3.3: Respiration
Co-enzymes
Coenzymes bind with enzymes. They either help catalyse a reaction or help transfer molecules (electrons/hydrogen).
They transfer using oxidation and reduction reactions:
Oxidation is the loss of electrons/hydrogen and the gain of oxygen.
Reduction is the gain of electrons/hydrogen and the loss of oxygen.
There are three coenzymes used in respiration:
NAD - Is able to accept 1 proton/hydrogen and 2 electrons.
After this, it becomes known as reduced NAD or NADH.
It is used in glycolysis and the link reaction.
FAD - Is able to accept 2 protons/hydrogens and 2 electrons.
After this, it becomes known as reduced FAD or FADH+.
CoA (Coenzyme A) -
It is used in the link reaction.
Aerobic respiration
There are 4 steps:
Glycolysis - Occurs in the cytoplasm and produces pyruvate, ATP and reduced NAD.
Link reaction - Occurs in the mitochondrial matrix and pyruvate is converted to acetyl coenzyme A.
Krebs cycle - Occurs in mitochondrial matrix and creates carbon dioxide and reduced NAD and FAD.
Electron transport chain - Occurs on the cristae of the inner mitochondrial membrane, where the energy from protons and electrons generates ATP from ADP and inorganic phosphate.
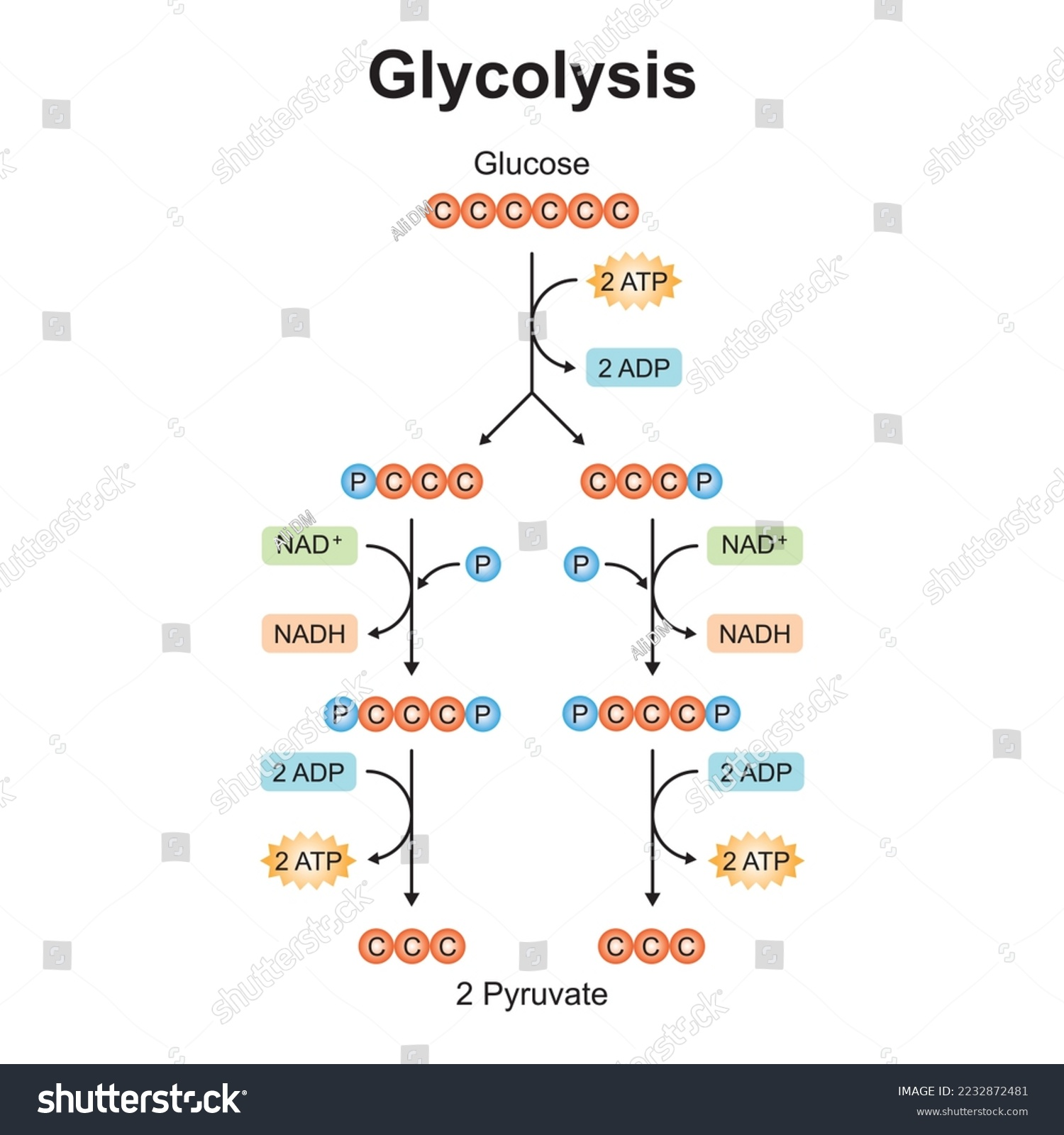
Glycolysis
This is the initial stage in both aerobic and anaerobic respiration, and occurs in the cytoplasm as it cannot pass through the mitochondrial membrane, and the enzymes necessary are not present in the mitochondria.
It occurs in 5 steps:
A glucose (6 carbon) molecule is phosphorylated by 2 phosphate groups, which requires 2 ATP molecules.
This is known as glucose diphosphate.
This is done to make the glucose more reactive to lower the activation energy required, and to make it polar and less likely to diffuse through the cell.
The glucose diphosphate is split into 2 molecules of triose phosphate, a 3 carbon sugar known as glyceraldehyde-3-phosphate.
Inorganic phosphate joins to both the triose phosphates.
