UNIT 3: Periodicity
Unit 3.1 The Periodic Table & Periodic Trends
3.1.1 The Periodic Table
Structure of the Periodic Table
The Periodic Table = A list of all known elements arranged in order of increasing atomic number (from 1 to 118)
Elements are in rows and columns
Atoms with the same # of shells are placed in the same row

Period = A row of elements
n = Period # = The outer energy level that is occupied by electrons
Group = Elements aligned vertically in columns
These elements share a similar outer-shell electron configuration
Valence Electrons = electrons in the outer shell
The Blocks of the Periodic Table
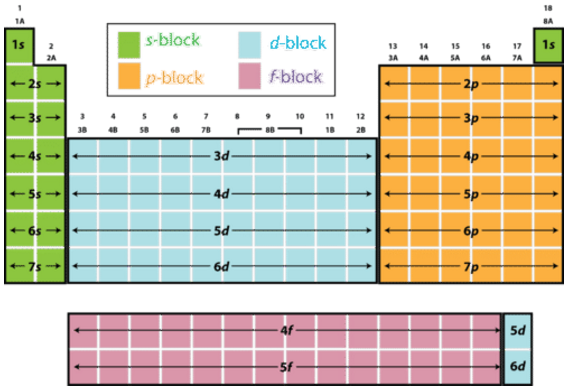
All elements are categorized into 4 main blocks
s-block = elements with only s electrons in the outer shell
p-block = elements with at least 1 p-electron in the outer shell
d-block = elements with at least 1 d-electron and at least 1 s-electron but NO f or p electrons in the outer shell
f-block = elements with at least 1 f-electron and at least 1 s-electron but NO d or p electrons in the outer shell
Deducing Electron Configurations
You can use the position of an element in the periodic table to help you figure out its electron configuration
ex. Germanium = [Ar] 3d104s24p2
Ge is in Group 4 → Tells you that there are 4 Valence Electrons
Ge is in Period 4 → Tells you that the 4 VE are in the 4th shell
The 2nd position in the p-block → Tells you that 2 electrons are in the p-subshell
3.1.2 Periodic Trends: Physical - Atomic & Ionic Radius
Atomic & Ionic Radius
Atomic Radius
Atomic Radius= the size of an atom = the distance between the nucleus of an atom and the outermost electron shell
Decreases across each period
Increases down each group
Electron Shell Theory
As you move across a period, the amount of protons increases, thus increasing the pull of the nuclei on the electrons, so the atoms become smaller
As you move down a group, more electrons are being added, so the radii increase
However, the outer electrons don’t feel the pull of the positive nuclei charge as much because the inner electrons repel them
Ionic Radius
Ionic Radius = the size of an ion
Trend down a group is the same as atomic radius: as the number of shells increases, the radius increases
However, the trend across a period depends on whether an ion is positive or negative
In general:
As the negative charge increases, the ionic radii increase
As the positive charge increases, the ionic radii decrease
Electron Shell Theory
Negative ions are formed when an atom accepts extra electrons which experience repulsion between the other valence electrons
So that causes a larger ionic radius
Positive ions are formed when an atom loses electrons
Since there are fewer electrons while the nuclear charge remains the same, each remaining electron feels the pull of the nucleus more strongly than before
Thus, the ionic radius decreases
3.1.3 Periodic Trends: Physical - Ionization Energy
First Ionization Energy
Ionization Energy (IE) = The amount of energy required to remove 1 mole of electrons from 1 mole of atoms (in the gaseous state) to form 1 mole of gaseous ions
Measured in kilojoules per mole (kJ mol-1)
First Ionization Energy = the amount of energy to remove the first mole of electrons
Ionization Energies: Trends
Increases across a period
Decreases down a group
Trends influenced by 4 factors:
Size of the Nuclear Charge
As the atomic number (# of protons) increases
→ the nuclear charge increases
→ greater attractive forces between positive nucleus and outer electrons
→ more energy is required to overcome these strong attractive forces when trying to remove an outer electron
Distance of Outer Electrons from the Nucleus
Electrons in shells further from the nucleus feel its pull less
→ So the further out electrons are, the lower ionization energy they have
Shielding Effect of Inner Electrons
= When the electrons in the inner shells repel those in the outer shells, so they don’t feel the attractive forces of the nucleus as much
The greater the shielding, the lower the ionization energy of the outer electrons
Spin-Pair Repulsion
Paired electrons in the same atomic orbital in a subshell repel each other more than electrons in different atomic orbitals, so it’s easier to remove an electron
Tip: This is the reason why the first ionization energy is always the lowest
Ionization Energy (IE) Across a Period
IE increases across a period because:
The nuclear charge across a period increases
The distance between the nucleus and outer electrons is the same
The shielding of the outer electrons by the inner is the same
Exceptions
IE decreases suddenly between the last element in one period and the first element in the next period because:
