Neoplasia: Chapter 5 Notes
Neoplasia: Chapter 5 Notes
Neoplasia= new growth
Terminology and Classification of Tumors
Naming Convention: Tumors are generally named and classified based on the cells and tissues from which they originate.
Nomenclature Variability: Tumor nomenclature is not entirely uniform, but certain generalizations can be made.
Exceptions to Naming Rules:
Lymphoid tumors.
Skin tumors arising from pigment-producing cells within the epidermis.
Certain tumors with mixed cellular components.
Specific types of tumors composed of primitive cells commonly found in children.
General Principles of Naming Tumors (Table 10-3):
Polyp, Papilloma: Any benign tumor that projects from surface epithelium.
Suffix "-oma": Indicates a benign tumor. The prefix usually denotes the primary tissue of origin (e.g., Lipoma for fatty tissue).
Carcinoma: A malignant tumor originating from surface, glandular, or parenchymal epithelium. This excludes tumors from endothelium or mesothelium.
Sarcoma: A malignant tumor arising from any primary tissue other than surface, glandular, and parenchymal epithelium (e.g., connective tissues, bone, muscle).
Leukemia: A neoplasm specifically involving blood cells.
Biochemistry of Cancer Cells
Loss of Contact Inhibition: Cancer cells lack the biological factor that inhibits growth leading to:
Continued division well beyond a single layer, forming clumps of overlapping cells.
Unlike normal mammalian cells, they do not exhibit anchorage dependence or density-dependent inhibition.
Differentiation and Adaptation:
They are generally less differentiated compared to normal cells.
More adapted to survive under unfavorable conditions, requiring less oxygen.
Cellular Organelles and Enzymes:
Contain fewer mitochondria.
Have less rough endoplasmic reticulum (RER).
Possess fewer specialized enzymes.
Immortality: Cancer cells can be grown indefinitely in culture, often referred to as being "immortal."
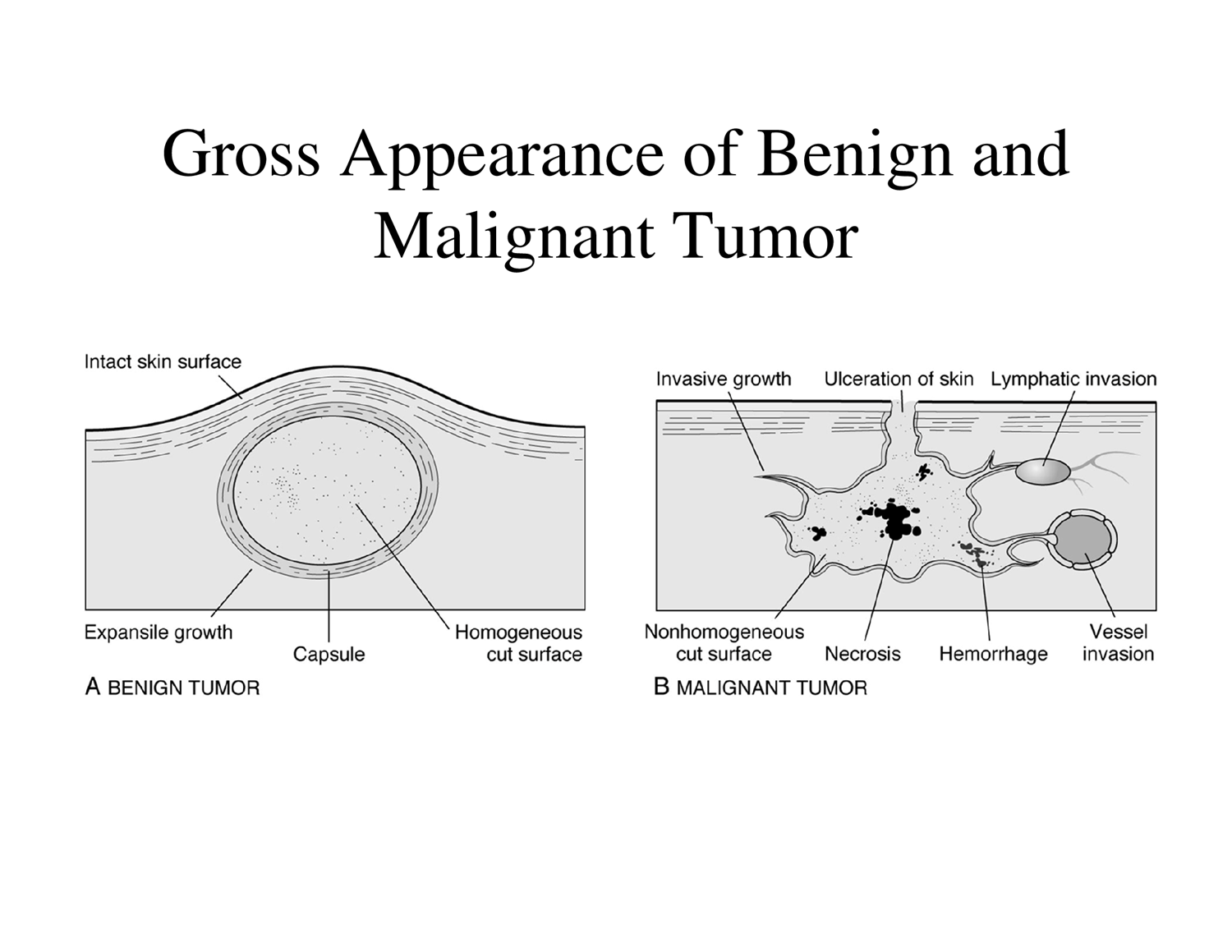
Gross Appearance of Benign and Malignant Tumors
Benign Tumor Characteristics:
Growth Pattern: Expansile growth, pushing against surrounding tissue.
