Human Pathology, Chapter 7. Fluid and Hemodynamic Disorders
Fluid and Hemodynamic Disorders
Blood Composition and Components
Blood constitutes approximately 8\% of total body weight, while other fluids and tissues make up the remaining 92\%.
Whole Blood (percentage by volume):
Plasma: 55\% of whole blood.
Formed Elements: 45\% of whole blood.
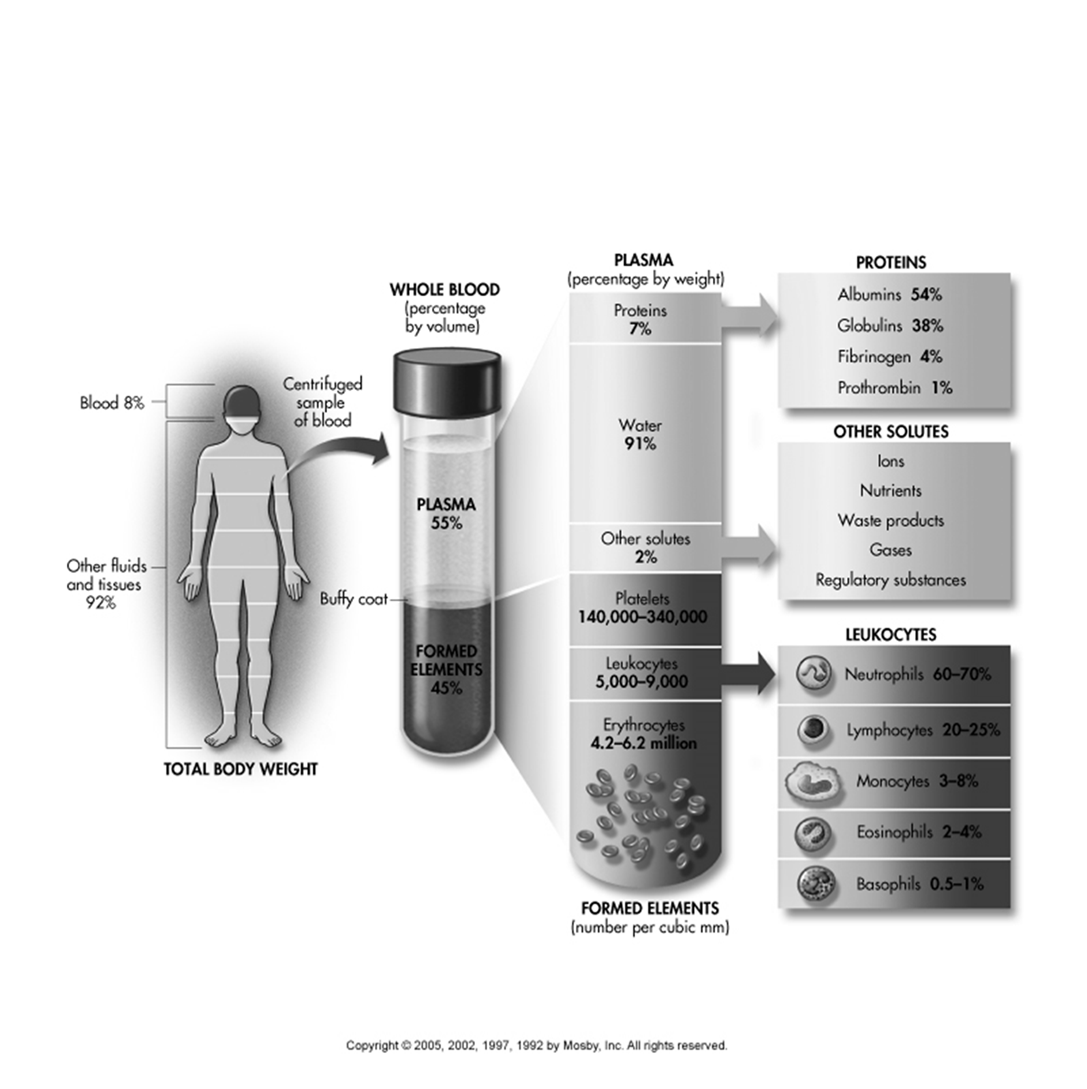
Plasma (percentage by weight)
Two main parts of blood. Fluid & formed elements. Fluid is plasma and mostly protein- 55% of blood.
Water: 91\%.
Proteins: 7\%.
Albumins: 54\%.
Globulins: 38\%.
Fibrinogen: 4\%.
Prothrombin: 1\%.
Other solutes: 2\%. These include ions, nutrients, waste products, gases, and regulatory substances.
Formed Elements (number per cubic mm):
Erythrocytes (Red Blood Cells): 4.2 \text{ to } 6.2 million.
Leukocytes (White Blood Cells): 5,000 \text{ to } 9,000 (found in the 'Buffy coat').
Neutrophils: 60 \text{ to } 70\%.
Lymphocytes: 20 \text{ to } 25\%.
Monocytes: 3 \text{ to } 8\%.
Eosinophils: 2 \text{ to } 4\%.
Basophils: 0.5 \text{ to } 1\%.
Platelets: 140,000 \text{ to } 340,000 (found in the 'Buffy coat').
Body Fluid Compartments
Blood is composed of two main parts: Fluid and Formed elements.
The fluid component is known as plasma, which is mostly protein and accounts for 55\% of the blood volume.
Capillary Exchange (Normal Physiology)
In a normal capillary, fluid moves out of the arterial end at a rate of 14 \text{ mL/min}.
Fluid re-enters the venule end at a rate of 12 \text{ mL/min}.
This results in a net fluid movement of 2 \text{ mL/min} into the interstitial space, which is then drained by the lymphatic system.
