The Veterinary Practice Laboratory – Key Terminology (VET 152)
Safety and OSHA
OSHA mandates specific laboratory practices and must be incorporated into the laboratory safety policy; some states have regulations superseding federal OSHA guidelines.
Safety Program
A comprehensive safety program is essential and must be in writing and accessible in the laboratory.
No eating or drinking signs; use and maintenance of equipment; location of safety equipment and supplies:
Eye wash station
Fire extinguishers
Spill clean up kits
Biohazard disposal containers
Protective gloves
Hazard Communication Standard
Employees must know exposure hazards.
Proper labeling on containers.
Safety Data Sheets (SDS) must be accessible to everyone.
Zoonotic Pathogens and PPE
Protocols to prevent exposure.
PPE to avoid hazards from chemicals or infectious pathogens through contact, inhalation, or absorption:
Eye protection
Protective clothing
Shields/Barriers (gloves, shoe covers, masks)
Proper disinfection of materials and surfaces; proper disposal of infectious material.
Shipping Hazardous Materials
Regulations – U.S. Department of Transportation
Any specimen with potential microorganism to cause disease in animals or humans.
Category A: higher risk; known or likely to cause disability, life-threatening, or fatal disease (example: Bacillus anthracis).
Category B: lower risk; not life-threatening in healthy individuals.
Most samples in veterinary patients fall under Category B.
Specific labeling: must have infectious substance label; specimens sealed in leak-proof containers; wrap in a layer of watertight material; absorbent material placed between each layer.
The Laboratory Layout and Work Area
What components make a workable lab?
Dedicated area, out of main traffic flow, separated from other areas of the hospital.
Large enough to accommodate equipment and personnel.
Sink, storage, electrical supply, internet access.
Counter space should be smooth, easily cleaned, and free of clutter; large enough to perform needed tasks; storage for supplies and reagents; sink and running water; ability to rinse, drain, and dispose of fluid specimens.
Floors: easily cleaned; nonslip mats in front of sinks.
Electrical outlets: more than you anticipate needing.
Refrigerator/freezer for storage of reagents and specimens; internet access; access to the diagnostic lab.
Equipment in the Laboratory
Minimal equipment needs:
Refractometer
Microhematocrit centrifuge
Clinical centrifuge
Microscope
Additional needs: incubators, aliquot mixers, pipettes, test tubes, cell counters, blood chemistry analyzers.
Refractometer
Measures refractive index of a solution; used for fluid-specific gravity, protein concentration, urine SG, spinal fluid, synovial fluid & other fluids.
Operates via prism and bending of light; read at the light-dark interface.
Calibration and Care of the Refractometer
Distilled water used for calibration with an adjustment screw.
Calibrated weekly to 1.000.
Clean after each use; never tap fluid onto the prism.
Centrifuges
Vital instrument used to separate cells and particulates from fluids; spins at high speed.
Heavier components form sediment at the bottom; liquid components rise to the top (supernatant).
Types:
Microhematocrit centrifuge (for hematocrit tubes)
Clinical centrifuge (for hematocrit and centrifuge tubes; horizontal or swing-arm; angled)
Angled centrifuge head holds tubes at a fixed angle; higher speeds; usually takes one tube size.

Swinging-arm centrifuge (cups hang vertically at rest and swing out horizontally during centrifugation).

Speeds: typically set by RPM; there are presets and timers; spins must be managed to avoid cell damage or incomplete separation.
Balancing: tubes must be counter-balanced with equal-weight tubes opposite each other; spills must be cleaned immediately.
Centrifuge Operation and Safety
Spinning too long or fast can rupture cells and cause changes in morphology
Spinning too slow or not long enough can prevent complete separation
Use preset RPM and timer; ensure proper balancing; avoid over- or under-spinning to protect cell morphology.
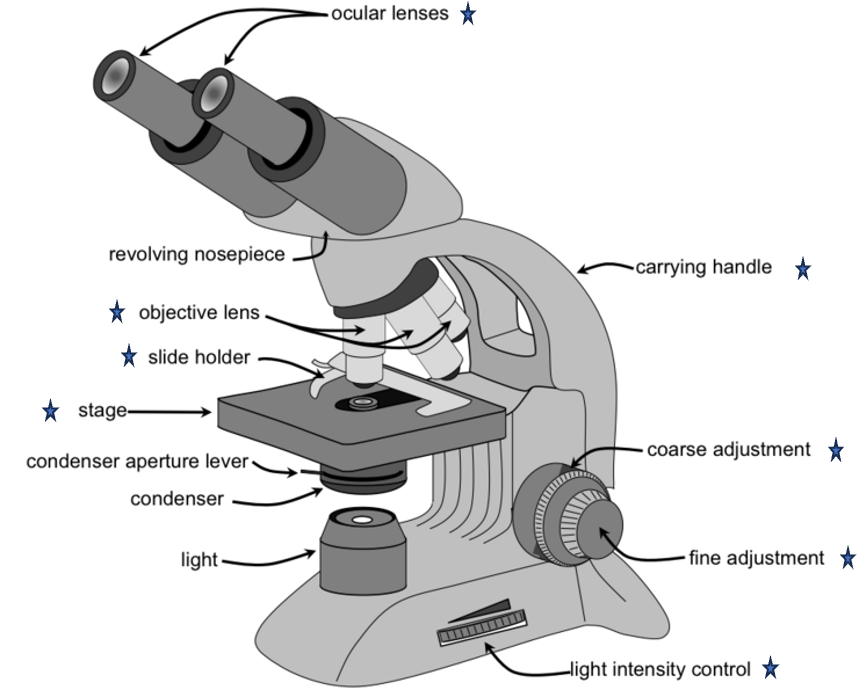
Microscope
Key components: ocular lenses, revolving nosepiece, stage, objective lenses, condenser, illumination controls, coarse and fine adjustment knobs, and slide holder.
Operating the microscope (oil-immersion 100x):
Locate area with 10x objective.
Rotate to 40x (high-power) and refocus with fine adjustment.
Move back to halfway between high-power and oil-immersion.
Apply a drop of immersion oil on the slide.
Bring oil-immersion lens into place and focus with the fine adjustment only; do not use coarse focus with oil.
When finished: turn off light, lower stage, remove specimen, clean oil-immersion lens if necessary, cover the microscope.
Care and Maintenance of the Microscope
Cleaning lenses: use only lens paper.
Clean oil immersion lens with methanol or a specially designed product; excess oil can be removed with xylene.
Do not use other oils; never use mineral oil, baby oil, etc.; cover when not in use.
Incubators
Microbiology incubation typically at (37 degrees C) is optimal for many bacteria.
Incubators are fitted with a thermometer inside; may have built-in humidity control; if not, add a small dish of water.