The distance between the nucleus and the outer electrons increases
The inner electrons’ shielding increases
These 2 factors outweigh the increased nuclear charge
IE slightly decreases between beryllium and boron since the 5th electron in boron is in the 2p subshell (which is further away from the nucleus than the 2s subshell of beryllium)
beryllium = 900 kJ mol-1
boron = 801 kJ mol-1
IE slightly decreases between nitrogen and oxygen because there is spin-pair repulsion in the 2p subshell of oxygen
nitrogen = 1402 kJ mol-1
oxygen = 1314 kJ mol-1
Ionization Energy (IE) Down a Group
As you go down a group, IE decreases because
The distance between the nucleus and the outer electrons increases
Electron shielding increases
The effective nuclear charge decreases as shielding increases
Successive Ionization Energies of an Element
Successive ionization energies (the amount of energy when you keep taking off electrons) increases because removing the next electron always takes more energy than the previous
In other words, it is harder to remove an electron from an already positive ion than a neutral atom
For several reasons:
As you remove more electrons, the shielding decreases, so the attractive forces increase along with an increased proton to electron ratio (so each electron feels the pull of the nucleus more and more)
Note: increases in successive ionization energy is NOT constant, but dependent on each atom’s electron configuration
3.1.4 Periodic Trends: Physical - Electron Affinity
Anions = negative ions formed when electrons gain electrons
Electron Affinity (EA) = The amount of energy released when 1 mole of electrons is gained by 1 mole of atoms of an element in the gaseous state to form 1 mole of gaseous ions
Think of it as the opposite of Ionization Energy
Measured in kilojoules per mole (kJ mol-1)
The periodicity of Electron Affinity has a similar pattern to Ionization Energies (but inverted)
The strongest “pull” on electrons → The greater amount of energy released when negative ions are formed
EA decreases down a group because:
As the atoms take on more electrons, the attraction for more decreases because the shielding increases and the effective nuclear charge decreases
3.1.5 Periodic Trends: Physical - Electronegativity
Electronegativity: Definition
Electronegativity = The ability of an atom to attract an electron pair in a covalent bond
Caused by the positive nucleus being able to pull the negative electrons closer
The Pauling Scale = A way to measure the value of electronegativity for each atom (on an arbitrary scale from 0.0 to 4.0)
 Electronegativity: Factors
Electronegativity: Factors
Nuclear Charge
The more positively charged protons in the nucleus, the more nuclear attraction between them and the negatively charged electrons
Thus, an increase in nuclear charge means an increase in electronegativity
Atomic Radius
Atomic Radius = The distance between the nucleus and valence electrons
As the atomic radius increases, the valence electrons are farther away, so they have a decreased attraction to the nucleus and less electronegativity
Electronegativity: Trends
Down a Group
Electronegativity decreases down a group
Although more protons are added and you would expect the electronegativity to increase with the increased nuclear attraction, this isn’t the case due to electron shielding:
As more electrons are being added, more shells are being filled, so more shells means more distance between the nucleus and valence electrons
In this case, the increased distance (that causes a decreased electronegativity) matters more than the increased nuclear charge (that would have caused an increase in electronegativity)
Across a Period
Electronegativity increases across a period
As you go across a period, the number of protons increases, while the shielding remains the same across a period, since no new shells are being added
Therefore, the increased nuclear charge matters more in this case
So the increased nuclear charge causes
Decrease in atomic radius (pulls electrons closer to nucleus)
Increase in attraction between nucleus and valence electrons
Increase in Electronegativity
3.1.6 Periodic Trends: Chemical
Metallic & Non-metallic Properties
Property | Metals | Non-metals |
|---|---|---|
Electron Arrangement | 1-3 valence electrons | 4-7 valence electrons |
Bonding | Metallic by loss of valence electrons | Covalent by sharing of valence electrons |
Electrical Conductivity | Good conductors | Poor conductors |
Type of Oxide | Basic (a few are amphoteric) | Acidic (Some are neutral) |
Reactions with Acids | Many react | None react |
