Chapter 22- Alkanes
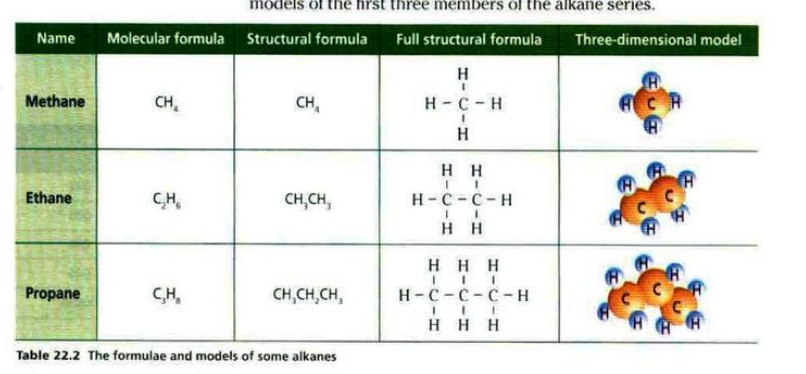
Alkanes are hydrocarbons that contain only single covalent bonds between carbon atoms.
They have the general formula: C
nH2n+2A structural formula is the formula which shows how atoms are arranged in a molecule.
Alkanes are saturated hydrocarbons because they contain only single carbon-carbon bond.
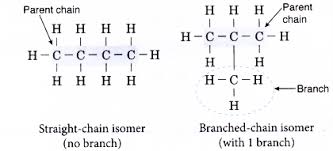
ISOMERISM
Compounds that have the same molecular formula but different structural formula are isomers.
Branched alkyl groups are isomers of alkanes.
PROPERTIES OF ALKANES
- As alkane molecules get bigger, the attractive forces become stronger.
- Melting and boiling points, and density of alkanes increases as their molecular sizes increases.
- Viscosity also increases as it becomes difficult to pour out bigger molecule chains.
- The flammability decreases as the carbon chains become longer.
- Complete combustion of alkanes produces carbon dioxide and water while incomplete combustion produces carbon monoxide and water.
CRACKING
- Longer chains of alkanes are broken down to smaller chains of hydrocarbons which are more in demand. This process is cracking.
SUBSTITUTION REACTIONS
Alkanes are generally unreactive but they react with halogens.
One mole of chlorine substitutes one hydrogen atom. Another product of hydrogen chloride is formed.
