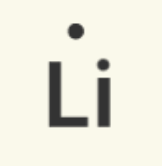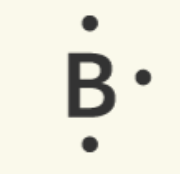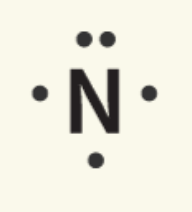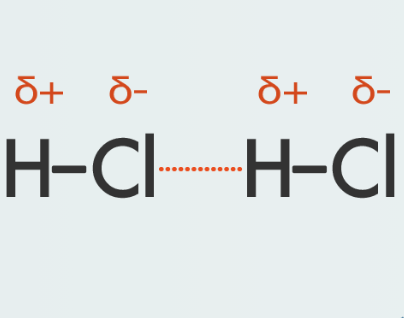FLVS Chemistry: Module 3 Notes (w/ Flashcards)
03.01 Valence Electrons:
Chemical Bonding:
According to the octet rule, the atoms of all elements have between one and eight electrons in their outermost, or valence, shell.
The most chemically : of all the elements are the noble gases.
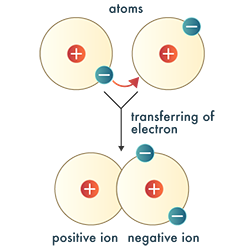
The atoms of other elements can lose, gain, or even share electrons to achieve the stable configuration of eight electrons in their valence shell. This transfer of electrons is what we know today as a chemical reaction and results in bonds between atoms, forming chemical compounds.
Valence Electron: an electron in the outermost shell of an atom that can be lost to or shared with another atom
Ionic bonds | Covalent bonds |
An ionic bond is a chemical bond that results from the electrostatic attraction between positive and negative ions.
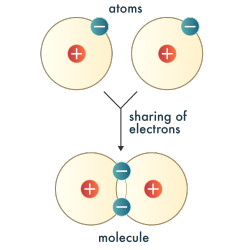
| In a covalent bond, electrons are shared between two atoms, neither atom completely gaining or losing electrons.
|
What’s the Valence?:
The outermost, or valence shell, has the highest energy level that contains electrons in an atom.
Element | Electron Configuration | Valence Electrons | Visuals |
Beryllium | 1s22s2 | 2 |
|
Nitrogen | 1s22s22p3 | 5 |
|
Magnesium | 1s22s22p63s2 | 2 |
|
Lewis Dot Notation:
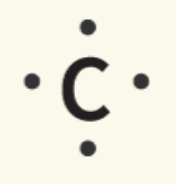
Lewis dot notation is often used to represent the valence electrons of an atom. This notation uses the symbol of the element, surrounded by dots.
Element | Lewis Dot Notation |
Carbon |
|
Lithium |
|
Boron |
|
Nitrogen |
|
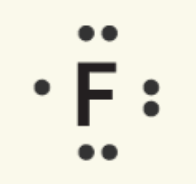
Fluorine |
|
Eight is the Magic:
According to the octet rule, atoms for elements in the main element groups prefer to have up to eight valence electrons.
An element is inert if the valence electrons equal eight, meaning that it doesn't react with other substances.
Chemical Reactivity:
Valence electrons are often found in the s and p sublevels of the outermost energy level in an atom.