Pathogenesis of Edema
Edema is the accumulation of excess fluid in the interstitial space and can result from several mechanisms:
Increased Hydrostatic Pressure: Elevated pressure within blood vessels forces fluid out.
Decreased Oncotic Pressure: Reduced plasma protein concentration (especially albumin) diminishes the osmotic pull of fluid back into the vessels.
Increased Permeability: Damage to capillary walls allows proteins and fluid to leak into the interstitial space.
Lymphatic Obstruction: Blockage of lymphatic drainage prevents the return of interstitial fluid to the circulation
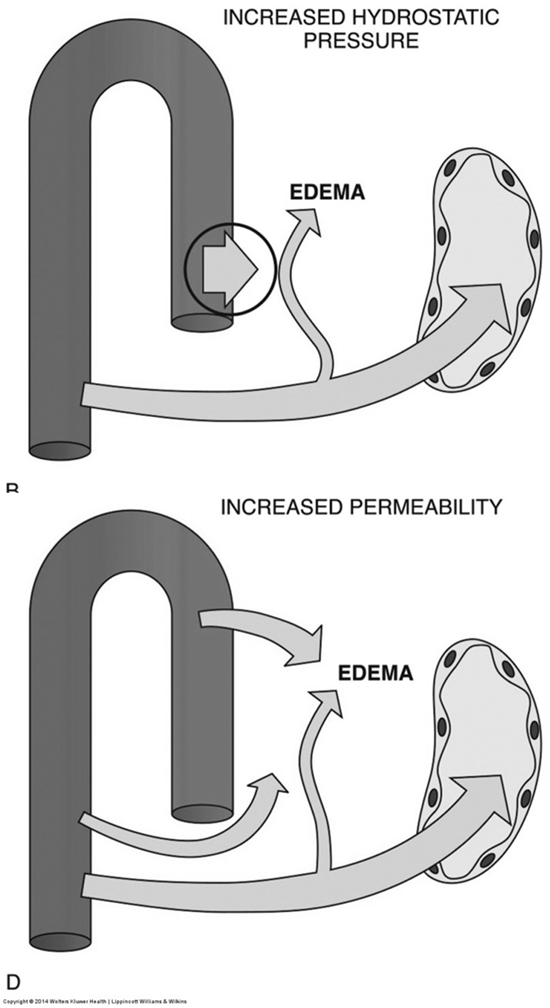
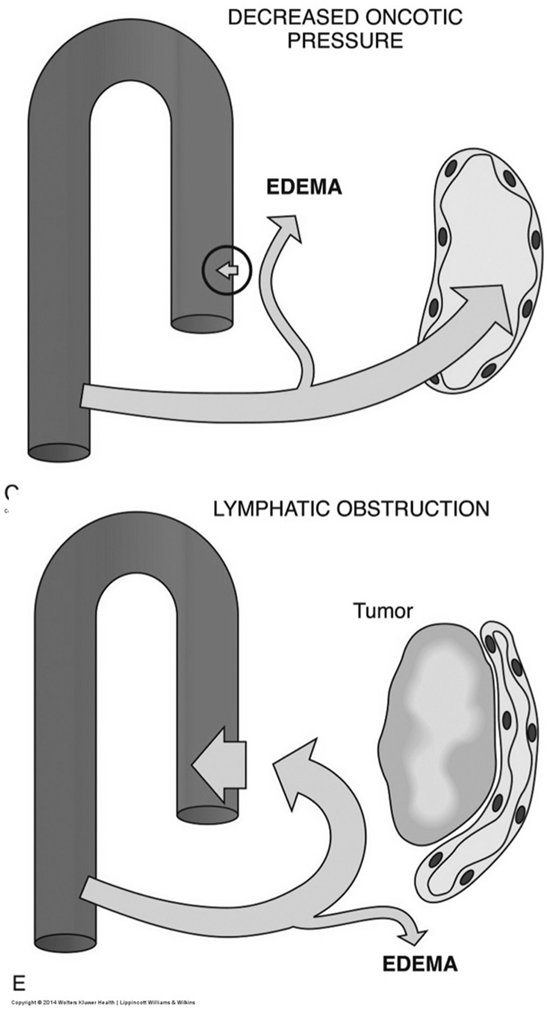
Clinical Manifestations of Edema
Cerebral edema: Swelling of brain tissue.
Pulmonary edema: Fluid accumulation in the lungs, impairing gas exchange.
Pitting edema of the lower extremities: Indentation remains after pressure is applied to the swollen area of the legs.
Periorbital (facial) edema: Swelling around the eyes or face.
Hydrothorax: Fluid accumulation in the pleural cavity (space around the lungs).
Severe edema of the arm: Often results from long-standing lymphatic obstruction, such as after a mastectomy where lymph nodes are removed, leading to swelling of the upper extremities.
Hyperemia
Active Hyperemia: Characterized by the dilatation of arterioles, leading to increased blood flow to an area.
Examples include blushing, physiological changes during exercise, and the initial stages of inflammation.
Passive Hyperemia (Congestion): Occurs due to a decrease in venous flow, leading to accumulation of blood in a tissue or organ. It is often associated with hydrostatic edema.
