Cholinergic Drugs
Agonist
Cholinergic System
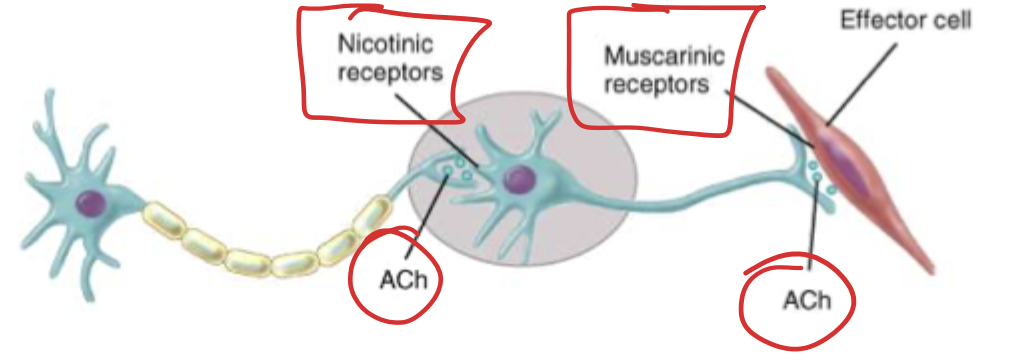
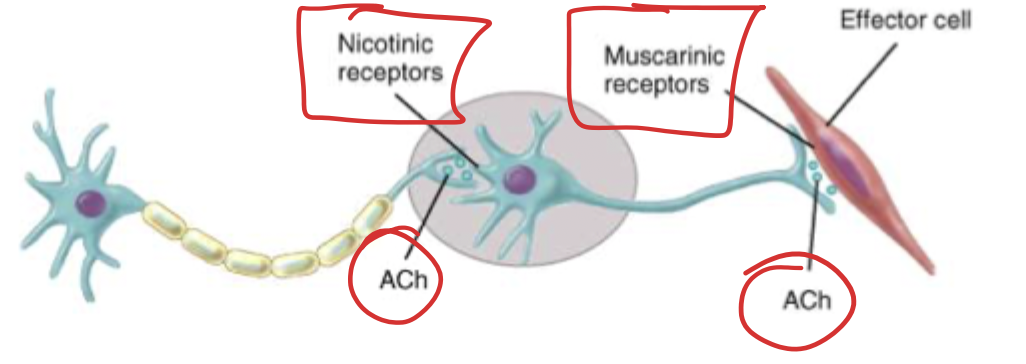
The system where the neurons are releasing the acetylcholine (ACh). There are cholinergic pathways in the cerebellum that affect cognitive functions. Drugs tend to work at target sites to directly stimulate (usually muscarinic (GPCRs)) receptors or by inhibiting the enzyme that breaks down acetylcholine. The somatic nervous system also has cholinergic functions in the somatic motor neurons, where ACh acts on motor neurons on skeletal muscles (causing contraction) → these are nicotinic receptors (Nm). Nicotinic receptors are also found between all the preganglionic and postganglionic neurons (these are Nn).
For ALL neurons that leave the CNS, they all make and release ACh and act on nicotinic receptor, what happens after that synapse is what differs. Therefore if you give something that impacts the Nn receptors you affect the entire ANS (parasympathetic and sympathetic).
Parasympathetic
Parasympathetic fibers from cranial nerves stimulate specific things.
CN III → pupillary muscles → Miosis (pupillary contraction).
CN VII → lacrimal/salivary/pterygopalatine glands → salivation and lacrimation.
CN IX → parotid gland → salivation.
Vagus → work on viscera like heart → slows conduction of AV node → bradycardia and decreased CO. ACh acts on M3 receptors on the endothelial cells of the blood vessels leading to the production of NO → vasodilation.
Vagus → smooth muscles of bronchioles → bronchoconstriction.
Vagus → secretions of HCL in stomach and other molecules in intestines, increased motility of GI tract.
Sacral (S2-S4) parasympathetic fibers → lower GI tract → increase motility and defecation. Increase motility of detrusor muscles to increase urination.