03.02 Nomenclature:
Nomenclature: the devising or choosing of names for things, especially in a science or other discipline
Naming Ionic Compounds:
Binary Ionic Compound: a compound composed of two different elements that bond due to electrostatic attraction and the transfer of electrons
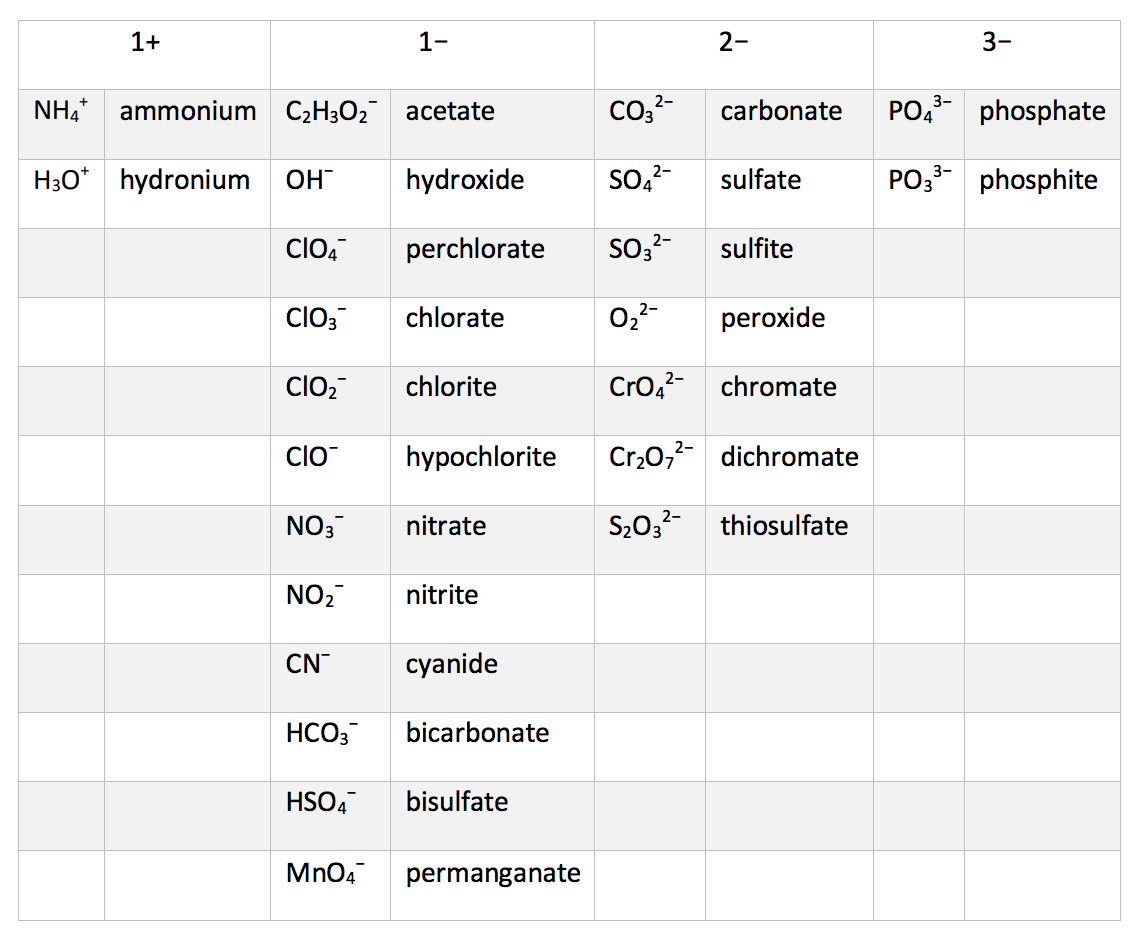
Polyatomic Ions: a charged group of covalently bonded atoms
Naming Binary Ions:
The first element is always the metal (cation). Do not change the ending.
The second element is always the non-metal (anion). Change the ending to of the element to-ide.
Naming Polyatomic Ions:
The first ion is always the metal or a polyatomic cation. Do not change the ending.
The second ion is always the non-metal or polyatomic anion. Change the ending to -ide or use the polyatomic ion chart to determine the ending.
Naming Covalent Compounds:
Subscript Number | Prefix |
1 | mono- |
2 | di- |
3 | tri- |
4 | tetra- |
5 | penta- |
6 | hexa- |
7 | hepta- |
8 | octa- |
9 | nona- |
10 | deca- |
Steps to Naming:
Add a prefix to the name of the first element to represent the number of atoms of the element in the bond (needed if there is more than one).
Add a prefix to represent the number of atoms of the second element to the root of the second element's name.
Add -ide to the last element.
03.03 Ionic Bonding:
Limiting factors of ionic compounds:
There are a limited number of elements available on the periodic table to use in compounds.
Some elements are less reactive than others or do not react at all (e.g., transition metals and noble gases)
Some element pairs cannot react (e.g. lithium will not react with sodium, both are metals)
Ionic Charges:
Positive Ions–Cations | Neutral Atoms | Negative Ions–Anions |
In an atom that loses electrons, protons outnumber the electrons and the atom becomes a positively charged cation. | Atoms are neutral when the numbers of protons and electrons are equal and their charges balance each other. | If the atom gains electrons, the electrons outnumber the protons and form a negatively charged anion. |
Metals have a positive charge equal to the number of valence electrons they lose, whereas nonmetals have a negative charge equal to the number of electrons they need to fill their valence shell.