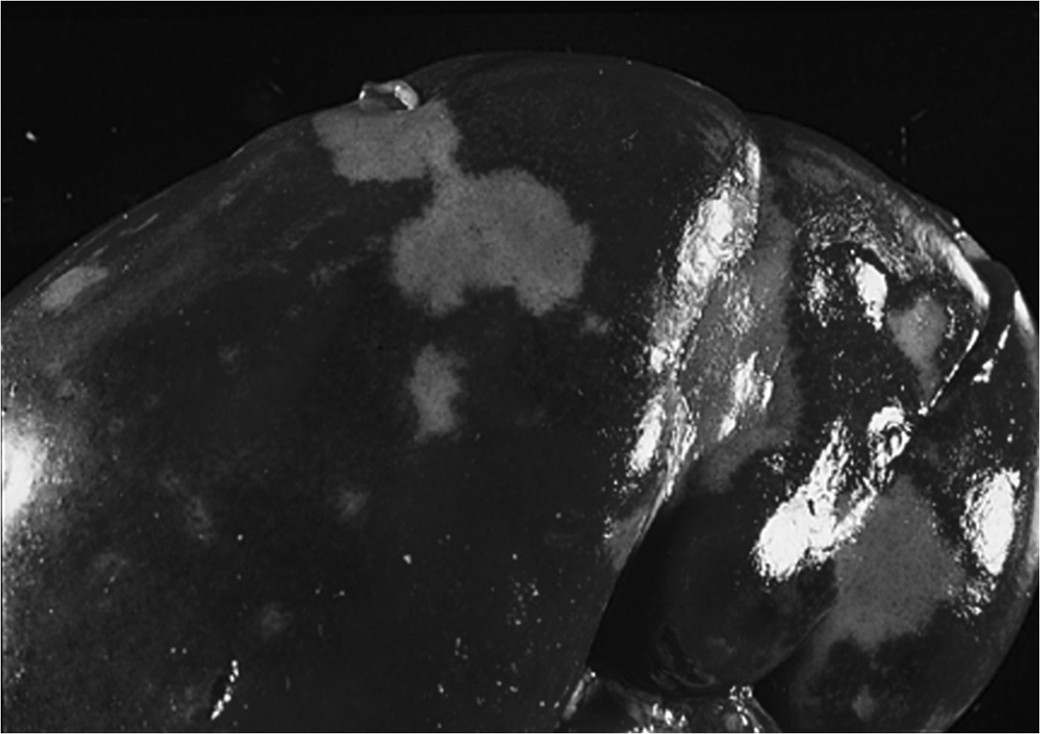
Chronic Passive Hyperemia of the Liver: In patients with right ventricular failure, the liver pathology is known as 'Nutmeg liver'. This characteristic appearance is due to multiple red, depressed cells resulting from prolonged congestion.
Chronic Passive Hyperemia of the Lung: In patients with left ventricular failure, chronic congestion often leads to alveolar fibrosis, also known as 'brown induration of the lung'. The macrophages found in the lung tissue during this condition are referred to as 'Heart failure cells' due to their hemosiderin content from phagocytosed red blood cells.
Elephantiasis
A condition caused by Filaria (parasitic worms) transmitted by mosquitoes.
These parasites develop and create blockages within lymphatic tissue, preventing proper fluid drainage.
This disruption leads to extreme localized swelling in the affected area.
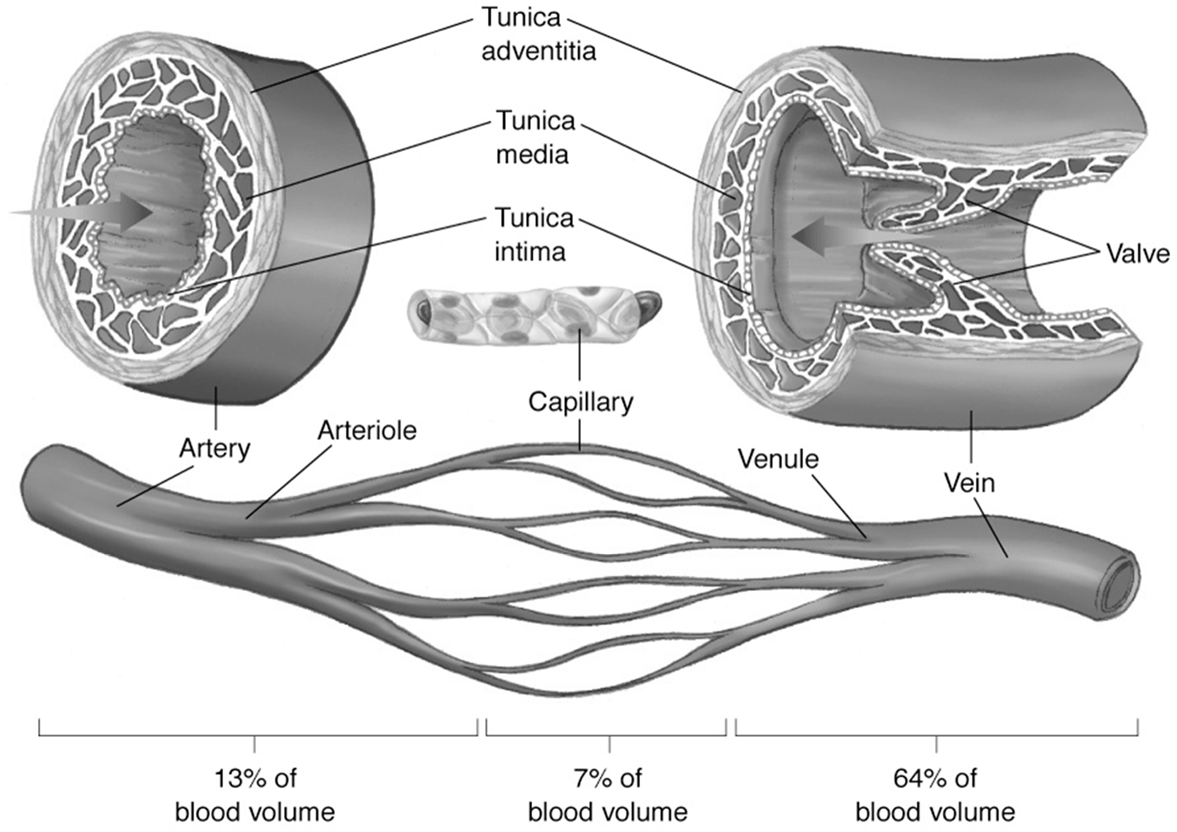
Circulatory System Blood Volume Distribution
Arteries: Contain approximately 13\% of the total blood volume.