Margin: Confined and well-demarcated.
Capsule: Often surrounded by a fibrous capsule.
Mobility: Usually movable within the tissue.
Cut Surface: Homogeneous appearance.
Malignant Tumor Characteristics:
Growth Pattern: Invasive growth, infiltrating into surrounding tissues.
Margin: Attached to surroundings, poorly demarcated.
Surface Manifestations: Can lead to ulceration of the skin.
Invasion: Frequent lymphatic and vessel invasion.
Cut Surface: Nonhomogeneous appearance due to areas of necrosis and hemorrhage.
Progression to Carcinoma in situ:
Normal Tissue \rightarrow Progressive Dysplasia \rightarrow Carcinoma in situ (non-invasive, confined to the epithelial layer) \rightarrow Poorly Differentiated Bronchial Carcinoma (invasive).
Adenocarcinoma: A malignant, gland-based tumor characterized by:
Loss of contact inhibition.
Anaplasia: cells lose their original shape and differentiation.
Metastasis and Angiogenesis
Metastasis: The process where cancer cells invade a blood vessel or lymph vessel and travel to another region of the body to form secondary tumors.
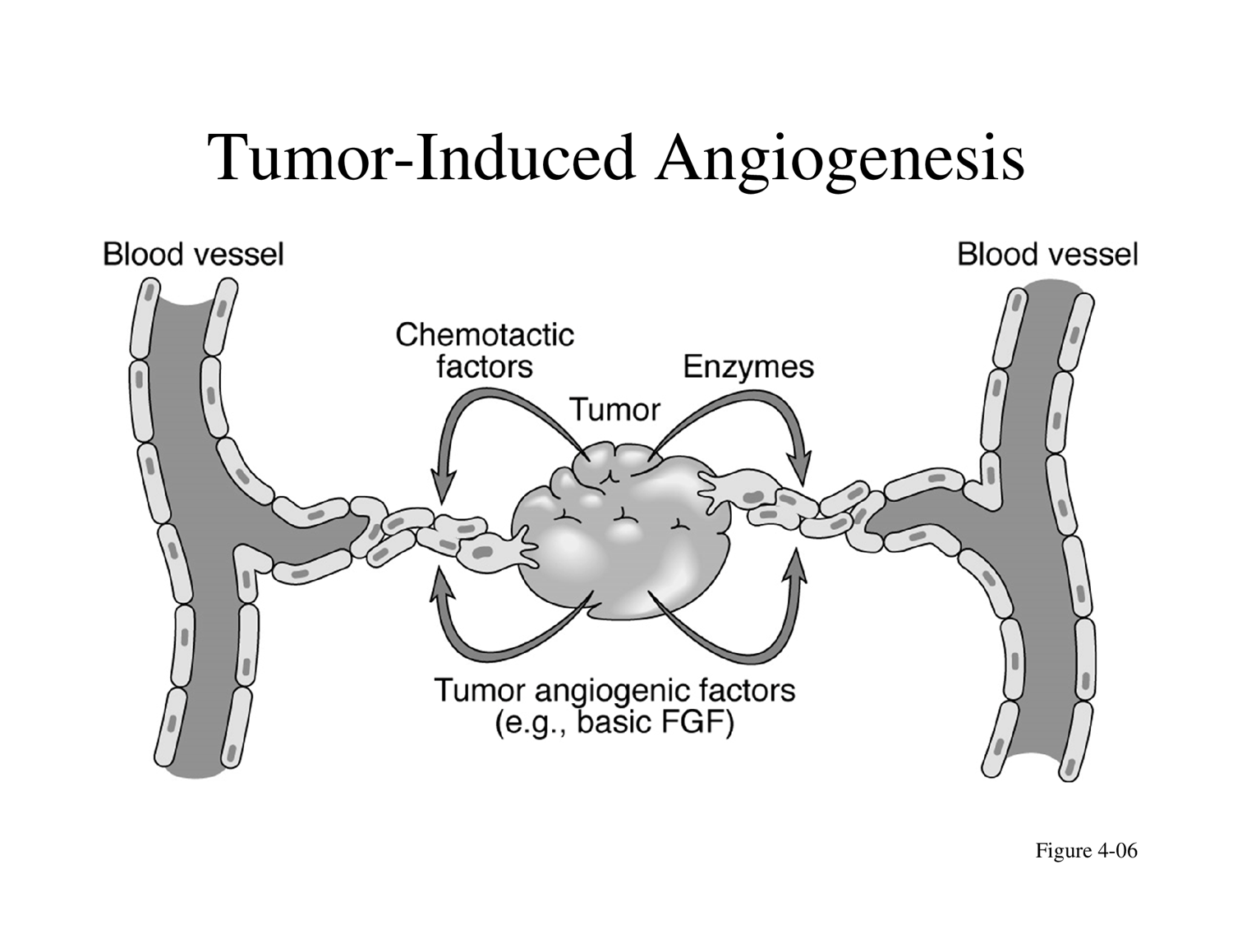
Tumor-Induced Angiogenesis:
Tumors stimulate the formation of new blood vessels from pre-existing ones to supply themselves with nutrients and oxygen.