Sympathetic (T1-L2)
In all preganglionic acetylcholine is released, and generally post-gang we release epi. EXCEPT for the skin, sweating is induced by preganglionic → acetylcholine → postganglionic → acetylcholine
Cholinergic Receptors and Agonist
ACh is made with choline which we get from our diet. Choline uptake is the rate limiting step. In the mitochondria, Choline transferase (CHAT) takes acetyl-COA (made from vitamin B5 (panthothenate)). and choline and combines them. The ACh is put into vesicles. When the neuron is activated the voltage-gated calcium channels open and the vesicles can fuse to the membrane due to the Ca2+ rushing in. When ACh gets out of the cell via exocytosis, it can bind the nicotinic receptor (ligand gated channels) on the somatic muscles (or between the neurons). When these bind the “gates” open sodium rushes into the neuromuscular junction, leading to depolarization inducing contraction. To the get the muscle to relax we have to remove the ACh, we can use the acetylcholinesterase to break it down into choline and acetyl groups. ACh levels drop and muscle relaxes. Choline and Aceytl groups can be recycled and reuptaken by the cell. In the case of 2 Chainz neurons, the release of ACh into the synapse leads to the continuation of signal down the chain.
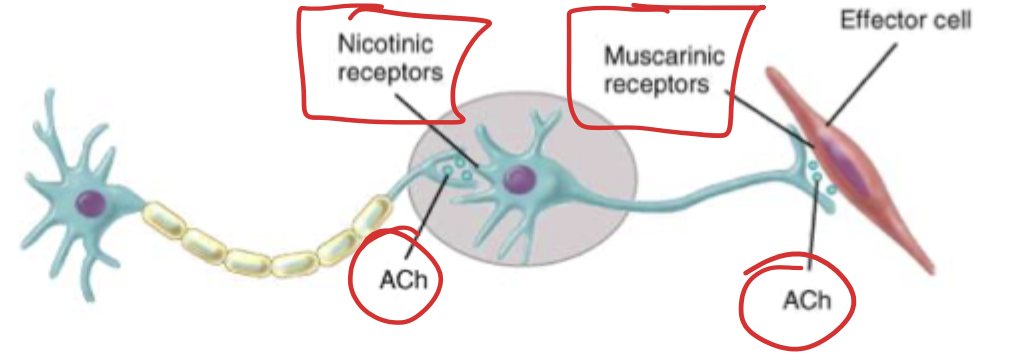
Nicotinic
Nicotine is an agonist (duh). 12 subtypes that are all ligand activated cation channels. Ligand channels is a pentameric structure when closed, when ACh binds sodium can enter the cell and depolarization can occur. Different tissues are going to have different subtypes (selectivity bb). These have a weak affinity for muscarine. These are found either on somatic muscles (Nm) or between the nerves (Nn).
Muscarinic
Muscarine is an agonist. 5 subtypes that are all GPCR (the important ones are M1, 2, 3). These are localized to parasympathetic effector sites! Principle cholinergic receptor in smooth/cardiac muscle and the glands. These show weak affinity for nicotine.
Inhibitory
in muscarinic receptors, choline is brought into the synaptic terminal (this is the rate limiting step). This combines with acetyl-COA (same enzyme (CHAT)) → ACh is born. Action potential leads to the exocytosis of the vesicles containing the ACh. ACh can bind the muscarinic receptors which can be inhibitory or activating. When we bind inhibitory receptors (AKA the G-inhibitory pathway (M2/4 receptor)) we get a conformational change, a G-protein gets activated, GDP is released, GTP binds activating Adenylate cyclase and inhibits cAMP so protein kinases cannot be activated, and a decrease in phosphorolation of proteins. In the nodal cells of the heart, we can use this concept to slow the heart rate because we aren’t opening those ligated sodium channels, no ion flow in, no action potential. G-inhibitory subunits have 3 parts, alpha, beta, gamma. Beta and gamma can bind potassium channels to further lower the activity level of the cell so we slow down action potential rate.
Stimulatory
Stimulatory receptors (Gq (M1,3,5)), when ACh binds it stimulates the GPCR, GDP leaves, GTP binds activating the receptor leading to the activation of phospholipase-c which breaks down PIP2 into DAG which activates protein kinase C and IP3 which increases the Calcium outflow. Both of these will increase phosphorlyation of proteins, increasing the response and the inflow of ions.