Capillaries: Contain about 7\% of the total blood volume.
Veins: The largest reservoir, holding approximately 64\% of the total blood volume. Veins also contain valves to prevent backflow of blood.
Blood vessel structure includes three layers: Tunica intima, Tunica media, and Tunica adventitia.
Hemorrhage
Hemorrhage is bleeding, either external or internal. The anatomical source and amount of blood loss determine its seriousness.
Types of External Hemorrhage
Results from soft tissue injury and accounts for nearly 10 million emergency department visits in the United States each year.
While most soft tissue trauma with mild hemorrhage is not life-threatening, it can carry significant risks of patient morbidity and disfigurement.
The seriousness depends on:
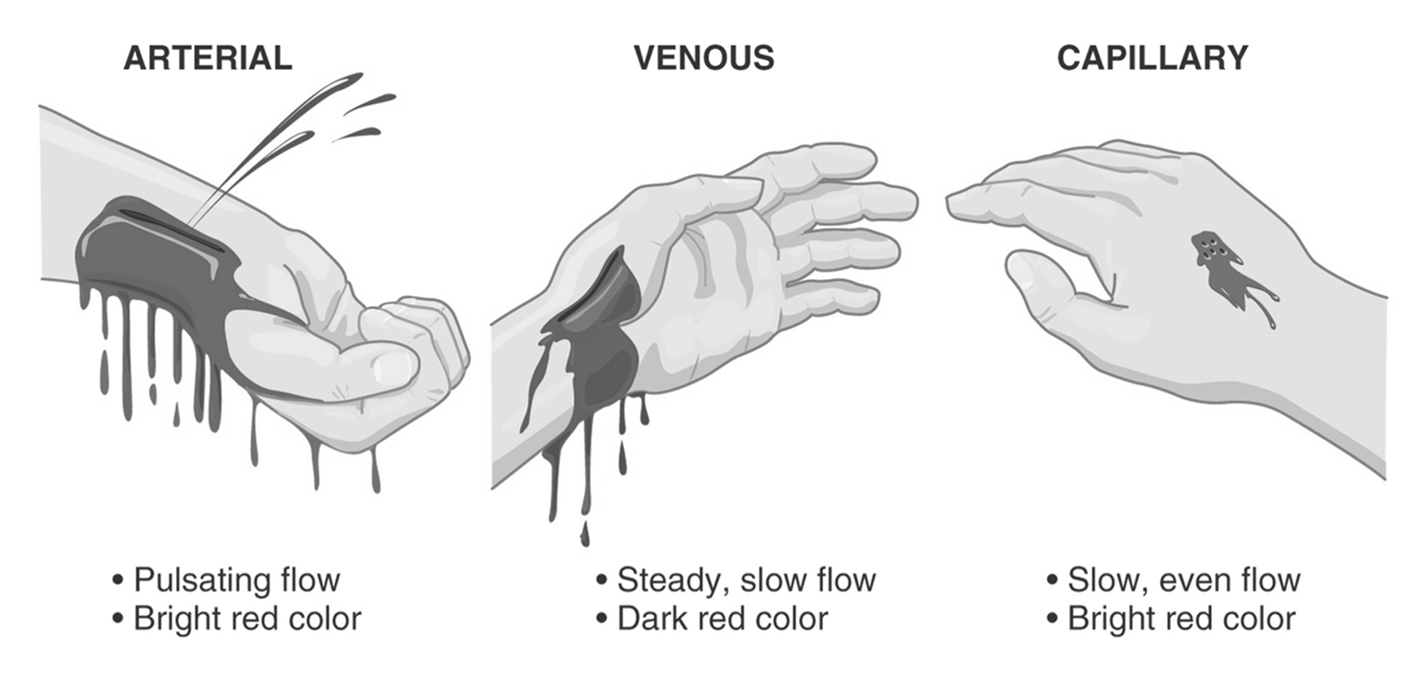
Anatomical source: Arterial, venous, or capillary.
Arterial hemorrhage: Bright red blood, flows rapidly from a wound.
Venous hemorrhage: Darker red blood, flows slowly.
Capillary hemorrhage: Bright red blood, flows slowly.
Amount of blood loss that can be tolerated by the patient.
Internal Hemorrhage
Can result from blunt or penetrating trauma, or acute or chronic medical illnesses.
Internal bleeding causing hemodynamic instability usually occurs in one of four body cavities:
Chest
Abdomen
Pelvis
Peritoneum
Clinically Important Forms of Hemorrhage
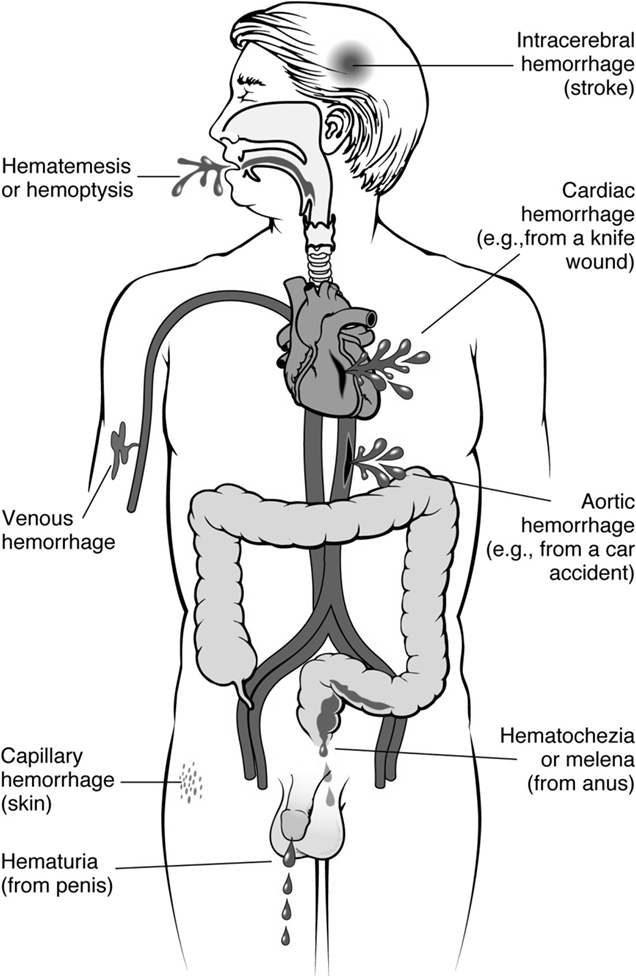
Intracerebral hemorrhage: Bleeding within the brain, often leading to stroke.