This is achieved by releasing tumor angiogenic factors (e.g., basic FGF) and enzymes.
Chemotactic factors are also released, attracting blood vessels towards the tumor.
Specific Tumor Types
Lipoma: A common benign tumor composed of fatty tissue.
Tumors of Blood Cells and Lymphocytes:
Leukemia.
Lymphoma.
Multiple myeloma.
Tumors of Neural Cells: Neuroblastoma.
Tumors of Glial and Neural Supporting Cells:
Glioma.
Meningioma.
Germ Cell Tumors: Teratoma.
Blastomas: Tumors composed of embryonic cells, originating from embryonic primordia.
Retinoblastoma: Affects the eye.
Neuroblastoma: Arises from the adrenal medulla or immature neural cells.
Hepatoblastoma: Affects the liver.
Nephroblastoma: Affects the kidney.
Eponymic Tumors: Named after the physicians who first described them.
Hodgkin's disease.
Ewing's sarcoma.
Kaposi's sarcoma.
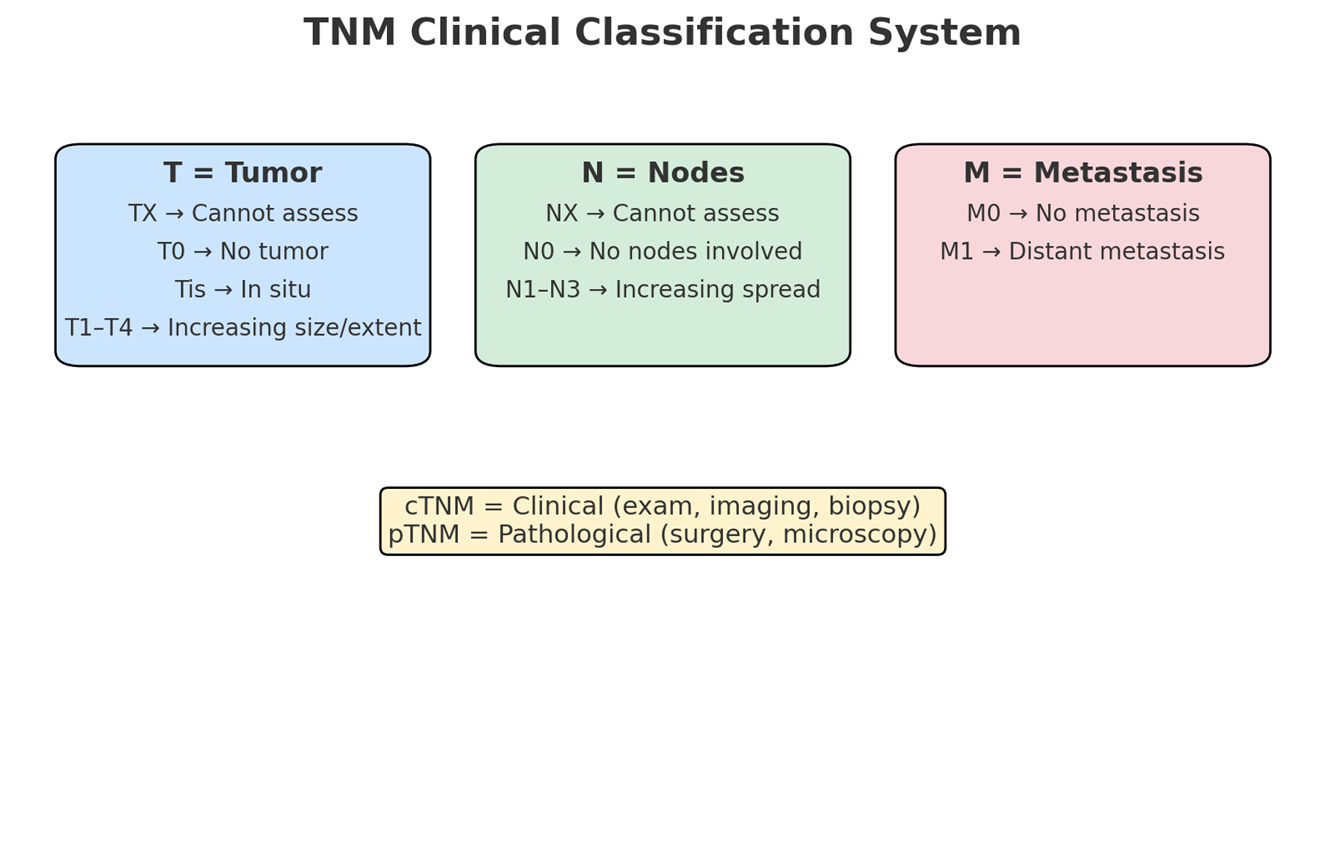
Tumor Grading and Staging (TNM Clinical Classification)
TNM System: A standardized system used to describe the extent of cancer, consisting of T (Primary Tumour), N (Regional Lymph Nodes), and M (Distant Metastasis).