Miscellaneous Equipment
Aliquot mixer (rocker), Speci-Mix, Bamstoad/ Thermolyne, cell counter, pipettes.

Test Tubes and Other Glassware
Glass or plastic test tubes in various sizes; microhematocrit tubes; larger tubes for blood collection; conical tubes.
Laboratory Tests and Uses
Common areas of evaluation include:
Hematology
Urinalysis
Parasitology
Cytology
Microbiology
Quality Control in the Laboratory
Accuracy of results depends on:
Consistent technique
Equipment maintenance
Calibration of equipment
Record keeping
Quality Control Concepts
Accuracy: how closely results approach the true value.
Precision: reproducibility and the magnitude of random errors.
Reliability: ability of a method to be accurate and precise.
Factors Affecting Results
Pre-analytic variables: sample collection, patient-related factors (breed, age, gender), fasting status, labeling, etc.
Analytic variables: instrument maintenance, inaccurate standards, sera, reagent stability.
Other factors: electrical surges/drops, human error, maintenance and calibration of equipment.
Running Controls & Equipment Maintenance
Controls are run regularly to assess technicians and instruments for valid results.
Include both normal and abnormal concentrations for each test.
Document results in a log/chart or via the instrument itself.
Errors
Pre-analytic errors are those that occur before the sample is analyzed. These can significantly impact test results and often include:
Biologic factors
Inherent to patient, such as breed, age, gender
Collection error – non-fasted animal
not fasted— lipemic sample
Non biologic factors
Clerical errors – incorrect labeling, etc.
Sample collection and handling
Analytic errors occur during the testing process itself within the laboratory. These errors can arise from:
Procedural issues: This includes deviations from standard operating procedures (SOPs), such as incorrect sample dilution, improper mixing of reagents, incorrect incubation times or temperatures, adding reagents in the wrong sequence, or inaccurate pipetting techniques.
Instrument maintenance neglects: A lack of regular maintenance can lead to equipment malfunctions, such as dirty optics in a spectrophotometer, clogged lines in an analyzer, or general machine breakdown, all of which compromise test accuracy.
Incorrect standards or controls: Using expired calibration standards or quality control materials, standards with incorrect concentrations, or contaminated control samples will lead to inaccurate calibration and unreliable results.
Reagent stability issues: Reagents that are expired, improperly stored, or have degraded can lose their reactivity or specificity, leading to false-positive or false-negative results.
Analytic errors involve procedural issues, instrument maintenance, incorrect standards, reagent stability, etc.
Laboratory Records
Standard Operating Procedures (SOPs) define test kit instructions.
Records include logs, request forms, patient ID, presenting signs, date, method of collection, pertinent history, tests desired, special notes.
Lab reports, necropsy reports, and supply inventory/order forms are maintained.
Notes on Specific Panels (Representative Coding Page Content)
CBC-Complete Blood Count and other panels are tracked with instrument methods such as laser flow cytometry and immunoassays; multiple tests and submission protocols may be listed in lab protocols.
Sample handling protocols include specific submission requirements, special handling flags, and designated submission instructions.
Quick Reference Summary
Safety: OSHA compliance, written program, PPE, eye wash, fire extinguishers, spill kits, biohazard disposal.
Hazard Communication: labeling, SDS accessibility.
Zoonoses: PPE, disinfection, disposal.
Shipping: Category A/B, labeling, leak-proof and absorbent packaging.
Lab layout: dedicated area, sink, storage, electrical, internet.
Equipment: refractometer, centrifuges, microscope, incubators, pipettes, cell counters, analyzers.
Refractometer: calibrate to 1.000 weekly; read at light-dark interface.
Centrifuges: balance tubes; avoid over-spinning; RPM/timing; sediment vs supernatant.
Microscope: oil immersion technique; careful lens care; avoid improper adjustments.
QC: run controls; maintain logs; document all results.
Records: SOPs, logs, patient data, test requests, lab reports.
Pre-analytic/analytic: monitor variables to ensure accuracy and reliability.