Cardiac hemorrhage: Bleeding in the heart, e.g., from a knife wound.
Aortic hemorrhage: Bleeding from the aorta, often due to high-impact trauma like a car accident.
Skin hemorrhage: Capillary bleeding visible on the skin.
Hemoptysis: Coughing up blood from the respiratory tract.
Hematemesis: Vomiting blood (originating from the upper gastrointestinal tract).
Hematuria: Blood in the urine (often indicative of a urinary tract infection or kidney issue).
Metrorrhagia: Utero-vaginal bleeding outside of normal menstruation.
Melena: Dark, tarry stools indicating upper gastrointestinal bleeding (digested blood).
Hematochezia: Bright red blood in stool, usually indicating lower gastrointestinal bleeding.
Thrombi and Emboli
Thrombus: A blood clot formed within a blood vessel or heart chamber. Adhering to the vessel or chamber walls
Embolus (plural: emboli): A free-movable mass or clot, typically generated from a thrombus, that travels from its origin to another anatomical location.
Anticoagulants (Blood Thinners)
Newer oral anticoagulants (NOACs) like Xarelto (rivaroxaban) and Eliquis:
Work by blocking Factor Xa (10\text{a}), an enzyme necessary for blood clots to form, thus slowing down clotting.
Are not considered active blood thinners in the same way as Warfarin or Heparin.
Key facts about Xarelto: No regular blood tests needed, no known dietary restrictions (patients can eat vitamin K-rich foods), and most people maintain the same dosage (adjustments only for kidney problems).
Xarelto is proven to reduce the risk of atrial fibrillation (AFib)-related stroke for AFib not caused by a heart valve problem.
Important Safety Information: Xarelto can cause serious bleeding, rarely leading to death, and may increase bruising and prolong bleeding time. Risk of bleeding is higher if taken with other medicines that increase bleeding risk.
Traditional anticoagulants: Warfarin and Heparin are considered the only actual blood thinners in the traditional sense.
Warfarin: Interferes with Vitamin K, a nutrient essential for blood clot formation.
Key facts about Warfarin: Requires regular blood tests (e.g., INR monitoring), has dietary restrictions (foods high in Vitamin K like spinach, cabbage, broccoli can affect its efficacy), and dosage adjustments are common based on blood test results.
Virchow Triad in Thrombosis
The formation of a thrombus is influenced by three primary factors, collectively known as Virchow's Triad:
Endothelial Injury: This is the single most important factor. Injury to endothelial cells can alter local blood flow and affect coagulability.
Abnormal Blood Flow: Either stasis (slow blood flow) or turbulence (disrupted, irregular flow) can contribute to endothelial injury and promote clot formation.
Hypercoagulability: An increased tendency of blood to clot.
These factors may act independently or combine to cause thrombus formation.
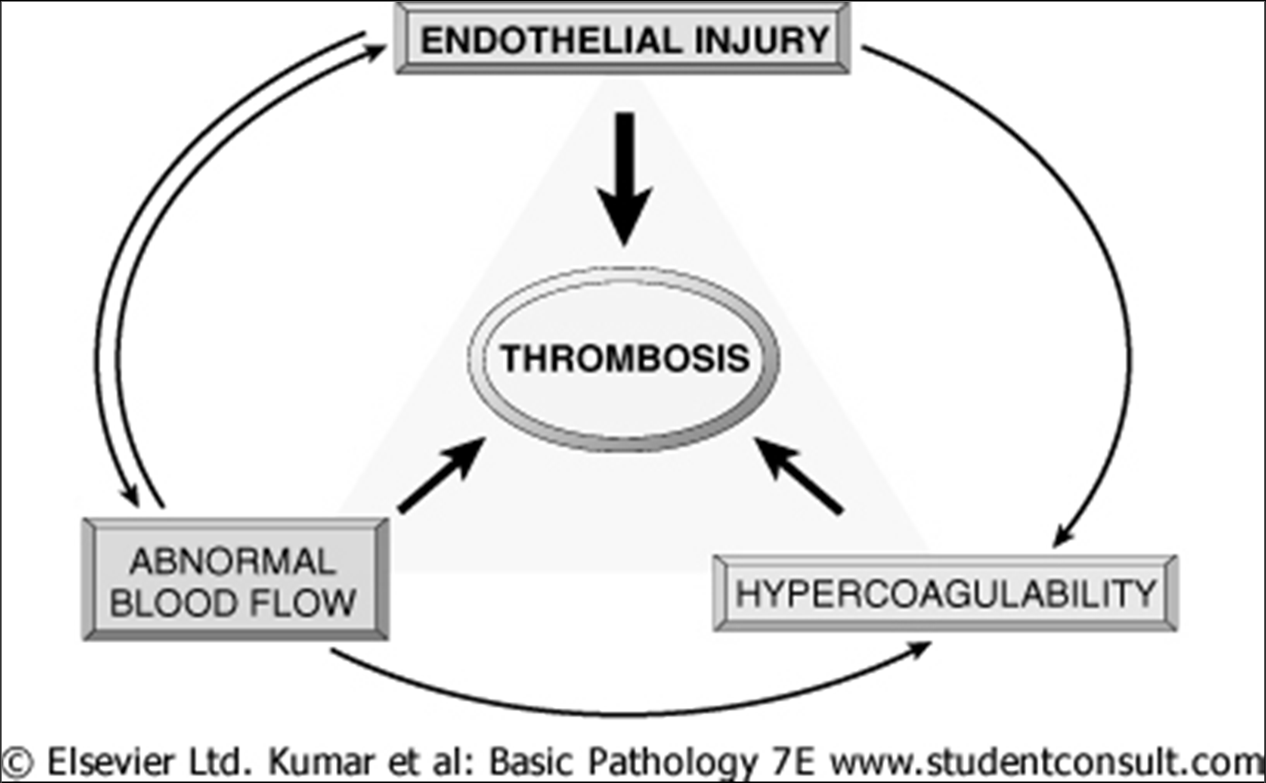
Virchow triad in thrombosis. Endothelial integrity is the single most important factor. Injury to endothelial cells can also alter local blood flow and affect coagulability. Abnormal blood flow (stasis or turbulence), in turn, can cause endothelial injury. The factors may act independently or may combine to cause thrombus formation.
