AP Chemistry: UNIT 5 THERMOCHEM
1) The Nature of Energy (KE, PE, heat, and work)
Energy
allows us to do work or produce heat
heat transfer from chemical processes
JOULES

amount needed to accelerate
Law of Conservation
energy can be converted from one form to another but can be neither creater nor destroyed
total energy content of the universe is constant
Types of energy
Potential energy
energy due to position or composition (chemical bonds)
can result from attractive and repulsive forces
stored energy
Kinetic energy
energy due to the motion of an object (atoms or molecules)
depends on the mass of the object (m) and its velocity (v)
KE = ½ mv2
Conversion of energy
transfering energy through physical contact
Methods of transfering energy
heat
transfer of energy between two objects due to a temperature difference (hot to cold)
temperature reflects random motion of particles in a substance (kinetic energy) - C and K
energy absorbed = increase temperature
energy released = decrease temperature
work
force acting over a distance
work = force x distance = F x ^h
Parts of the universe
universe = system + surroundings
system
part of the universe on which one wishes to focus their attention
in the lab (where you are)
reactants and products of a reaction in their container
3 types
open system
open system can exchange mass and energy, usually in the form of heat with its surroundings
closed system
which allows transfer of energy (heat) not mass
isolated system
does not allow the transfer of either mass or energy
surroundings
include everything else in the universe
things other than the reactants and products in their container
Types of Reactions
Exothermic
results in the evolution of heat (energy)
energy flows out of the system
Endothermic
results in the absorption of energy from the surroundings
heat flows into a system
Reaction mechanism
energy gained by the surroudings must be equal to the energy lost by the system
endothermic reactions result from a lowered potential energy of the reaction system
must add energy from surroundings
PE products > PE reactants
exothermuc reactions, potential energy stored in chemical bonds is converted to thermal energy via heat
energy must be released to surroundings
PE products < PE reactants
change (triangle) PE
stored in the bonds of products as compared with the bonds of reactants
exo - more energy is released while forming new bonds than is consumed while breaking the bonds in the reactants
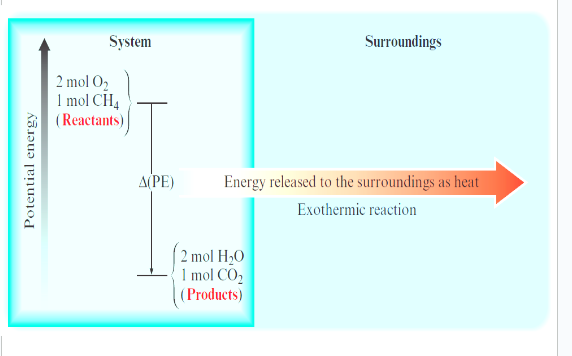
endo - energy that flows into the system as the heat is used to increase the potential energy of the system (energy needed to break bonds)
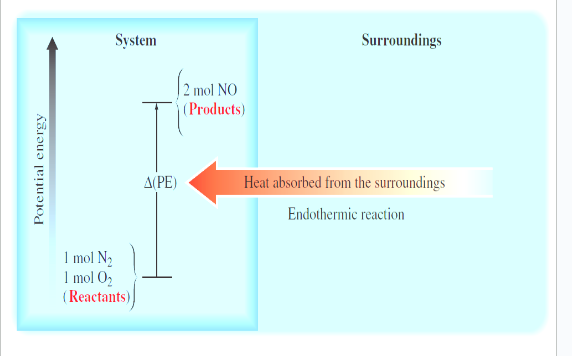
thermodynamics
study of energy and its interconverstions
1st law of thermodynamics
energy of the universe is constant (law of conservation of energy)
Internal energy (E) of a system
sum of kinetic and potential energies of all particles in a system
can be changed by flow of work, heat, or both

Parts of thermodynamic quantities
number
gives the magnitude of chnage
sign
indicates the direction of flow (endo/exo)
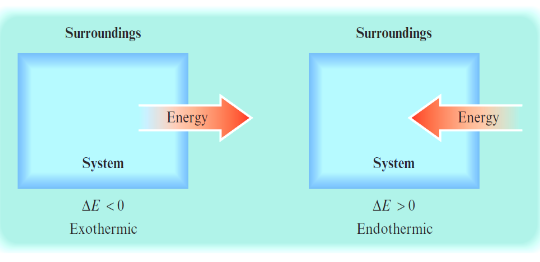
reflects the systems point of view
in endo
q = +x
when the surroundings do work on the system
w is positive
in exo
q = -x
when a system does work on surroundings
w is negative
Work
associated with a chemical process
work done by a gas through expansion
work done to a gas through compression
equation
F=force
P= pressure (force per unit of area)
P= F/A
H= height
A = area

