AP Bio Unit 1 Review
Properties of Water
Polarity: Water molecules exhibit polarity, with hydrogen atoms carrying a slight positive charge and the oxygen atom carrying a slight negative charge. This polarity is crucial for the formation of hydrogen bonds.
Hydrogen Bonding: The ability of water molecules to form hydrogen bonds results in unique properties such as high surface tension and the ability to dissolve many substances.
Cohesion and Adhesion: Cohesion refers to water molecules sticking to each other, which contributes to surface tension. Adhesion is the attraction of water molecules to other surfaces, facilitating processes like capillary action in plants.
Cohesion allows water to move up roots as water evaporates from leaves, hydrogen bonding pulls more water up
High Specific Heat: Water's high specific heat capacity allows it to absorb significant amounts of heat without a large change in temperature, which is vital for maintaining stable environmental conditions for organisms.
Universal Solvent: Water's ability to dissolve a wide range of polar and ionic substances makes it essential for biochemical reactions and cellular processes.
Ice Floats: The crystalline structure of ice makes it less dense than liquid water, allowing it to float and insulate aquatic environments during cold weather.
Mnemonic: Paul hides cats and ants holding umbrellas inside
Carbon: The Backbone of Life
Covalent Bonds: Carbon can form four covalent bonds, allowing it to create diverse and complex molecules essential for life.
Organic Molecules: Carbon is the fundamental building block of organic molecules, including carbohydrates, lipids, proteins, and nucleic acids.
Functional Groups: Various functional groups (e.g., hydroxyl, carboxyl, amino, phosphate) can attach to carbon chains, altering their chemical properties and reactivity.
Isomerism: Carbon compounds can exist as isomers, which are molecules with the same molecular formula but different structures, leading to different properties.
Macromolecules: Carbon's ability to form long chains and rings allows for the creation of macromolecules, which are essential for biological functions.
Biological Significance: The versatility of carbon is crucial for the complexity of life, enabling the formation of diverse biological structures and functions.
CHNOPS
Most common elements found in living organisms
Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, Sulfur
🧱 Macromolecules: Building Blocks of Life
Carbohydrates: Composed of monosaccharides (e.g., glucose), they provide energy and structural support.
Examples include starch (energy storage in plants), glycogen (energy storage in animals), cellulose (plant cell walls), and chitin (fungal cell walls and exoskeletons).
Contain C, H, O in a 1:2:1 ratio
Lipids: Made from fatty acids and glycerol, lipids store energy, provide insulation, and form cell membranes.
Examples include triglycerides (energy storage), phospholipids, and steroids (hormones).
Phospholipids are amphipathic with two hydrophobic tails made of fatty acids and a hydrophilic head
Ideal molecule for cell membrane structure, allows cells to compartmentalize their functions
Ex: Cholesterol, a hydrophobic molecule
Mainly contain C, H, O
H and O are typically in a ratio of greater than 2:1 (H:O)
Can also contain nitrogen, sulfur, and phosphorus
Proteins: Composed of amino acids, proteins serve as enzymes, structural components, and transport molecules (ion channels and active transport). Examples include enzymes (catalysts), hemoglobin (oxygen transport), and collagen (structural support).
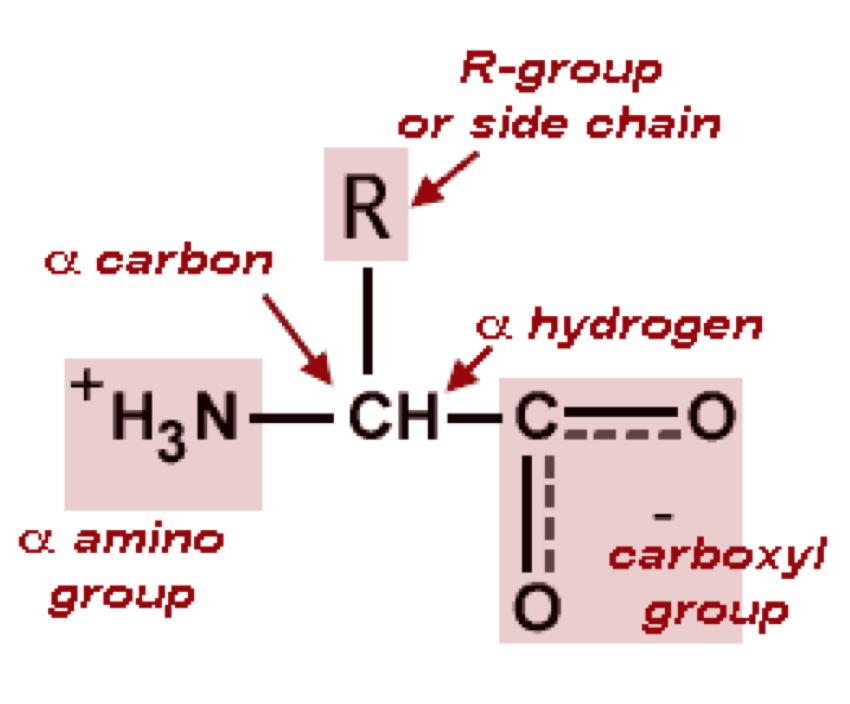
Amino acids are made up of a Carboxyl group and amino group
Contain C, H, O, N, and some contain S, ratio is not fixed
Nucleic Acids: Made from nucleotides, nucleic acids store and transmit genetic information. Key examples include DNA (genetic blueprint) and RNA (protein synthesis).