In smooth muscle of GI tract, when ACh binds we depolarize the cell → increasing motility. So we can give an ACh lookalike or we can give something that inhibits ACh-esterase so it’s not broken down as fast, increases the levels of ACh indirectly
Direct Agonist (Ach look-a-likes)
These work directly at the nicotinic or muscarinic receptor. Acetylcholine is obviously a full agonist.
Nicotine is a full agonist at nicotinic receptors and has both central and peripheral effects. Central effects include arousal, increased mental performance, activation of the dopamine system (reward) so can be addictive. Peripheral effects are sympathmimetic because it activates the adrenals to release NE and Epi → increased BP and HR. When we’re trying to get people
Muscarine → hits muscarinic receptors (that’s how they were named), found in some poisonous mushrooms.
Bethanechol → nonselective muscarinic only lacks nicotinic actions; (heart, glands, smooth muscle, pupil). Used to treat urinary retention by stimulating the detrusor muscle. May also treat neurogenic atony like in toxic megacolon. ADRs include generalized cholinergic stimulation: DUMB BELSS (diarrhea, urination, miosis, bradycardia, Bronchiospasm, Excitation of nervous/muscles, lacrimation, Salivation, Sweating) and nausea
Methacholine → muscarinic only; M3 receptors (bronchioles). Methacholine can be use in bronchiole provocation test (testing for asthma, maybe COPD). We would see intense bronchospasm to decrease FEV to less than 1%
Pilocarpine → muscarinic only; M3 receptors and can cross the BBB. Not super selective. In the lacrimal or salivary glands to increase lacrimation/salivation which is useful in xerostomia and Sjogren’s. In both open angle and closure-angle Glaucoma, we have an issue draining our aqueous humor so we can give pilocarpine to pull the ciliaris to improve drainage into canal of Schlemm or constrict the pupil to decompress the canal of Schlemm. This reduces the intraoccular pressure. (Usually the acute treatment and last 4-8 hours). ADRs include blurred vision, night blindness, brow ache, diaphoesis, salivation. Counteracted by atropine
Carbachol → Both; not broken down by AChE. Has profound affects on the GI tract and cardiovascular system because it can cause the release of epi from the adrenal medullas. In Narrow angle Glaucoma, we have an issue draining our aqueous humor so we can give carbachol to pull the ciliaris to improve drainage into canal of Schlemm or constrict the pupil to decompress the canal of Schlemm. This reduces the intraoccular pressure. (Usually the acute treatment, most likely using pilocarpine). Not really used because it is nonselective. Few ADRs with ophthalmologic use.
Cevimeline → Stimulates salivation and lacrimation which is useful in xerostomia and Sjogren’s.
Indirect Agonist - Acetylcholine esterase inhibitors (AChE)
Work on the acetylcholine esterase to raise the levels of ACh indirectly → reversible. Ach accumulates in the synapse, ACh levels raise because nothing is getting broken down.
Endrophonium → Short acting (10-20 minute max) and cannot cross BBB (quaternary amine). Myasthenia Gravis an autoimmune attack against nicotinic receptors so patients cannot contract their muscles and develop weakness. We can utilize edrophonium to inhibit the ACh esterase to raise the levels of synaptic ACh and overcome antibodies that are bound to the receptors (raise our army → overcome the enemy). When we give edrophonium to help diagnose MG this is known a the tensilon test. Edrophonium is NOT used to treat, only diagnose. In the case of neuro-muscular blocking agents, such as tubocurarine, we block nicotinic receptors and inhibiting them, to reverse this competitively with edrophonium (can act in a small degree as an agonist). Edrophonium is used to distinguish myasthenic vs. cholinergic crisis. NO LONGER USED!
