organization_of_the_periodic_table
Unit 0 (*take picture)
Unit 1
Periodic Table of Elements
Overview
Organizes elements based on increasing atomic number.
Elements are categorized into groups (families) and periods.
Elements Groups
Groups/Families: Vertical columns.
Elements in the same group share similar chemical properties.
Periods: Horizontal rows.
Properties change significantly across a period.
Families of Elements
Family IA (Alkali Metals):
Properties: 1 valence electron, highly reactive, soft metals.
Example Elements: Lithium (Li), Sodium (Na), Potassium (K).
React violently with water.
Family IIA (Alkaline Earth Metals):
Properties: 2 valence electrons, not found uncombined in nature.
Example Elements: Magnesium (Mg), Calcium (Ca).
Transition Metals:
Include groups labeled B (e.g., iron, copper).
Good conductors of heat/electricity, typically colorful compounds.
Boron Family:
3 valence electrons, includes boron (metalloid) and aluminum (metal).
Carbon Family:
4 valence electrons, includes non-metal carbon crucial for organic life.
Nitrogen Family:
5 valence electrons, includes nitrogen (major atmospheric component).
Share electrons in bonding.
Oxygen Family:
6 valence electrons, oxygen highly reactive and abundant.
Halogen Family:
7 valence electrons, most reactive nonmetals.
Example Elements: Fluorine, Chlorine.
Noble Gases:
Full outer energy level, extremely unreactive.
Example Elements: Helium, Neon, Argon.
Properties of Elements
Metals:
Good conductors of heat and electricity, shiny, ductile, malleable, reactive especially with water (corrosion).
Non-Metals:
Poor conductors, dull, brittle in solid form, gases at room temperature.
Metalloids:
Properties intermediate between metals and non-metals; can be shiny or dull, ductile, and conduct electricity better than non-metals.
Special Categories
Lanthanides: Rare earth elements, used in high technology applications.
Actinides: Mostly man-made elements; includes radioactive elements.
Hydrogen
Unique, sits atop Family IA but is not a member.
Gas at room temperature, 1 valence electron, needs 2 electrons to fill its shell.

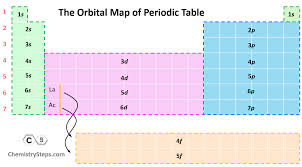
*Always go left to right
*Remember very top is BOTH 2s
*Pattern is : ___ S ___D ____ P
@ Rows on the very bottom are 4F and 5F
Day five
Types of Trends: Atomic Radii, Ionization Energy, electronegativity and Ionic Radii.
Describing Trend: property that either increases or decreases.
Group trends- changes from top to bottom.
Period trends-changes from left to right.
trends occur during the Coulombic Forces of attraction = Attraction of oppositely charged particles. Depends on size of the charges and *the distance between the charges.
(Basic three rules of Periodic trends)
1.Electrons are attracted to protons —>A. The closer the electron is to the nucleus(more strong attraction) B. The more protons, more strongly electrons get attracted to nucleus (called nuclear)
2.Electron ← → (Repelled) by another electron inside an atom. The tendency(Move in a particular direction) of electrons in the inner energy levels that blocks the attractions of the nucleus for the valence electrons(outer ring ) is known as the shielding effect.
Completed p sublevels are very stable(so example 2p ^ 6).Atoms prefer to add or subtract valence electrons(outer ring) to create complete p sublevels if possible
Atomic Radii is half the distance of two nuclei(center region of the nucleus )to each other.
distance from an atom’s nucleus to the outermost orbital of electron.
Large atoms are located on the periodic table on the left side and decrease when going to the right
larger atoms, the valence electrons are further from the pull of the nucleus.
Review
1. How did Mendeleev arrange the elements in the periodic table?
Mendeleev arranged the elements by increasing atomic mass, grouping them by similar chemical properties.
2. What prompted Mendeleev to leave gaps in the periodic table for unknown elements?
Mendeleev noted patterns in the properties of elements and predicted the existence of unknown elements based on gaps that indicated similar properties would exist for undiscovered elements.
3. How did Moseley arrange the elements in the periodic table?
Moseley rearranged the periodic table by increasing atomic number rather than atomic mass, resolving discrepancies in Mendeleev's organization.
4. Define period and group in the context of the periodic table.
Period: Horizontal rows where properties change progressively.
Group (or Family): Vertical columns where elements share similar chemical properties.
5. How do metals differ from nonmetals?
Metals are good conductors of heat/electricity, are malleable, ductile, and generally shiny. Nonmetals are poor conductors, often dull, and brittle in solid form, with most being gases at room temperature.
6. What is a metalloid, and where are they located?
Metalloids possess properties intermediate between metals and nonmetals; they are located along the zigzag line on the periodic table separating metals from nonmetals.
7. List one distinctive characteristic for each of the main groups on the periodic table.
Alkali Metals (Group 1): Highly reactive, with 1 valence electron.