Contain C, H, O, N, and P, ratio varies
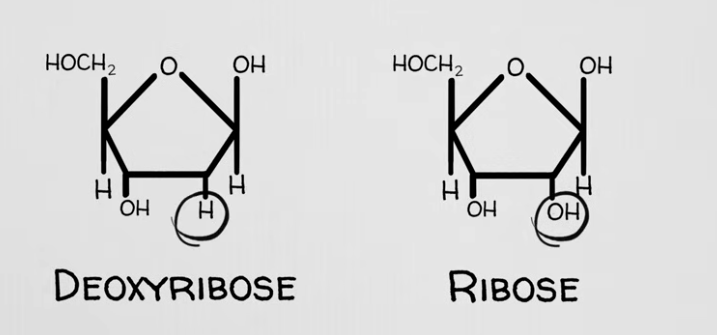
Sugars forming the sugar-phosphate backbone of DNA and RNA:
RNA can also form base pairs even though its single stranded because it must be able to act as a template to synthesize more
Dehydration Synthesis and Hydrolysis: Macromolecules are formed through dehydration synthesis (removal of water) and broken down by hydrolysis (addition of water).
Functional Diversity: Each class of macromolecule plays a unique role in biological systems, contributing to the complexity and functionality of living organisms.

Protein Structure
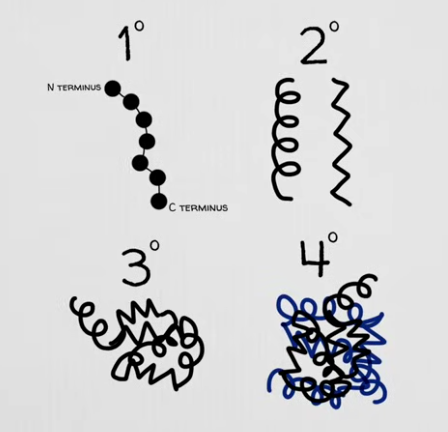
Primary Structure - sequence of amino acids linked together by peptide bonds (covalent bond between amino acids) to form a polypeptide chain
Formed during the process of translation at a ribosome
Secondary Structure - local folding patterns of the polypeptide chain, stabilized by hydrogen bonds between the backbone atoms
α-Helices (alpha helices) - A coiled structure where the polypeptide chain forms a right-handed spiral.
β-Pleated Sheets (beta sheets) - A sheet-like structure formed when two or more strands of the polypeptide chain align side by side and are held together by hydrogen bonds.
Tertiary Structure - three-dimensional shape of a single polypeptide chain, resulting from interactions between the amino acid side chains (R-groups).
Gives proteins their overall shape
Quaternary Structure - exists in proteins that consist of more than one polypeptide chain (more than one subunit)
subunits can interact through the same forces seen in tertiary structure (hydrophobic interactions, hydrogen bonds, ionic bonds, etc.)
Enzymes: Catalysts of Life
Catalytic Role: Enzymes are proteins that act as catalysts, speeding up chemical reactions without being consumed in the process.
Active Site: The specific region of the enzyme where the substrate binds, facilitating the conversion of substrates into products.
Induced Fit Model: The enzyme undergoes a conformational change upon substrate binding, enhancing the fit and increasing the likelihood of a reaction.
Factors Affecting Activity: Enzyme activity is influenced by temperature (higher temperatures can lead to denaturation), pH (each enzyme has an optimal pH), and substrate concentration (increased concentration can enhance reaction rates until saturation).