Most elements in taller columns have set charges; however, the charge of elements in the fourth main group depends on the bond they form with other elements.
Elements, in groups 3 through 12, do not have common charges listed on the periodic table.
Formula Units:
Chemical Formula: a formula giving the number of atoms of each of the elements present in one molecule of a compound
Formula Unit: the formula of an ionic compound that represents the simplest whole number ratio of ions in the compound, not the actual number of each ion present in the crystal
Steps to Write Chemical Formulas for Binary Ionic Compounds:
Identify the charge of each of the ions in the compound.
Write the cation or metal first, followed by the anion or nonmetal.
Determine the ratio of positive to negative ions needed to make the compound neutral (a net charge of zero). You can use the "crossing the charges" method, which means the number of the charge (not the sign) of the cation becomes the subscript of the anion, and vice versa.
Make sure the subscripts represent the lowest whole number ratio for the ionic compound.
Check your work: Make sure the net charge of the formula is zero. Take the charge of each ion multiplied by its subscript, and then add them together.
Polyatomic ions are a group of atoms covalently bonded but with an overall positive or negative charge. These ions can bond with metals, nonmetals, and other polyatomic ions.
Within chemical formulas, polyatomic ions are sometimes enclosed by parentheses. The parentheses are needed if there is more than one polyatomic ion in the formula unit.
03.04 Covalent Bonding:
Covalent Bonds:
Nonmetal atoms share electrons by overlapping half-filled orbitals from their valence shells. This gives both atoms in the covalent bond a full valence shell of eight electrons.
Elements with a full valence shell are more stable.
The gases, liquids, and organic solids around us do not have repeating lattices. The gases, liquids, and organic solids around us are discrete molecules structured around covalent bonds.
Covalent bonds occur when two nonmetal atoms share electrons to achieve a stable configuration of the noble gases.
If two hydrogen atoms come close together, the electron of each atom is attracted to the positively charged nucleus of the other. The atomic orbitals begin to merge, and the two nuclei share the two electrons, each achieving a stable configuration with two electrons in the first principle energy level.
On paper, we represent each valence electron with a dot; a shared electron pair is shown as a horizontal line.
Double covalent bonds involve two shared pairs of electrons.
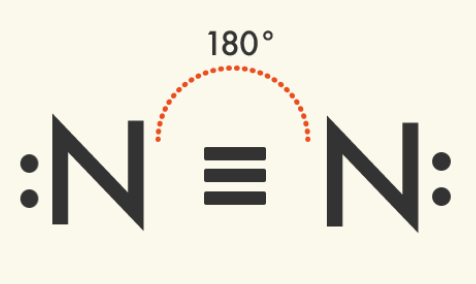
Triple bonds involve three shared pairs of electrons.
Single, Double, and Triple:
Diatomic Molecule: a molecule composed of only two atoms of the same or different chemical elements.
Single bonds | Double bonds | Triple bonds |
Hydrogen has one electron and only needs one additional electron to fill its valence shell. Therefore, each hydrogen atom shares its electron with the other to form a single bond in H2. | Each oxygen atom needs two additional electrons to fill its valence shell. Two oxygen atoms form a double bond. | Each nitrogen atom can share three electrons with another nitrogen atom to fill their valence shells with eight electrons. This means there are a total of six shared electrons to form a triple bond in the N2 molecule. |
When more electrons are shared between the two atoms, the bond is stronger and the atoms are pulled closer together, making the bond shorter.
Molecular Lewis Structure:
The number of covalent bonds an atom can form is usually equal to the number of electrons it needs to fill its valence shell.
To form a covalent bond, each element shares at least one valence electron. Elements that can form more than one bond do so by making multiple single bonds, a double bond, or a triple bond.