Common Sites of Thrombus Formation
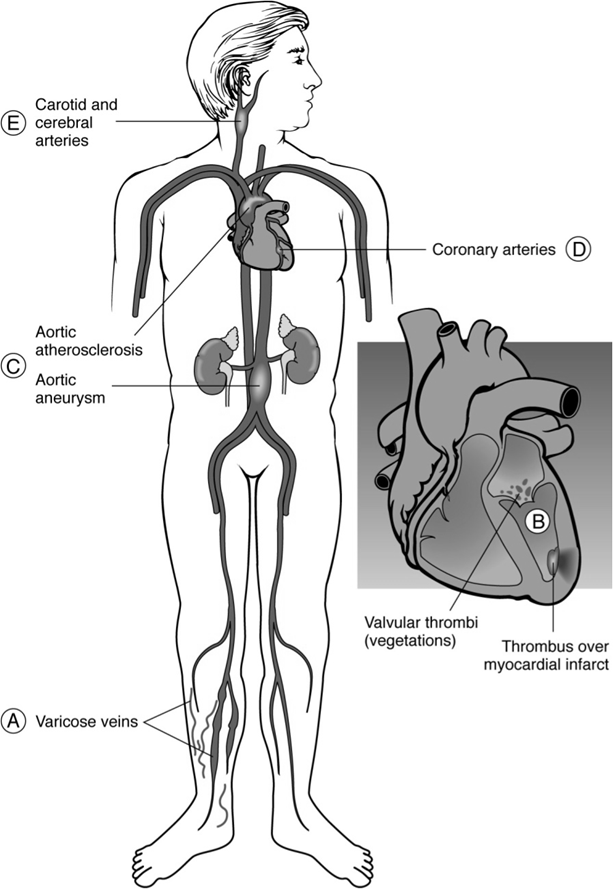
Example: Thrombosis of the inferior vena cava, where superficial collateral veins dilate to allow blood return from legs to the right atrium.
Arterial and Venous Thrombi
Lines of Zahn: Microscopic laminations seen in thrombi, consisting of alternating layers of fibrin and platelets, which are responsible for the layered shape of the thrombus. These are typically more prominent in arterial thrombi.
Fate of Thrombi
Fibrinolysis via TPA (Tissue Plasminogen Activator):
TPA is released from the endothelial cells of blood vessels.
It functions to cleave or dissolve fibrin, thereby breaking down the clot.
TPA is a protein that is used acts as a clot buster by dissolving the clot, think of drain-o for a clogged sink
American Heart Association (AHA) recommendations for TPA administration:
For cardiac patients (e.g., acute myocardial infarction), TPA should ideally be administered within 6 hours of the event.
For stroke patients (ischemic stroke), TPA should ideally be administered within 3 hours of the event. This time frame has been modified and extended in some cases due to advances in surgical techniques and patient selection criteria.
Thrombi can also obstruct blood vessels, propagate, embolize, or undergo organization and recanalization.
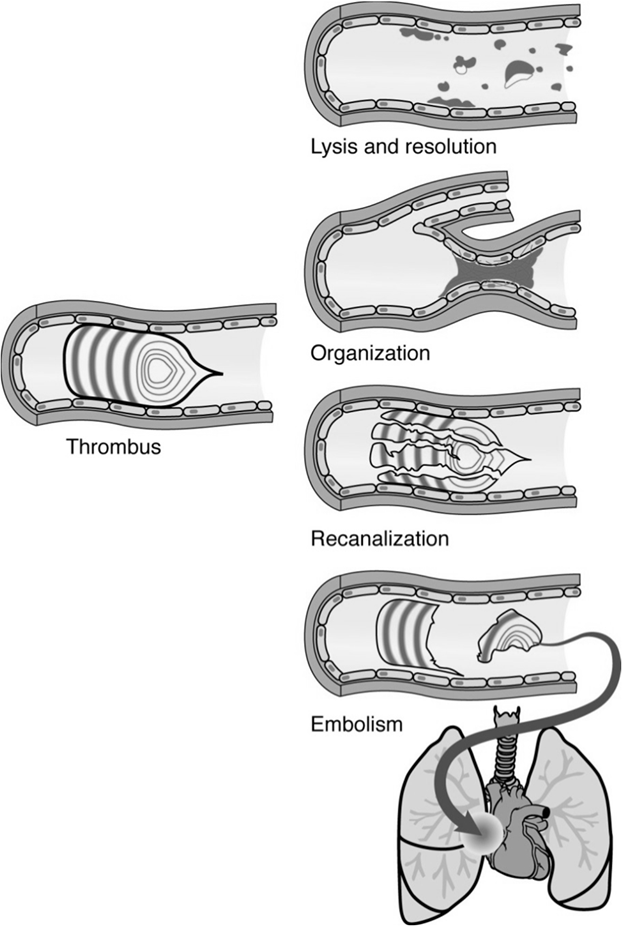
Embolism
An embolus is a detached intravascular solid, liquid, or gaseous mass that is carried by the blood to a site distant from its point of origin.