The molecules are then dehydrogenated/oxidated. The hydrogens join NAD, creating two reduced NADs.
This creates enough energy to produce 4 ATPs, which are formed by substrate-based phosphorylation, with the phosphates on the triose phosphate converting ADP to ATP.
This produces pyruvate.
One cycle of this produces:
4x ATP.
2x reduced NAD.
1x pyruvate, which contains a high amount of chemical potential energy.
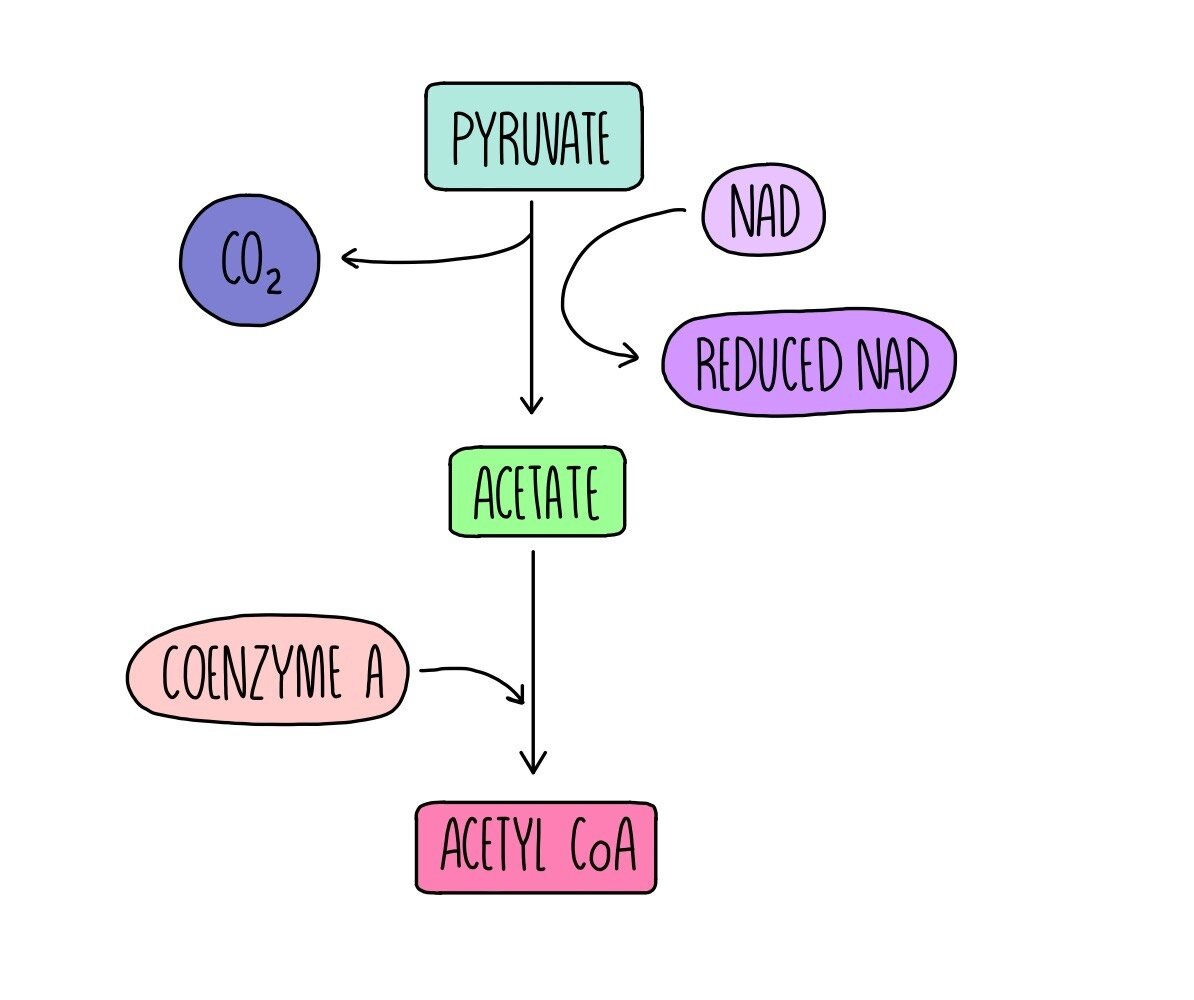
Link cycle
This links glycolysis to the Krebs’ cycle, and occurs within the mitochondrial matrix:
It has 4 steps:
Pyruvate diffuses from the cytoplasm into the mitochondrial matrix.
Pyruvate is then dehydrogenated/oxidised, this hydrogen is then accepted by NAD to form reduced NAD.
It is also decarboxylated, meaning a molecule of carbon dioxide is removed from it.
This leaves a 2 carbon acetate group which combines with coenzyme A (CoA) to make acetyl coenzyme A (AcCoA) which enters the Krebs’ cycle.
One cycle of this produces:
1x AcCoA.
1x carbon dioxide.
1x reduced NAD.