Physical Characteristics | Malleable (can be bent and shaped) High melting and boiling point | Flaky/Brittle Low melting and boiling point |
What causes these different properties? Trends in:
Atomic Radius
Ionic Radius
Electron Affinity (EA)
Ionization Energy (IE)
Electronegativity (EN)
Low EN and low IE of metals allow their valence electrons to be able to move away from the nucleus or “delocalize”
High EN and high EA of non-metals allows for their electrons to be shared and form covalent bonds
Similarities in EN for the elements in the diagonal band separating metals from non-metals explains their behavior as" “metalloids”
Unit 3.2 Oxides, Group 1 & Group 17
3.2.1 Periodic Trends: Oxides Across a Period
Oxides Across a Period
Oxide = a binary compound that contains oxygen and another element
ex. Carbon dioxide CO2
Since oxides are acid-base they show their chemical trend: they change from basic to amphoteric to acidic as you move across a period
Amphoteric = having the ability to react chemically as either an acid or a base
ex. Aluminum oxide (so it can react with an acid like HCl or with a base like NaOH)
Period 3 Oxides
Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO2 and SO3 |
|---|---|---|---|---|---|
Basic | Basic | Amphoteric | Acidic | Acidic | Acidic |
The reason for the different acidic or basic natures is because of their structure, bonding, and electronegativity
Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO2 and SO3 | |
|---|---|---|---|---|---|---|
Basic | Basic | Amphoteric | Acidic | Acidic | Acidic | |
Structure | Giant Ionic | Giant Ionic | Giant Ionic | Giant Covalent | Simple Molecular | Simple Molecular |
Bonding | Ionic | Ionic | Ionic/Covalent | Covalent | Covalent | Covalent |
Na | Mg | Al | Si | P | S | Cl | O | |
|---|---|---|---|---|---|---|---|---|
Electronegativity | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 3.5 |
Electrons will be transferred to oxygen when forming oxides and providing an ionic bond because of the highest difference in electronegativity between oxygen and Na/Mg/Al
On the other hand, Si, P, and S (elements with the least difference in EN) will share the electrons with oxygen to form covalently bonded oxides
3.2.2 Periodic Trends: Group 1 - The Alkali Metals

Group 1 Metals = “Alkali Metals” = metals that form alkaline solutions with high pH when they react with water
All elements in group 1 end with the electron configuration: ns1
Physical Properties
Soft and easy to cut (more so as you move down the group)
Shiny
Conduct heat/electricity
Low melting points (decreases more going down the group since atomic radius increases so metallic bonding gets weaker)
Low Density
Chemical Properties
Reactive with oxygen and water in the air
Often kept under oil to prevent reactions
Highly reactive with water
And produces an alkaline metal hydroxide solution and hydrogen gas
3.2.3 Periodic Trends: Group 17 - The Halogens
The Halogens

Group 17 non-metals
Poisonous
Diatomic = forming molecules of 2 atoms
Have 7 valence electrons
Form halide ions by gaining one more electron to complete the octet rule
Halogen | Physical State @Room Temp | Color (In solution) |
|---|---|---|
Fluorine | Gas | Yellow |
Chlorine | Gas | Pale Green (Green-Blue) |
Bromine | Liquid | Red-Brown (Orange) |
Iodine | Solid | Black (Dark Brown) |
Physical Properties Trends (Down the Group)
Density, Melting/Boiling Points of the halogens increase going down the group
Reactivity decreases down the group
Why?
Halogens electron configurations all end in ns2np5
When the 7 valence electrons react, they need one more to have a full shell
Electron Affinity decreases
Atomic Radius increases
→ # of Shells increases
→ Shielding increases
→ Distance to nucleus increases
→ Weaker electrostatic forces to attract the 8th electron
Therefore, as you go down the group, the reactivity decreases (because it is harder to attract the 8th electron)
Reaction of Halogens with Halid Ions in Displacement Reactions
Halogen Displacement = When a more reactive halogen displaces a less reactive halogen from an aqueous solution of its halide
Halogen Displacement Reactions
Chlorine and Bromine
Chlorine + colorless potassium bromide = bromine (Orange Solution)
Since chlorine is above bromine in group 17, it is more reactive
So chlorine will displace bromine
2KBr (aq) + Cl2 (aq) → 2KCl (aq) + Br2(aq)
potassium bromide + chlorine → potassium chloride + bromine
Bromine and Iodine
Since bromine is higher than iodine in group 17, it is more reactive
Thus, bromine will displace iodine
Br2 (l) + 2NaI (aq) → 2NaBr (aq) + I2 (aq)
bromine + sodium Iodide → sodium bromide + iodine