Physosigmine → Short acting (30 min - 2 hours); Tertiary amine (highly lipid soluble → penetrate BBB). Muscarinic action leads to GI muscle contraction, miosis, bradycardia, hypotension. Nicotinic action leads to muscle twitches, fasciculations, and at higher doses skeletal muscle paralysis. In the case of anti-cholinergic overdose (TCA, atropine, atypical antipsychotics) these are trying to block the ACh and decrease activity. We can give physostigmine to displace the antagonist and get more ACh into the system. ADRS: convulsions, bradycardia, accumulations of ACh may lead to skeletal muscle paralysis
Neostigmine → Acts for 2-4 hours; cannot cross BBB. Greater affect on skeletal muscle than physosigmine. GI motility/bladder contractility post-op/post-partum/DM (gastroparesis) → we can give neostigmine to increase motility. Myasthenia Gravis an autoimmune attack against nicotinic receptors so patients cannot contract their muscles and develop weakness. We can utilize neostigmine to inhibit the ACh esterase to raise the levels of synaptic ACh and overcome antibodies that are bound to the receptors (raise our army → overcome the enemy). Neostigmine has a shorter duration of action when compared to pyridostigmine. In the case of neuro-muscular blocking agents, such as tubocurarine, we block nicotinic receptors and inhibiting them, to reverse this competitively with neostigmine(can act in a small degree as an agonist). ADRs: DUMB BELSS (diarrhea, urination, miosis, bradycardia, Bronchiospasm, Excitation of nervous/muscles, lacrimation, Salivation, Sweating)
Pyridostigmine → Longer lasting when compared to Endroponium, physosigmin, neostigmine. Myasthenia Gravis an autoimmune attack against nicotinic receptors so patients cannot contract their muscles and develop weakness. We can utilize Pyridostigmine to inhibit the ACh esterase to raise the levels of synaptic ACh and overcome antibodies that are bound to the receptors (raise our army → overcome the enemy). Pyridostigmine has a longer duration so it may be better for chronic longterm management. Patients may be taking their pyridostigmine correctly but the symptoms are still getting worse, this could be due to myasthenia crisis (MG is progressing intense anitbody attacks and the drug isn’t enough) or a cholinergic (too much pyridostigmine so ACh are through the roof leading to excessive stimulation of the muscle → weakness). So you have to either have to gamble on which is occurring or we can give edrophonium. If edrophoium works and symptoms improve its a myasthenia crisis and we raise the dose of pyridostigmine, but if the weakness gets worse its a cholinergic crisis and we lower the dose of the pyridostigmine.
Donepezil → Tertiary amine (highly lipid soluble → penetrate BBB). In the CNS, the nucleus of meynut provides cortical stimulation which is important with cognitive function. In Alzheimer’s this pathway ACh levels are lower which decreases cognitive function. In these cases we can use Donepizel to inhibit the ACh-esterase, raising ACh levels improving function and memory → does not stop the Alzheimer’s just slows progression. Have not been shown to reduce healthcare costs or delay institutionalism. Can be used in combination memantine for moderate-severe disease. Side effects are mainly GI related.
Rivastigmine → Tertiary amine (highly lipid soluble → penetrate BBB). In the CNS, the nucleus of meynut provides cortical stimulation which is important with cognitive function. In Alzheimer’s this pathway ACh levels are lower which decreases cognitive function. In these cases we can use Rivastigmine to inhibit the ACh-esterase, rasing ACh levels improving function and memory → does not stop the Alzheimer’s just slows progression. Have not been shown to reduce healthcare costs or delay institutionalism. Can be used in combination memantine for moderate-severe disease. Side effects are mainly GI related.
Galantamine → reversible. In the CNS, the nucleus of meynut provides cortical stimulation which is important with cognitive function. In Alzheimer’s this pathway ACh levels are lower which decreases cognitive function. In these cases we can use galatamine to inhibit the ACh-esterase, rasing ACh levels improving function and memory → does not stop the Alzheimer’s just slows progression. Have not been shown to reduce healthcare costs or delay institutionalism. Can be used in combination memantine for moderate-severe disease. Side effects are mainly GI related.
Tacrine → eversible. In the CNS, the nucleus of meynut provides cortical stimulation which is important with cognitive function. In Alzheimer’s this pathway ACh levels are lower which decreases cognitive function. In these cases we can use tacrine to inhibit the ACh-esterase, rasing ACh levels improving function and memory → does not stop the Alzheimer’s just slows progression. Have not been shown to reduce healthcare costs or delay institutionalism. Can be used in combination memantine for moderate-severe disease. Not used anymore due hepatotoxicity.