Pulmonary Thromboembolism: A common and serious consequence of deep venous thrombosis (DVT), where a clot from a deep vein (e.g., in the leg) travels to the lungs.
A saddle embolus is a large pulmonary embolus that straddles the bifurcation of the main pulmonary artery, causing severe obstruction and often shock.
Venous Emboli: Typically originate from deep venous thrombosis and commonly travel to the lungs, causing pulmonary emboli. Other sources include infected venous catheters, tumor emboli (e.g., renal cell carcinoma), amniotic fluid embolism (rare but severe), fat embolism (from fractures), or air/foreign material infections.
Arterial Emboli: Usually originate from the left side of the heart (e.g., left ventricle after myocardial infarct, valvular thrombi, AFib) and can travel to various organs, causing infarction.
Common sites of impact: Brain (stroke), Retina, Spleen, Kidney, Small intestine, and Lower legs.
Infarction (Tissue Death)
Infarction refers to tissue death (necrosis) caused by ischemia, resulting from an obstruction of its arterial supply or venous drainage.
White (pale) infarct: Occurs in solid organs with a single arterial blood supply (end-arterial circulation) and relatively sparse tissue structure, where blood cannot readily diffuse into the necrotic area. Examples include the spleen, kidney, and heart.
Red (hemorrhagic) infarct: Occurs in organs with dual blood supply (e.g., lung, small intestine), in venous occlusions, or in loose tissues where blood can collect in the infarcted area after necrosis. The dual supply and spongy nature of the lung allow blood to passively flow into the necrotic zone, making it hemorrhagic.
The fate of infarcts depends on several factors:
Their anatomic site.
The type of cells forming the tissue (e.g., neurons are more susceptible to ischemia than fibroblasts).
The circulatory status of the organism (e.g., presence of collateral circulation).
The extent of necrosis.
SHOCK: Inadequate Tissue Perfusion
Shock is a life-threatening condition characterized by INADEQUATE TISSUE PERFUSION, meaning that the body's tissues are not receiving enough oxygen and nutrients to meet their metabolic demands.
Stages of Shock (Cellular Level)
Stage 1: Vasoconstriction
The body's initial compensatory response involves widespread vasoconstriction to redirect blood flow to vital organs.
If circulatory volume is not promptly restored (e.g., with intravenous fluids or blood), shock progresses.
Stage 2: Capillary and Venule Opening
This stage occurs with a 15\% \text{ to } 25\% decrease in intravascular blood volume.
The sympathetic nervous system (fight-or-flight response) is activated, increasing heart rate, respiratory rate, and capillary refill time. Blood pressure may still appear normal.
Postcapillary sphincters remain constricted, causing blood to pool and stagnate in the capillary system, leading to capillary dilation.
Progressive hypoxemia (low oxygen) and acidosis (build-up of acid levels) lead to the opening of more venules and capillaries, vastly expanding the vascular space.
Sluggish blood flow and reduced oxygen delivery result in increased anaerobic metabolism and lactic acid production.
The respiratory system attempts to compensate for acidosis by increasing ventilation to blow off carbon dioxide (CO_2).
As acidosis increases and pH falls, red blood cells may cluster together (rouleaux formation), further impeding microcirculation.
This stage often progresses to the third stage if fluid resuscitation is inadequate, delayed, or overshadowed by trauma or sepsis.
Stage 3: Disseminated Intravascular Coagulation (DIC)
A life-threatening condition marked by widespread activation of the blood clotting system, leading to the formation of small blood clots (microthrombi) throughout the body.
The time of onset depends on the degree of shock, patient age, and pre-existing medical conditions.
This stage occurs with a 25\% \text{ to } 35\% decrease in intravascular blood volume (equivalent to 15-20 liters in an adult).
Hypotension becomes evident at this stage.
Blood replacement is usually required.
Stage 3 is often resistant to treatment (refractory shock) but is still considered reversible with aggressive intervention.
Distal tissue cells rely heavily on anaerobic metabolism, leading to a significant increase in lactic acid production and more severe acidosis.
Stage 4: Multiple Organ Failure (MOF)
The amount of cellular necrosis required to cause organ failure varies by organ and its underlying health.
Typically, hepatic (liver) failure occurs first, followed by renal (kidney) failure, and then heart failure.
If capillary occlusion persists for more than 1 \text{ to } 2 hours, the cells nourished by that capillary undergo irreversible necrotic changes.
At this stage, blood pressure falls dramatically (to levels of 60 \text{ mmHg} or less).
Cells can no longer utilize oxygen effectively, and cellular metabolism ultimately ceases.