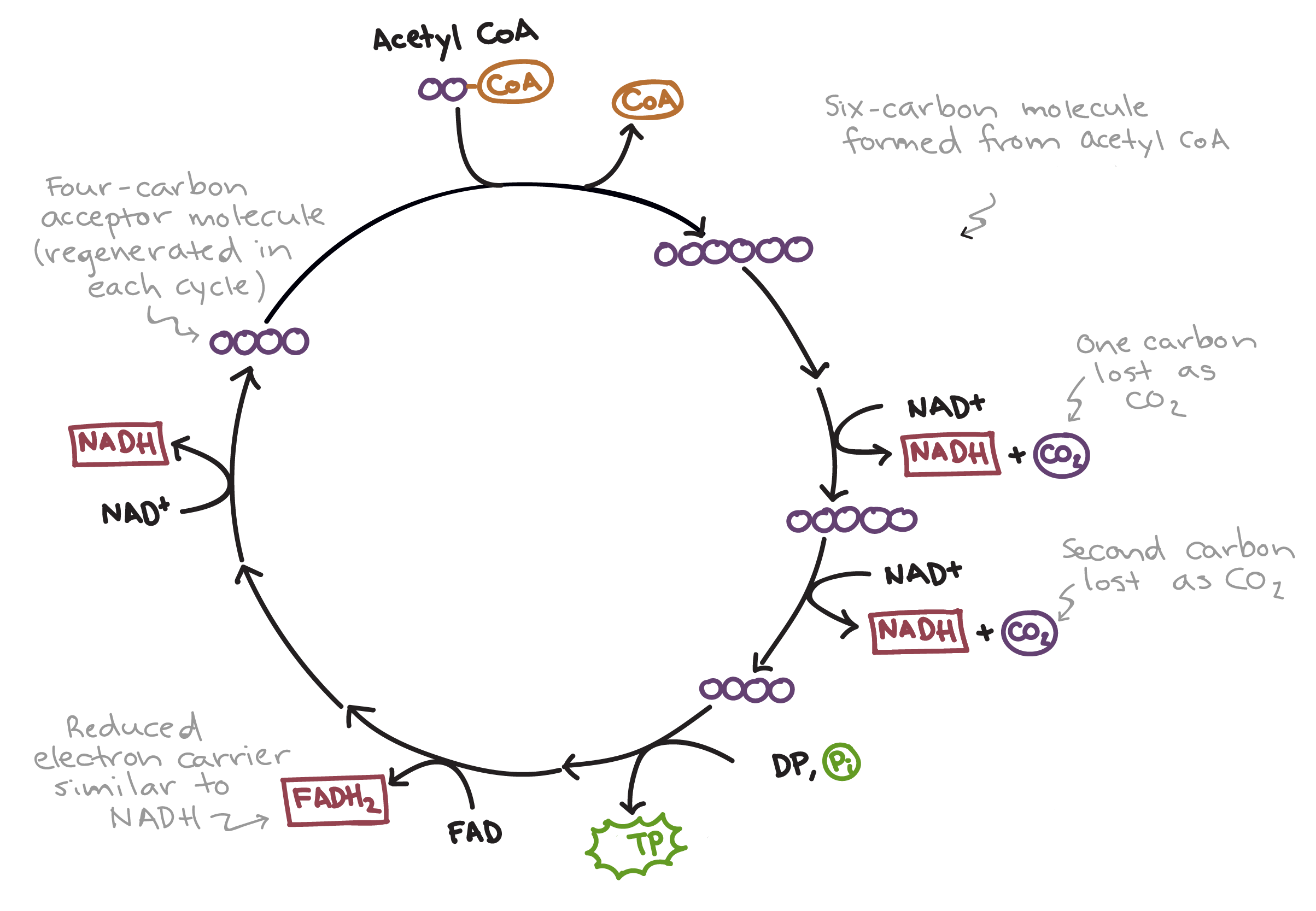
Krebs cycle
Occurs within the mitochondrial matrix, as a way to release energy from carbon bonds, using carbon-carbon, carbon-hydrogen and carbon-hydroxide bonds. It happens in 4 main steps:
AcCoA enters the Krebs cycle and combines with a four-carbon acid, forming a 6-carbon compound.
The CoA is regenerated back to the link reaction, to carry more carbon.
The 6 carbon compound is decarboxylated, and then dehydrogenated/oxidised, making a reduced NAD.
This leaves a 5-carbon compound.
This is then decarboxylated and dehydrogenated/oxidised once, making a reduced NAD.
It then creates one ATP via substrate-phosphorylation.
This leaves a 4-carbon compound.
This is then dehydrogenated/oxidised twice, created one FAD and one NAD.
Then it combines with AcCoA, repeating the cycle.
There are two enzymes involved:
Decarboxylase helps with decarboxylation, by removing carbon dioxide from the -COOH groups of the Krebs cycle intermediates (the 4, 6 and 5 carbons)
Dehydrogenase helps with dehydrogenation.
The acetate group from the original glucose is broken down into carbon dioxide and water.
The original energy from the glucose bonds is carried by electrons in the hydrogen atoms in the reduced NADs and FAD.