Enzyme Regulation: Enzymes can be regulated by inhibitors (which decrease activity) or activators (which increase activity), allowing for fine-tuning of metabolic pathways.
Biological Importance: Enzymes are crucial for metabolic processes, including digestion, energy production, and biosynthesis.
Energy and Metabolism
Metabolism: The sum of all chemical reactions occurring within an organism, encompassing both anabolic and catabolic pathways.
Anabolic Pathways: These pathways build complex molecules from simpler ones, such as in photosynthesis where glucose is synthesized from carbon dioxide and water.
Catabolic Pathways: These pathways break down complex molecules to release energy, such as in cellular respiration where glucose is oxidized to produce ATP.
ATP (Adenosine Triphosphate): The primary energy carrier in cells, ATP releases energy when hydrolyzed to ADP, driving various cellular processes.
Energy Transfer: The conversion of energy from one form to another is essential for maintaining cellular functions and supporting life.
Key Terms: Understanding terms like dehydration synthesis, hydrolysis, and enzyme activity is crucial for grasping metabolic processes.
Key Terms and Concepts
Dehydration Synthesis: A chemical reaction that forms bonds between molecules by removing water, crucial for macromolecule formation.
Take hydroxl from one molecule and hydrogen from the other to get a water molecule
Hydrolysis: A reaction that breaks bonds by adding water, essential for the breakdown of macromolecules.
Cohesion and Adhesion: Properties of water that allow it to stick to itself and other surfaces, important for biological processes.
Enzymes: Biological catalysts that speed up chemical reactions, vital for metabolism and cellular function.
Active Site: The specific region of an enzyme where substrate binding occurs, critical for enzyme function.
Denaturation: The loss of protein structure and function due to environmental factors like temperature or pH changes, affecting enzyme activity.
Functional Group: a specific group of atoms within a molecule that gives that molecule its characteristic chemical properties, essentially determining how it will react with other molecules and influencing its overall biological function
Ex: hydroxyl (-OH), amino (-NH2), carboxyl (-COOH), carbonyl (C=O), phosphate (-PO4), and sulfhydryl (-SH)
Trace elements: Essential elements required by organisms in very small amounts, such as iron (Fe), iodine (I), and zinc (Zn).
Nonpolar covalent bond: A type of covalent bond in which electrons are shared equally between two atoms, resulting in no significant charge difference across the molecule.
Polar covalent bond: A covalent bond in which electrons are shared unequally, leading to partial positive and negative charges on different ends of the molecule (e.g., water).
Cohesion: The attraction between molecules of the same substance, often due to hydrogen bonding, as seen in water molecules sticking together.
Adhesion: The attraction between molecules of different substances, which allows water to cling to surfaces like plant cell walls.
Capillary action: The movement of liquid in narrow spaces against gravity due to cohesion and adhesion, such as water traveling up plant roots.
Glycerol: A three-carbon molecule that serves as the backbone of lipids like triglycerides and phospholipids.
Organic compounds: Molecules primarily composed of carbon and hydrogen, often including oxygen, nitrogen, phosphorus, and sulfur, and found in living organisms (e.g., proteins, lipids, carbohydrates).
Inorganic compounds: Chemical compounds that do not contain carbon-hydrogen (C-H) bonds, such as water (H₂O), salts, and minerals.
Glycosidic linkage: A covalent bond formed between two monosaccharides during a dehydration reaction, creating disaccharides or polysaccharides.
Carboxyl group (-COOH): A functional group found in organic acids (e.g., amino acids and fatty acids) consisting of a carbonyl (C=O) and hydroxyl (-OH) group.
R-group: A variable chemical side chain in amino acids that determines the properties and function of the amino acid in a protein.
Side chain: Another term for the R-group in amino acids; it influences protein structure and function.
Dipeptide: A molecule consisting of two amino acids joined by a peptide bond.
Chaperone proteins (chaperonins): Proteins that assist in the proper folding and assembly of other proteins, preventing misfolding and aggregation.
Triglycerides: Lipids composed of one glycerol molecule and three fatty acids, serving as a major form of stored energy in organisms.
Phospholipids: A type of lipid that contains a glycerol backbone, two fatty acid tails (hydrophobic), and a phosphate group (hydrophilic), making up cell membranes.
Polyunsaturated: Refers to fatty acids with two or more double bonds in their hydrocarbon chain, often found in plant oils and considered healthy fats.
Amphipathic molecule: A molecule that has both hydrophobic (water-repelling) and hydrophilic (water-attracting) regions, such as phospholipids in cell membranes.