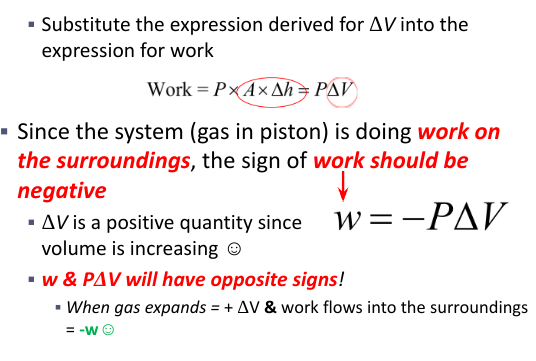
Gas expansion
delta V positive
W is negative
Gas compression
delta V is negative
W positive
2) Enthalpy and Calorimetry
Enthalpy
delta H- the heat content of a system
a state function that is defined as
H=E+PV
E= internal energy of system
P= pressure of the system
V= volume of the system
enthalpy is a state function because it does not depend on the pathway between states
at constant pressure enthalpy change (delta H = qp )
qp = heat at constant pressure
delta H = enthalpy of products - enthaply of reactant
positive = endo
negative = exo
Calorimetry
science of measuring heat (q)
based on observations of temperature change when a body absorbs or discharges energy in form of heat
calorimeter
device used to determine the heat associated with a chemical reaction
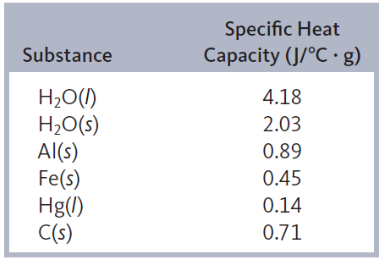
heat capacity ( C )
heat absorbed/increase in temperature
specific heat capacity
energy required to raise the temperature of one gram of a substance by one degree celisius
j/c*g or j/k*g
molar heat capacity
energy required to raise the temperature of one mole of a substances by one degree celsius
j/c*mol or j/k*mol
for metals different from water - less energy
Constant - pressure calorimetry
atmospheric pressure remains constant during the process
used to determine enthalpy changes in reactions in a solution
delta H = qp
calculation of heat (q) for a neutralization reaction: released= absorbed
specific heat capacity x mass of solution x change in temperature
s x m x delta T
heat of reaction is an extensive property - depends on amount tho
Constant - volume calorimetry
used in conditions when experiments are to be performed under constant volume
no work done since V must chnage for PV to work to be performed
bomb calorimeter
delta E = q + w = q = qv
3) Hess’s Law
Enthalpy change as a state function
going from reactants to products the ehthalpy (delta H) is the same whether the reaction takes place in one step or in a series of steps
If the reaction is reversed the enthalpy is also reverse (endo and exo)
magnitude of enthalpy is directly proportional to the quantities of reactants and products in a reaction
if the coefficients in a balanced reaction are multiplied by an interger the value of enthalpy is multiplied by the same integer
somehow combine all of the the equations and combine the enthalpy values
watch out for coefficients
4) Standard Enthalpies of Formation
Delta Hfo

change in the enthalpy that accompanies of one mole of a compound from its elements with all substances in their standard states
standard state
precisely defined reference states
Gas state
1 atm
Pure substance Condensed state (l or s)
pure liquid or solid
in Solution
1M
Standard state of an element
form where element exists under conditions of 1 atm and 25C
Oxygen = standard state O2(g)
^H in any lone element is 0
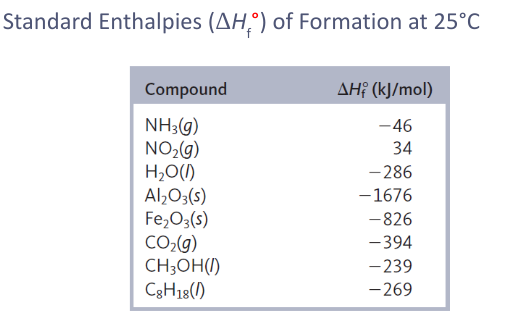
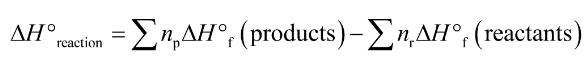
degree symbol on thermodynamic function shows that the corresponding process that is carried out under standard conditions
elements in its standard state is 0
5) Sources of Energy
w
e
e
e
ee