There are 4 products of each full Krebs cycle:
1x ATP.
1x reduced FAD.
2x carbon dioxide.
3x reduced NAD.
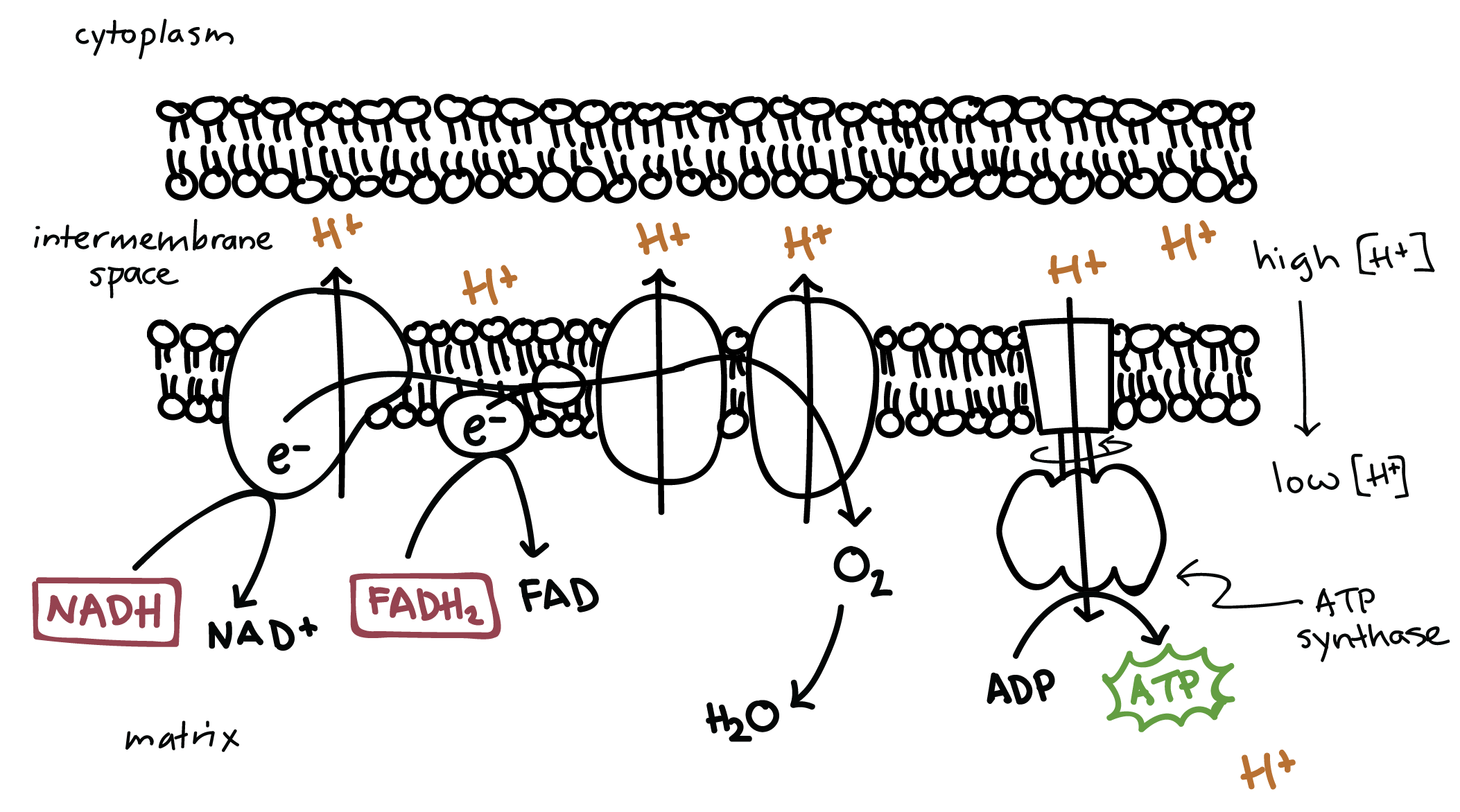
Electron transport chain
Occurs on the cristae of the inner mitochondrial membranes, and is also sometimes referred to as the cytochrome chain due to the redox reactions (one molecule is oxidised and the next in the chain is reduced).