T - Primary Tumour:
TX: Primary tumor cannot be assessed.
T0: No evidence of primary tumor.
Tis: Carcinoma in situ (non-invasive).
T1, T2, T3, T4: Indicate increasing size and/or local extent of the primary tumor.
N - Regional Lymph Nodes:
NX: Regional lymph nodes cannot be assessed.
N0: No regional lymph node metastasis.
N1, N2, N3: Indicate increasing involvement of regional lymph nodes.
Note: Direct extension of the primary tumor into lymph nodes is classified as lymph node metastasis (N). Metastasis in any lymph node other than regional ones is classified as a distant metastasis (M1).
M - Distant Metastasis:
M0: No distant metastasis.
M1: Distant metastasis is present.
Application of TNM system (Example):
A 30-year-old female with a breast tumor (2.0 cm) localized to the top left breast, spread to two adjacent lymph nodes, but no distant metastasis.
TNM Description: T2, N2, M0.
Types of TNM Information:
cTNM: Clinical classification based on examination, imaging, and biopsy.
pTNM: Pathological classification based on surgical findings and microscopic examination.
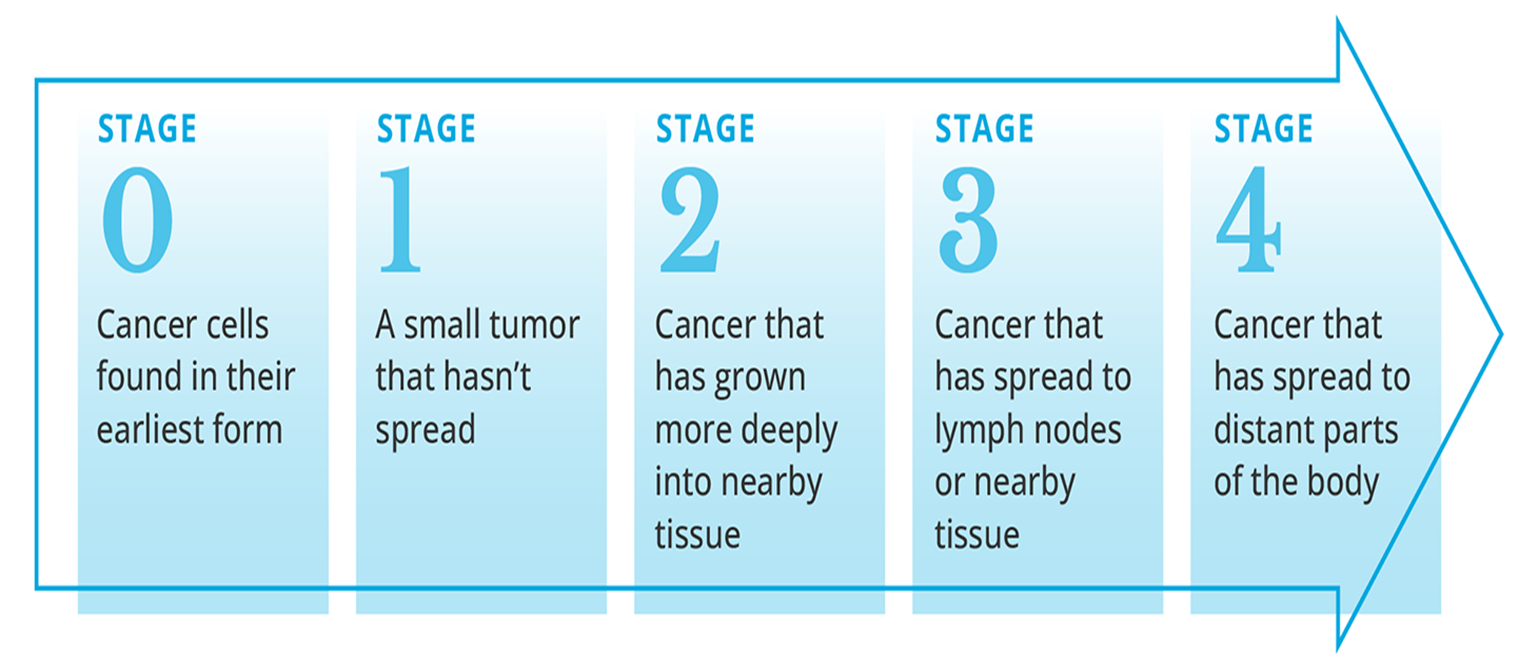
Cancer Staging
Stage 0: Cancer cells found in their earliest form, typically non-invasive (e.g., carcinoma in situ).