Echothiophate → Similar to Sarin/pesticides its an organophosphate, lots of ADRs, IRREVERSIBLE inhibitors (long duration 1 week). Once our own enzymes breakdown the alkyl group that was added to the ACh-esterase the antidote no longer works (AGING). The only way to overcome this is to make more enzymes which takes time. When treating open-angle glaucoma, you can consider echothiophate → not a 1st draft pick and not used anymore. Actions include paralysis of motor function and convulsions. Can reverse atropine toxic ingestions if given fast enough. Organophosphates (insecticides) are irreversible AChE inhibitors. Potent biological warfare. Since we have increase synaptic ACh we see similar effects to that of a cholinergic crisis.
Adverse Effects of Agonist
If a patient has a cholinergic crisis (to much ACh) we ramp it up → increased miosis, increased lacrimation, increase salivation, intense drop of heart beat and CO → Hypotension, intense bronchiospasm leading to SOB and wheezing, Diarrhea, urinration increases, excessive skeletal muscle contraction (weakness), aggitation, restlessness, tremors, convulsions, increased sweating. However, if we undertreat a MG patient, you still are going to have weakness (myasthenic crisis). To reverse a cholingic crisis we can use atropine and parlidoxime. Pradlidoxime can reactivate AChE and atropine will displace the agonist and block muscarinic receptors. Diazepam can decrease convulsion and act as a supportive measure
DUMB BELSS (diarrhea, urination, miosis, bradycardia, Bronchiospasm, Excitation of nervous/muscles, lacrimation, Salivation, Sweating).
Naturally, some snakes have AChE inhibitors in their venom which can paralyze their prey. Organophosphates (insecticides) are irreversible AChE inhibitors. Potent biological warfare.
Antagonist
Cholinergic Physiology
Basal ganglia which are used to help us regulate movement processes. This mediated by a balance of dopamine and ACh.
Muscarinic receptors are present on the emetic center in the brain → trigger vomiting.
In the eye, muscarinic receptors are presents on the pupils to get them to constrict and on the ciliaris to contract increasing aqueous humor drainage.
In the lacrimal glands when the muscarinic receptors are activated we increase tear production (lacrimation).
In the salivary glands when the muscarinic receptors are activated we increase salivation.
When we stimulate VAGUS muscarinic receptors in the heart we slow the heart rate and decrease CO. In the lungs, we see bronchoconstriction (we’re resting, you don’t need that much air).
In the Liver, muscarinic activation can increase bile release.
In the GI tract you’d see increase motility and secretions.
In the skin, the muscarinic receptors lead to sweating.
In the bladder, the activation of muscarininc receptors leads to the contraction of detrusor muscle leading to urination.
Types of Muscarinic Antagonist → more clinically useful
The goal of these drugs is to block the reaction between ACh and muscarinic GPCR receptors.
M2/4 are inhibitory receptors when ACh binds these we decrease cAMP and causes K+ ion efflux. This leads to less cellular activation and a hyperpolarized cell. In the heart this is what causes a decrease HR and CO. Antagonist blunt these effects.
M1/3/5 is a stimulatory receptor so when ACh binds we increase cAMP and phosphorylation. This leads to secretion and contraction. In the bladder, these leads to an increase in contractility of the detrusor muscle that leads to urination. Antagonist blunt this effect.
No skeletal muscle effect because those are nicotinic receptors.
Tertiary Amines
Tertiary amines are lipophilic so it is easier for these to cross BBB.
Atropine
Nonselective. Generally last for 4 hours (unless we’re in the eye then its 4 days). High affinity for muscarinic receptors. Greatest effects are seen in bronchial tissue, salivary glands, sweat glands, heart. Metabolized by the liver and excrete in the urine.
Blocks the muscarinic receptors in the pupil and ciliaris. This will lead to pupil dilation and decreases the drainage of aqueous humor. This could be useful in retinal examination. Since we affect the drainage we can cause blurry vision and increase IOP. Be careful with glaucoma patients. Largely replaced due to long action time.
In the exocrine glands, we block lacrimation and salivation leading to dry eye and mouth. Useful in patients who may drool a lot or before intubation to make it easier. Salivary glands are extremely sensitive to atropine. Also blocks sweat glands → dangerous in children and elderly.
In the heart, at low doses we block M1 which will cause a slight drop in heart rate. At high doses we oppose the M2 receptor this will lead to an increase HR and CO. Useful in severe symptomatic bradycardia.
In the lungs, atropine causes bronchodilation → not the 1st draft pick.