This is the process where oxidative phosphorylation occurs.
The inner membrane is impermeable to protons.
It is comprised of a series of protein molecules known collectively as respiratory enzymes, individually known as carriers and pumps.
Carriers also include cytochromes, which are proteins which are bonded with iron, allowing the proteins to be oxidised and reduced. There are three main types of respiratory enzymes:
Electron carriers - Carry electrons and pass them along the chain of carrier molecules.
Proton pumps - Pump protons through the membrane.
Protein complexes - Associated with ATP synthetase and are used by protons to pass through. They are also known as stalked particles, as they have a stalk like structure.
They are nanomachines.
There are 7 steps to the process of the electron transport chain:
The coenzymes NAD and FAD reduced release hydrogen, which is split into electrons and protons. The coenzymes are oxidised, and the electron carriers where the electrons are donated are reduced.
NAD reduced uses three proton pumps, and each pair of hydrogen atoms produces enough energy for 3 ATPs.
FAD reduced uses two proton pumps, as it donates a pair of hydrogen atoms later on. It therefore produces enough energy for 2 ATPs.
Electrons then move down a series of electron carriers, providing energy for the proton pumps as they move. They reduce the pumps when entering, and oxidise them when leaving.
Reduced NAD’s hydrogen atom pair provides energy for 3 proton pumps, and reduced FAD’s hydrogen atom pair provides energy for 2 proton pumps.
The energy is used to pump the protons from the hydrogen atoms from the mitochondrial matrix to the intermembrane space.
The protons then accumulate in the intermembrane space.
This forms an electrochemical gradient, and a concentration gradient which is maintained by the continuous pumping of the proton pumps.
The protein complexes, which are associated with the enzyme ATP synthetase, are channels for the protons to diffuse through to reenter the mitochondrial matrix.
This movement is known as chemiosmosis, as it is down an electrochemical gradient.
This moves drives the synthesis of ATP using ADP and inorganic P.
For every 120 degree rotation, 1 ATP is produced. This requires 3 protons.
The rotor and stalk rotate, and the ATP is formed in the catalytic head.
Oxygen from the blood removes the protons and electrons to maintain the gradients. It is reduced by the hydrogen ions and electrons, creating water.
Oxygen is known as the final hydrogen/electron acceptor in the electron transport chain.
2H+ + 2e- + ½ O2 = H2O.
For each molecule of glucose which undergoes glycolysis, the electron transport system receives 10 NAD reduced and 2 FAD reduced, which generates 34 ATP synthesised altogether.
Inhibition
Disrupting the proton gradient causes cell death.
Apoptosis, programmed cell death, operates by preventing proton gradients across cell membranes from forming.
Examples of inhibitors:
DNP - a mitochondrial poison that prevents ATP synthesis.
It can be used by people to attempt to lose weight, as fats and carbohydrates are oxidised but all their energy is converted to heat, causing weight loss.
This can cause the body to overheat, sometimes fatally.
Cyanide - a non-competitive inhibitor of oxygen.
As this is the final carrier in the electron transport chain, it causes the accumulation of protons and electrons.
This destroys the proton gradient, preventing ATP synthetase from working. This cell dies quickly.
Anaerobic respiration
This occurs in the absence of oxygen, which causes a chain effect.
There is no oxygen to remove the protons and electrons in the ETC chain.
Reduced NAD can no longer be oxidised to release the hydrogen, and can therefore not be regenerated to pick up more.
Glycolysis is the only stage that can continue, but it needs an alternative method of removing hydrogen, pyruvate and regenerating NAD.
There are two methods of achieving this:
Lactic acid fermentation - Occurs in animals, often during vigorous exercise when muscle cells do not receive enough oxygen. Occurs in 3 stages:
Glycolysis occurs as normal.