Stage 1: A small tumor that has not spread.
Stage 2: Cancer has grown more deeply into nearby tissue.
Stage 3: Cancer has spread to lymph nodes or nearby tissue.
Stage 4: Cancer has spread to distant parts of the body.
Lung Cancer: Staging and Treatment
Recommended Treatment Options: Surgery, Radiation therapy, Chemotherapy.
Causes of Cancer (Carcinogenesis)
Exogenous Causes:
Chemical Carcinogens: Polycyclic aromatic hydrocarbons, aromatic amines, nitrosamines, and heavy metals (lead & mercury).
Physical Carcinogens: Ultraviolet (UV) radiation, X-rays, and radioactivity (ionizing radiation).
Biologic Carcinogens: Certain viruses.
Endogenous Causes:
Oncogenes.
Tumor suppressor genes (mutation or inactivation).
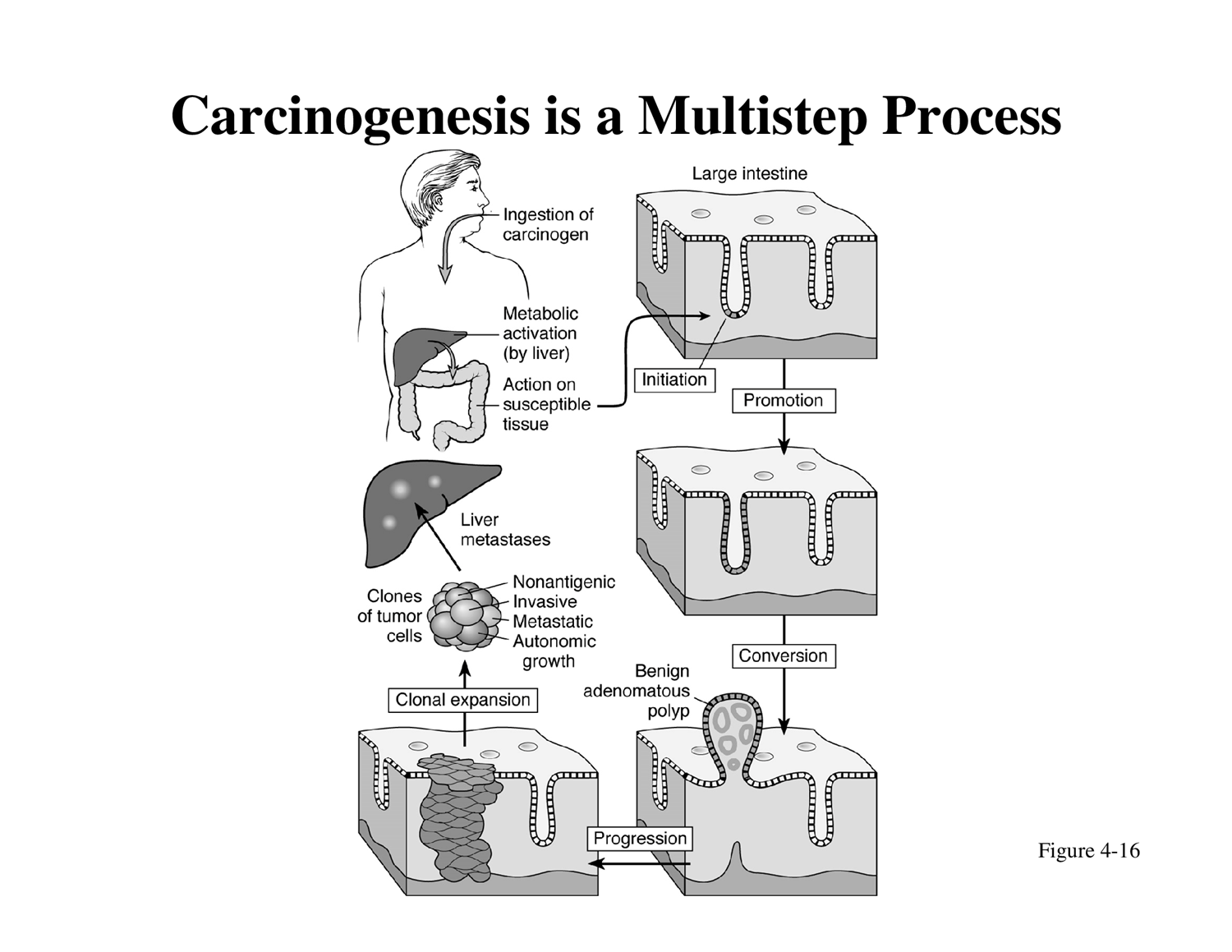
Chemical Carcinogenesis: A Multistep Process
Carcinogen Definition: A cancer-producing substance that can be breathed in or ingested.