Alkaline Earth Metals (Group 2): Not found uncombined in nature, with 2 valence electrons.
Transition Metals: Good conductors and typically colorful compounds.
Halogens (Group 17): Most reactive nonmetals with 7 valence electrons.
Noble Gases (Group 18): Full outer energy levels, extremely unreactive.
8. Classes of Elements:
francium: metal, alkali metal, solid
sulfur: nonmetal, gas
silicon: metalloid, solid
copper: metal, transition metal, solid
9. List the names of different orbital types.
s, p, d, f
10. What does the Pauli exclusion principle state about electrons?
The Pauli exclusion principle states that no two electrons in the same atom can have identical quantum numbers, limiting the number of electrons in an orbital to two, with opposite spins.
11. Number of orbitals and maximum electrons:
Orbital Number of Orbitals Number of Electrons | ||
3s | 1 | 2 |
4d | 5 | 10 |
6s | 1 | 2 |
3p | 3 | 6 |
4f | 7 | 14 |
5f | 7 | 14 |
2p | 3 | 6 |
3d | 5 | 10 |
2nd energy level | 4 | 8 |
4th energy level | 16 | 32 |
12. What is Hund's rule? Explain how it applies to an atom of nitrogen.
Hund's rule states that electrons will fill degenerate orbitals singly before pairing up. In nitrogen, which has 7 electrons, the 2p orbitals will each receive one electron before pairing occurs, resulting in three unpaired electrons.
13. Orbital diagrams and electron configurations:
Sulfur: 1s² 2s² 2p⁶ 3s² 3p⁴
Iron: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
14. Why does the 4s sublevel fill before the 3d sublevel?
The 4s sublevel fills before the 3d because it has a lower energy level than the 3d sublevel at the point when electrons are added to the respective orbitals.
15. Electron configurations corresponding to neutral atoms:
a. 1s²2s²2p⁶3s²: Magnesium (Mg)
b. 1s²2s²2p⁶3s²3p⁶: Argon (Ar)
c. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p³: Arsenic (As)
d. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d²: Zirconium (Zr)
16. Difference between an orbital diagram and an electron configuration:
An orbital diagram visually represents the orbitals and the distribution of electrons within them, showing unpaired and paired electrons, while an electron configuration provides a shorthand notation of the electrons in each orbital.
17. Not valid electron configurations:
1s²2s²2p⁶3s²3p⁶4s²4d¹⁰4p⁵ is not valid because it would require more than 8 electrons in the 4th energy level, violating the energy level capacity.
1s²2s²2p⁶3s³d⁵ is not valid because the 3d sublevel cannot accommodate additional electrons until the 4s sublevel has electrons assigned in accordance with the Aufbau principle.
19. Orbital blocks:
Nonmetals: p block
Metalloids: p block
Lanthanide series: f block
Actinide series: f block
Alkali metals: s block
Alkaline earth metals: s block
Halogens: p block
Transition metals: d block
Metals: d and s blocks
20. Noble-gas configurations:
Sodium: [Ne]3s¹
Iron: [Ar]4s²3d⁶
Sulfur: [Ne]3s²3p⁴
Uranium: [Rn]7s²6d⁴
Tin: [Kr]5s²4d¹⁰5p²
Iodine: [Kr]5s²4d¹⁰5p⁵
21. What are valence electrons? How is the number of valence electrons for a neutral atom determined?
Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. The number of valence electrons corresponds to the group number for elements in the main groups (1, 2, 13-18) of the periodic table.
22. Table for Group Number and Valence Electrons:
Group Number Valence Electrons | |
1 | 1 |
2 | 2 |
13 | 3 |
14 | 4 |
15 | 5 |
16 | 6 |
17 | 7 |
18 | 8 |
23. Predict the charge on stable ion:
Br: -1
Mg: +2
Cs: +1
N: -3
24. Why are the electron configurations of many ions isoelectronic with those of noble gases?
Many ions achieve a stable electron configuration that matches that of the nearest noble gas by losing or gaining electrons, thereby obtaining a full outer shell (octet).
25. Describe atomic radius and its overall trend.
Atomic radius is the distance from the nucleus to the outermost shell of electrons, which generally increases down a group and decreases across a period from left to right due to increasing nuclear charge.
26. Describe ionization energy and its overall trend.
Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period from left to right and decreases down a group due to increased distance between the nucleus and outer electrons.
27. What is the difference between atomic radius and ionic radius?
Atomic radius refers to the size of a neutral atom, while ionic radius is the size of an ion, which can differ based on whether the ion is positively charged (cations are smaller than their neutral atoms) or negatively charged (anions are larger than their neutral atoms).
28. Describe electronegativity and its overall trend.
Electronegativity is the ability of an atom to attract electrons in a bond. It generally increases across a period from left to right and decreases down a group due to increasing distance from the nucleus and increased shielding effect.
 Knowt
Knowt