Pyruvate becomes a hydrogen acceptor and is converted to lactate, regenerating NAD.
Once oxygen becomes available, this is respired into water and carbon dioxide, releasing its energy.
A buildup of lactic acid can cause muscle fatigue and soreness.
Alcohol fermentation - Occurs in various microorganisms and plant cells under certain conditions, for example in waterlogged soils. It has 3 stages:
Glycolysis occurs as normal.
Pyruvate is decarboxylated, forming ethanal and CO2. Ethanal is a hydrogen acceptor.
Ethanal is then reduced to ethanol using the hydrogen, and NAD is regenerated.
This is irreversible, meaning that even once oxygen rejoins, ethanol is not broken down. This can accumulate and reach toxic concentrations.
Energy budget
Process | H atoms carried by NAD | H atoms carried by FAD | ATP from ETC | ATP from substrate-level phosphorylation | Total |
|---|---|---|---|---|---|
Glycolysis | 2 | 0 | 6 | 2 | 8 |
Link reaction | 2 | 0 | 6 | 0 | 6 |
Krebs cycle | 6 | 2 | 22 | 2 | 24 |
Total | 10 | 2 | 34 | 4 | 38 |
This gives a total of 38 ATP created per molecule of glucose, across the stages of glycolysis, link reaction and the Krebs cycle, however this is only a theoretical number. In reality, cells produce on average 30-32 molecule of ATP due to:
ATP is necessary to move pyruvate, NAD and FAD reduced across the mitochondrial membrane.
Proton gradient can be compromised by proton leakage across the inner mitochondrial membrane, therefore not moving through and powering ATP synthetase.
Molecules can leak through membranes.
Glucose when combusted in oxygen produces 2880kj, making this the theoretical energy available.
Each molecule of ATP releases 30.6kj of energy, which when multiplied by the maximum amount of ATP that can be created, equals 1162.8.
To work out the efficiency of ATP production, energy made available ÷ theoretical energy made available, so 1162.8 ÷ 2880 × 100.
This equals 40.4%, which is considered quite high.
For anaerobic respiration, only glycolysis occurs, and oxidative phosphorylation can no longer occur. This means only two ATP can be created, 30.6 × 2 = 61.2kj.
61.2 ÷ 2880 × 100 = 2.1%
This is extremely inefficient compared to aerobic respiration.
Alternative respiratory pathways
The Krebs cycle is known as a metabolic hub as carbohydrates, fats and proteins can all be used.
AcCoA is the most significant molecule as it links the metabolism of all three macromolecules.
Lipids
Used when carbohydrates in the body are low.
However, is also used by tissues with a rich blood supply, such as the liver, and desert animals.
It occurs in three main steps:
Lipids are hydrolysed into their constituent molecules; glycerol and three fatty acids.
The glycerol is phosphorylated with ATP, then dehydrogenated/reduced by NAD and converted to triose phosphate, which then enters the glycolysis pathway.
Fatty acids are split into 2 carbon fragments that enter the Krebs cycle as AcCoA. Hydrogen is then released, picked up by NAD which enters the electron transport chain.
This can produce high ATP levels depending on the length of the hydrocarbon chain of the fatty acid. Additionally, longer chains have:
More carbon atoms, which produces more CO2 then muscle cells can remove quickly enough due to a limited blood supply.
More hydrogen atoms, so more NAD is reduced, producing more ATP. This is why cells with a rich blood supply respire fat, ATP they produce is readily transported around the body.
This also produces more water/metabolic water, which is important for animals who have access to less water such as desert animals.
Proteins
This is only used when carbohydrates and lipids are extremely low, such as during starvation.
Tissue protein is mobilised for this, and kidney and heart muscle is the first to break down.
This occurs in 3 stages:
Proteins are hydrolysed into their constituent amino acids, leaving carbon residue.
The amino acids are transported to the liver and deaminated (the amino group is removed) leaving -NH2. This is converted to urea.
The carbon residue is converted to AcCoA, pyruvate, or another Krebs cycle intermediate where it is dehydrogenated.