Absorption and Activation: A carcinogen is absorbed either preformed or in the form of a pro-carcinogen, which is then metabolically transformed into an active carcinogen (e.g., by the liver).
Stepwise Action (Know These Steps!):
Initiation: The active carcinogen causes irreversible genetic damage (mutation) in a cell.
Promotion: Subsequent exposure to promoting agents stimulates the proliferation of the initiated cells, but this step is reversible.
Conversion: The initiated cells undergo further changes, becoming overtly malignant.
Progression: Further genetic alterations occur, leading to increased genetic instability, invasiveness, and metastatic potential.
Clonal Expansion: Rapid and uncontrolled proliferation of the malignant cells, forming a tumor and potentially leading to metastases (e.g., liver metastases). These cells exhibit non-antigenic properties, invasiveness, metastatic potential, and autonomic growth.
Role of UV Light in Cancer
Hazardous Exposure: UV radiation exposure from sources like tanning equipment is hazardous to health.
Acute Effects: Overexposure causes skin and eye burns.
Cumulative Effects: UV effects are cumulative over time.
Increased Risks: Greater risks are associated with early and repeated exposure.
Synergistic Effects: Certain drugs and cosmetics can increase UV effects.
Long-Term Consequences:
Premature skin aging.
Increased risk of various skin cancers:
Melanoma (the most dangerous skin cancer).
Basal cell carcinoma.
Squamous cell carcinoma.
Misconception Alert: Tanning beds are not a safe way to get Vitamin D.
DNA Damage and Repair by UV Light
UV Light Induces Damage: UV light can cause various types of DNA damage, including single-strand breaks, double-strand breaks, and cross-linking.
DNA Repair Enzymes: The body's DNA repair enzymes attempt to fix this damage.
Outcomes of Damage/Repair:
Normal: Successful repair restores DNA integrity.
Point Mutation: Inaccurate repair leads to a change in a single nucleotide.
Cancer: If damage is severe or repair mechanisms fail, mutations can accumulate, leading to uncontrolled cancer cell growth and tumor formation.
Ionizing Radiation: Damage by ionizing radiation (e.g., from uranium atoms) involves particles striking cell nuclei and damaging DNA, leading to uncontrolled division and cancer.
Action of Tumor Viruses (Human Carcinogenic DNA and RNA Viruses)
Mechanisms of Viral Carcinogenesis:
A. Integration (DNA Virus): DNA viruses (e.g., HPV) integrate their viral DNA into the host cell's genome, leading to malignant transformation.
B. Transduction (Acute-Transforming RNA Virus): RNA viruses can acquire a host cell proto-oncogene, incorporate it into their own viral genome, and then reintroduce it into other host cells, causing rapidly-developing malignant transformation.
C. Insertion of RNA Promoter Gene (Slow-Transforming RNA Virus): RNA viruses can insert a viral promoter gene near a host cell's oncogene. The viral promoter then activates the adjacent cellular oncogene, leading to malignant transformation over time.
Human Carcinogenic DNA Viruses:
Human Papilloma Virus (HPV).
Epstein-Barr Virus (EBV).
Hepatitis B Virus (HBV).
Human Carcinogenic RNA Virus:
Human T-cell Leukemia/Lymphoma Virus (HTLV-1).
HPV and Vaccination (GARDASIL):
Prevalence: Approximately 80\% of women will have had genital HPV by the age of 50.
GARDASIL Vaccine: Helps protect against 4 types of HPV (Types 6, 11, 16, 18).
Types 16 and 18 are responsible for 70\% of cervical cancer cases.
Types 6 and 11 cause 90\% of genital warts cases.
Target Age Group: Primarily for girls and young women aged 9 to 26, as clinical trials focused on this demographic due to early exposure rates.
Important Considerations:
Contraindicated for individuals allergic to vaccine ingredients.
Not recommended for pregnant women.
Does not treat existing cervical cancer or genital warts.
