radial distribution and angular nodal structure.
Radial probability density (4πr^2|ψ|^2) shows where an electron is likely to be found on a sphere of radius r.
Higher $n$ → larger average radius and more radial nodes.
Nuclear penetration: density near the nucleus; s orbitals have greater near-nucleus density (penetration) than higher-ℓ orbitals.
Shapes, Orientation, and Key Concepts
s: spherical symmetry; one orbital per $n$ (for each $n$ there is 1s, 2s, 3s, …).
p: three lobes along axes; $m= -1,0,+1$ correspond to orientations along x, y, z (px, py, pz).
d: five shapes; more complex angular distributions.
f: seven shapes; even more complex.
For each type, increasing $n$ enlarges the orbital and adds radial nodes (e.g., 2s, 3s, 4s; 2p, 3p, 4p; 3d, 4d, 5d).
Energy ordering: within a given $n$, different $\ell$ states have different energies due to fine structure and penetration effects (conceptual; actual ordering depends on perturbations beyond the basic hydrogen model).
Quick Takeaways for Exam (Most Important Points)
Quantum numbers: $n$, $\ell$, $m$, $ms$ with definitions above; orbitals are labeled $\psi{n\ell m ms}$ and can be written as \psi{n\ell m ms}(\mathbf{r}) = R{n\ell}(r)\,Y\ell^{m}(\theta,\phi)\,\chi{m_s}.
Energy levels depend only on $n$ in the hydrogenic model: En = -\dfrac{RH}{n^2}.
For each $n$: \ell = 0,1,2,…,n-1; number of orbitals total for that $n$ is $n^2$.
Nodes: radial nodes n_r = n - \ell - 1; total nodes n - 1.
Orbital shapes: s (spherical), p (two-lobed/dumbbell along axes), d (clover/other complex shapes), f (more complex shapes).
Electron density is given by $|\psi|^2$; density near nucleus is called penetration, with s orbitals penetrating more than higher-$\ell$.
For each $n$, there exists an s orbital ($\ell=0$); as $n$ increases, orbitals grow in size and acquire more radial nodes.