Used as antispasmodic agents to relax GI tract.
We can use atropine as an antidote in a cholinergic crisis like in a organophosphate/sarin.
ADRs: dry mouth, sandy eyes, blurred vision, tachycardia, urinary retention, constipation, restlessness, confusion, hallucination, delirium. May progress to depression, collaspe of circulatory and respiratory systems → death. Geriatric patients are at a higher risk for CNS symptoms. Peds are also sensitive.
Scopolamine
Greater action on CNS and longer duration than atropine.
In the vestibular system (emetic center), we can use scopolamine to block the vomiting reflex. This is useful in motion sickness and post-op N/V. Blocks the M1 receptors in the brain. Can produce sedation at low doses and excitement at high doses (susceptible to abuse).
Used as antispasmodic agents to relax GI tract.
Similar ADRs to atropine.
Tropicamide and Cyclopentolate
Used as ophthalmic solutions for mydriasis and cycloplegia. Shorter duration than atropine.
Benztropine and Trihexypheidyl
In the basal ganglia, movement control is modulated by dopamine and Ach. In disease states (like Parkinson’s disease) where you have a massive drop in dopamine so now we have hella ACh. We can help with tremors, by blocking ACh from binding those receptors. Another indication is anti-psychotics which may produce extrapyramidal symptoms to alleviate those symptoms.
Oxybutynin, Tolteradine, Solfenacin, Darifenacin, Fesoterodine, Tropsium
Block M3 receptors in the bladder resulting in decreased intravesical pressure, increased bladder capacity, and reduced frequency of bladder contractions. Adverse effects are due to also blocking M3s in the eye, CNS, GI tract, and salivary glands. Darifenacin and Solifenacin are more selective. Half life is pretty long and is metabolized by CYP450s of the liver, except for tropsium which is hydrolyzed by the secondary liver pathway.
In the bladder, we can use these drugs to block muscarinic receptors to inhibit bladder contraction to alleviate some of the urinary incontenence/urgency/overactive bladder. Oxybutynin is more commonly used and has a transdermal patch form with less ADRs. In elderly patients use tropsium since its a quaternary amine that minimally crosses the BBB.
ADRs: dry mouth, constipation, blurred vision.
Diclyclomine, Hyoscyamine
We can use this in the treating diarrhea or GI tract spasms like in irritable bowel syndrome. This is going to block some of that GI motility which can alleviate symptoms.
Quaternary Amines
Hydrophilic so these have trouble penetrating that blood brain barrier.
Glycophyrrolate
Useful in patients who have a lot of drooling or respiratory secretions because we are blocking the muscarinic receptor from ACh. The goal is to inhibit these secretion. May be used pre-op or pre-intubation.
Ipratroprium, Tiotroprium
In the the smooth muscles of the bronchioles, ACh/muscarinic receptors lead to bronchoconstriction. This can lead to SOB especially in asthma and COPD. If we inhibit that bronchoconstriction, more air can get in the lungs. Ipratropium is more short acting, while tiotroprium is more long acting. These drugs are second line, therapeutic effects are specific due to inhalational application.
Ganglionic Blockers → block the 2 Chainz nicotinic receptor
Acts on both the parasympathetic and the sympathetic system with NO selectivity. Block the entire ANS.
Hexamethonium → originally designed to treat HTN. No longer used.
Anticholinergic Toxicity
This can be caused by antimuscarinic agent, tricyclic antidepressants, 1st generation antihistamine, antipsychotics, and the Bella Donna plant.
For the CNS, you’ll see an intense cognition change, AMS, delirium and rarely seizures.
In the eyes, you’ll see massive pupil dilating and blurry vision. Watch your patients with history of glaucoma.
In the glands, you’ll see intense dry eyes and mouth.
In the heart, you’ll see a massive increase and HR and CO (can lead to HTN)
In the lungs, you’ll see intense bronchodilation → not super important
In the skin, you’ll see no sweating → no evaporative cooling → fever
In the bladder, no bladder contractions leading to urinary retention. Watch your patients with BPH.
In the GI tract, you’ll see severe constipation. Watch your patients with history of SBO, ileus, or large bowl obstruction.
Okay team, how are we treating this → physostigmine (cholinergic agonist) and supportive care to allow for the drug to metabolize on its own.