May not provide full protection against all types of cervical cancer; routine screenings remain essential.
Does not protect against other HPV types or diseases not caused by HPV.
Oncogenes and Tumor Suppressor Genes
Proto-oncogene: A normal gene whose protein product is essential for healthy cell growth and differentiation.
Oncogene: A mutated proto-oncogene whose product results in exaggerated or aberrant cell growth and differentiation, favoring cancer development.
Suppressor Gene (Tumor Suppressor Gene): Actively counteracts growth stimuli and protects cells against activated or newly acquired oncogenes.
Activation of Cellular Oncogenes
Proto-oncogenes can be converted into oncogenes through various mechanisms:
Point Mutation: A change in a single nucleotide base pair that alters the protein product, leading to an oncogenic protein.
Amplification: An increase in the number of copies of a proto-oncogene, resulting in the overproduction of a normal protein.
Chromosome Rearrangement: Translocations or inversions that can lead to:
Overproduction of a normal protein (e.g., by placing the gene under a strong promoter).
Creation of an oncogenic fusion protein.
Viral Gene Insertion: Integration of viral genes that can activate nearby proto-oncogenes, leading to an oncogenic protein.
Hypothetical Model of Cancer Growth
Cancer Development is generally driven by:
Activation of Oncogenes.
Inactivation, mutation, or loss of Anti-oncogenes (Tumor Suppressor Genes).
Example: Activation of MYC Oncogene in Burkitt's Lymphoma:
A chromosomal rearrangement occurs, specifically a translocation between chromosome 8 and chromosome 14.
The myc gene (a proto-oncogene) on chromosome 8 is moved adjacent to the immunoglobulin (Ig) gene on chromosome 14.
This leads to increased synthesis of the MYC protein, which promotes cell proliferation and contributes to Burkitt's lymphoma.
Tumor Suppressor Genes: p53 and BRCA
Function: Tumor suppressor genes protect cells against the effects of activated or newly acquired oncogenes.
Key Tumor Suppressor Genes:
p53: Often called the "guardian of the genome."
A nuclear phosphoprotein that functions as a transcription factor.
Mechanism: In response to DNA damage, p53 switches off DNA replication to allow time for DNA repair.
Apoptosis Induction: If DNA repair fails, p53 can trigger cell suicide (apoptosis) to eliminate damaged cells.
Role in Cancer: Mutations in p53 are considered primary events in the evolution of normal tissue to a cancerous state.
Prevalence: Found mutated in over 50\% of many known human cancers.
Hypothetical Model of p53 Involvement:
Normal Cells: DNA damage activates p53 \rightarrow Cell cycle arrest \rightarrow DNA repair and synthesis \rightarrow Proliferation.
p53 Mutant Cells / Impaired p53 Regulation: DNA damage leads to a continuation of the cell cycle despite damage \rightarrow Accumulation of mutations \rightarrow Leading to Cancer.
BRCA-1, 2, and 3:
These genes are primarily associated with hereditary breast and ovarian cancers.
Mutations in these genes often run in families, significantly increasing the risk for these cancers.
Cancer Treatment
Immunotherapy:
Leverages the patient's own immune system to fight cancer.
Mechanism (PD-1/PD-L1 Pathway):
Normal Immune Response: T lymphocytes identify and destroy tumor cells.
Tumor Camouflage: Some tumor cells express PD-L1 molecules, which bind to PD-1 receptors on T lymphocytes, deactivating them and making the tumor "invisible" to the immune system.
Inhibitor Drugs: New antibody-based drugs block the interaction between PD-1 on T cells and PD-L1 on tumor cells.
Result: Lymphocytes are freed from inhibition, regain their anti-cancer potential, recognize and reduce cancer.
Other Immunotherapy Approaches: Isolation of immune cells from the patient, culturing them with activators (PAP, GM-CSF) or genetically engineering cancer-specific T-cells, and then reinfusing them.
Radiation Therapy:
Uses high-energy radiation (e.g., X-rays, gamma rays, particle beams) to kill cancer cells and shrink tumors.
How it Works:
Direct Damage: Therapeutic beams directly inactivate DNA within cancer cells.
Indirect Damage: Radiation interacts with water molecules to produce free radicals (e.g., Auger electrons, singlet oxygen) which then damage DNA.
Targeting: Advanced techniques (e.g., using implanted transmitters and "sat nav" signals in prostate cancer) ensure radiation precisely hits the target while minimizing damage to healthy tissue.
Chemotherapy:
Involves the use of nonselective cytotoxic drugs.
Mechanism: These drugs target vital cellular machinery or metabolic pathways critical to the rapid growth and division of both malignant and normal cells.
Stops cell division/replication processes.
Alters cell structure to create non-dividing cells.
Induces spontaneous cell death (apoptosis).
Can prevent blood vessel growth (anti-angiogenic effects).