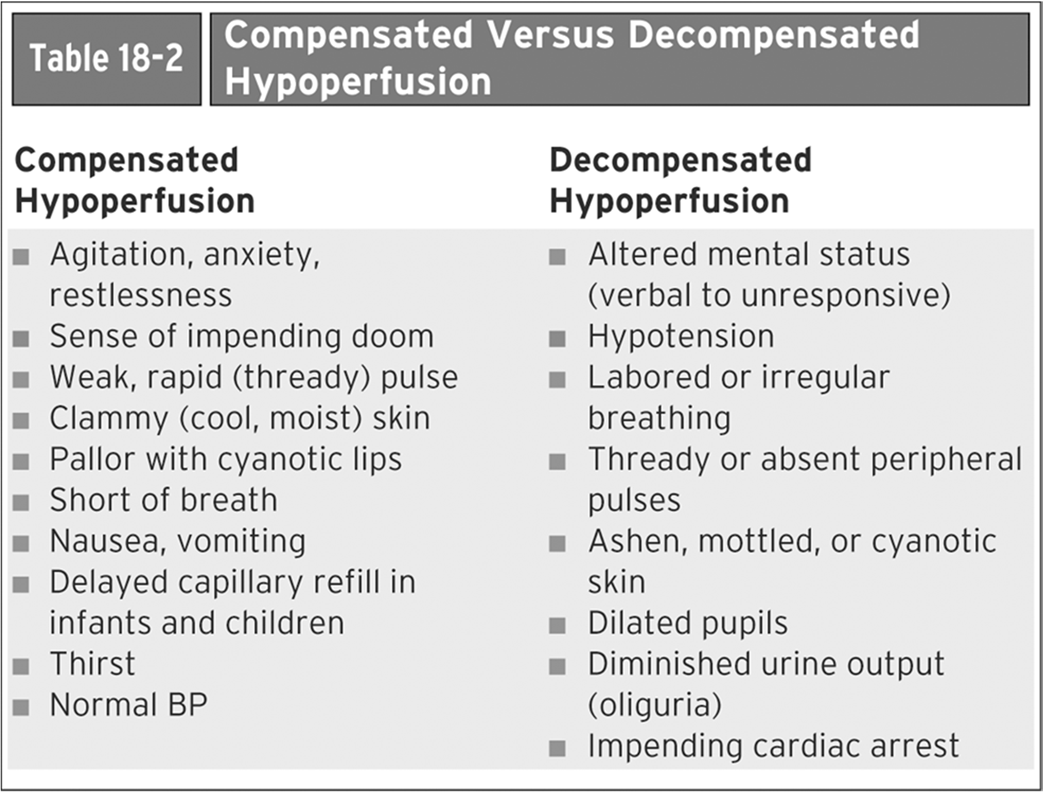
Phases of Shock (Compensated, Decompensated, Irreversible) used in the medical field
Compensated Hypoperfusion (Earliest Stage):
The body can still compensate for blood loss.
Blood pressure is maintained at or near normal levels.
Level of responsiveness is the best indicator of tissue perfusion.
Signs/Symptoms: Agitation, anxiety, restlessness, sense of impending doom, weak/rapid (thready) pulse, clammy (cool, moist) skin, pallor with cyanotic lips, shortness of breath, nausea/vomiting, delayed capillary refill (especially in infants and children), thirst, normal blood pressure.
Treatment at this stage typically results in full recovery.
Decompensated Hypoperfusion:
Blood pressure begins to fall.
Blood volume drops by more than 30\%.
Compensatory mechanisms begin to fail.
Signs and symptoms become more obvious.
Cardiac output falls dramatically.
Signs/Symptoms: Altered mental status (ranging from verbal cues to unresponsiveness), hypotension, labored or irregular breathing, thready or absent peripheral pulses, ashen/mottled/cyanotic skin, dilated pupils, diminished urine output (oliguria), impending cardiac arrest.
Treatment at this stage sometimes results in recovery.
Irreversible (Terminal) Phase of Shock / Multiple Organ Dysfunction Syndrome (MODS):
This is the last and often fatal stage of shock.
Systemic Inflammatory Response Syndrome (SIRS): A generalized manifestation of a severe local immune or inflammatory reaction. It is a hypermetabolic state featuring two or more signs of systemic inflammation such as fever, tachycardia, tachypnea, and leukocytosis.
Septic Shock: Defined as clinical SIRS so severe that it results in organ dysfunction and hypotension.
Compensated Anti-inflammatory Response Syndrome (CARS): In some patients, anti-inflammatory factors play a significant role, leading to a paralysis of the immune system and a poor outcome.
Compensated anti-inflammatory response (cars) acts to try to compensate systemic inflammatory response. however it usually makes it worse
The mortality of SIRS/MODS exceeds 50\%, making it responsible for most deaths in non-coronary intensive care units in the United States.
Organ Damage Manifestations: Fever, potential brain death, Acute Respiratory Distress Syndrome (ARDS), centrilobular hemorrhagic necrosis of the liver, acute tubular necrosis of the kidney, superficial hemorrhagic necrosis of the intestine, focal myocardial necrosis, congestion and hyperplasia of the spleen, stress (steroid) ulcers of the stomach, and widespread vasodilation with splanchnic pooling.
Types of Shock
Cardiogenic Shock: Caused by myocardial pump failure.
Causes: Myocardial infarction, myocarditis, cardiac tamponade, massive pulmonary embolus.
Hypovolemic Shock: Caused by a significant decrease in blood volume.
Causes: Hemorrhage, severe diarrhea, dehydration, extensive burns.
Septic Shock: Caused by severe infection, leading to widespread inflammation and vasodilation.
Anaphylactic Shock: A severe, systemic Type 1 hypersensitivity (allergic) reaction, causing widespread vasodilation and increased vascular permeability.
Neurogenic Shock: Caused by brain damage or spinal cord injury, leading to loss of sympathetic tone and widespread vasodilation.
Underlying Pathophysiological Mechanisms for all types of shock: Myocardial pump failure, decreased blood volume, widespread vasodilation, and increased vascular permeability.
ALL FORMS OF SHOCK LEAD TO HEART FAILURE