Goal: To eliminate enough tumor cells to achieve remission or control disease progression.
Clinical Manifestations of Cancer
General Symptoms:
Cachexia (severe wasting).
Loss of well-being.
Skin lesions.
Liver enlargement (hepatomegaly).
Splenomegaly (enlarged spleen).
Ascites (fluid accumulation in the abdomen).
Abdominal masses.
Bleeding (rectal, urinary tract, vaginal).
Thrombosis (blood clot formation).
Neurologic symptoms.
Obstruction of airways, digestive tube, or intestines.
Respiratory symptoms (dyspnea/shortness of breath, pneumonia)
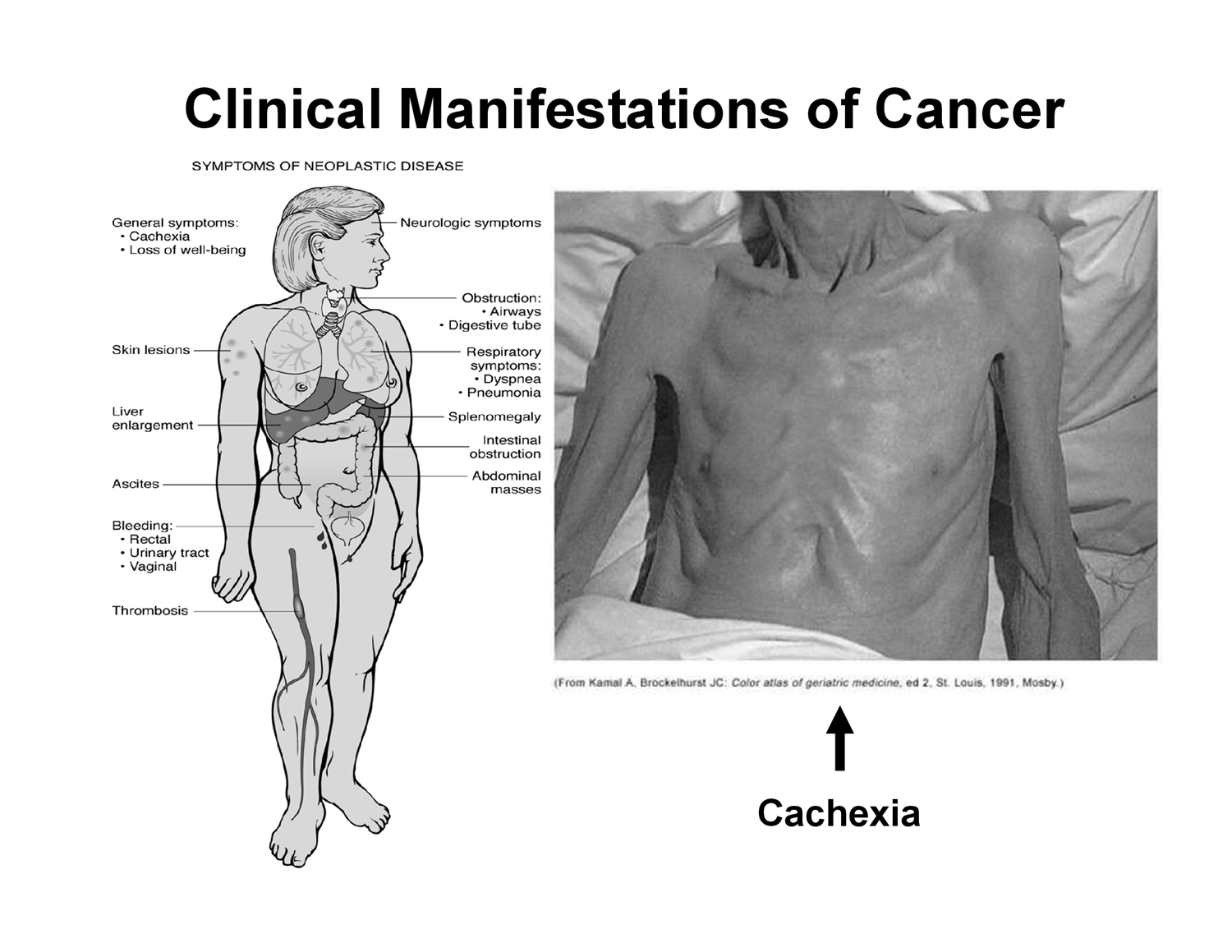
Syndrome of Cachexia (Hypermetabolic State):
The most severe form of malnutrition.
Present in 80\% of cancer patients at the time of death.
Cancer cells have spread and stole nutrition and ATP from normal cells. at this stage most patients don't eat
Terminal stage of cancer but can occur, essentially the malnutrition stage
Characteristics: Anorexia (loss of appetite), significant weight loss, anemia, asthenia (extreme weakness), taste alterations, and altered metabolism of proteins, lipids, and carbohydrates.