Element | H | C | N | O | F |
Symbol |
|
|
|
|
|
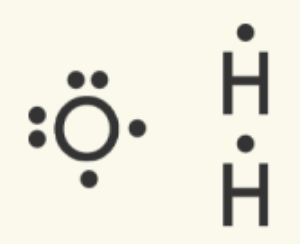
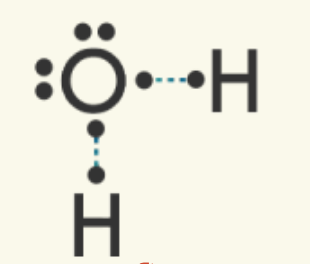
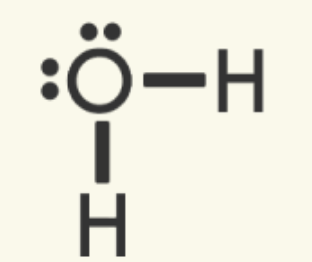
Let's determine the Lewis dot structure for H2O (water).
Step 1 | Step 2 | Step 3 |
Two hydrogen atoms and one oxygen atoms are present. Draw the dot structure for all three atoms to model their valence electrons. Determine how many electrons are needed to satisfy the octet rule for each atom. Here, the oxygen atom needs two electrons to reach eight, and each hydrogen needs one (remember, hydrogen only has the s orbital, which is filled with two electrons). | Oxygen can make the most bonds (needs more electrons) out of the two atoms present, so it will be the central atom. One oxygen atom can share a valence electron with each of the two hydrogen atoms. The electrons match up to make pairs (two electrons).
| A shared electron pair is represented by a line. One line is a single covalent bond. The other valence electrons pairs are called nonbonded, unshared, or lone pair electrons.
|
Covalent vs. Ionic Bonds:
Ionic Bonds | Covalent Bonds | |
Bonding | Transfer of Electrons | Sharing of Electrons |
Elements within Bond | Metals with Nonmetals | Nonmetals with Nonmetals |
Dissolve in Water | Yes | Varies |
Compound Consistency | Brittle | Soft |
Melting and Boiling Point | High | Low |
03.05 Molecular Structure:
VSEPR Theory:
Valence shell electron pair repulsion (VSEPR) theory is used to predict the geometry and shape of a molecule, which plays an important role in molecular interactions. This theory is based on the concept that valence electron pairs, whether bonded or non-bonded (lone pairs), repel one another. Lone pairs and bonded pairs will spread out and position themselves around the central atom so they are as far away from each other as possible.
In the molecular formula CH4:
Carbon has four electrons in its valence shell; hydrogen has one.
Carbon can share each of its valence electrons with one hydrogen atom.
All five atoms achieve a stable noble gas configuration, the carbon with eight electrons in its valence shell and the hydrogen with two electrons.
The bonding electron pairs have like charges and repel each other.They adopt a molecular arrangement that minimizes this repulsion. This gives the methane molecule the shape of a four-sided pyramid, a tetrahedron.
The negatively charged electron pairs are as far apart from one another while staying as close as possible to the positively charged nucleus of the central carbon atom; this theory is called the valence shell electron pair repulsion theory, or VSEPR for short.
In the formula H2O:
The shape of a water molecule is determined by the repulsion of all its electron pairs, including the oxygen atoms' unshared pairs of electrons.
This shape is called bent triatomic.
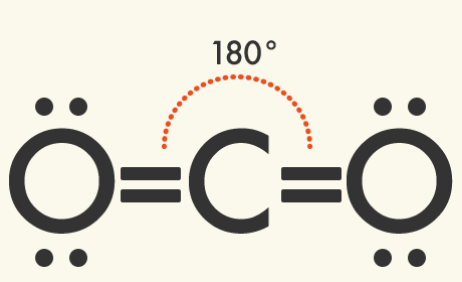
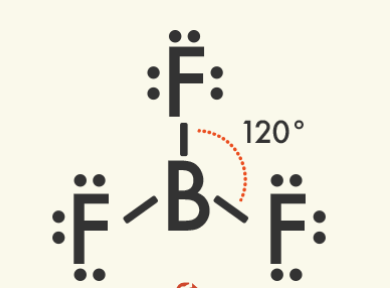
Some other commonly occurring molecular shapes include the linear triatomic structure of carbon dioxide (CO2), the trigonal planar structure of boron trifluoride (BF3), and the pyramidal structure of ammonia (NH4).
Shapes of Molecules:
The number of domains is the sum of bonded atoms and lone pairs of electrons surrounding the central atom. This total determines the shape of a molecule.
Lewis dot structures can be used to determine both VSEPR geometry and molecular shape.
With 0 lone pairs, the VSEPR geometry and molecular shape are the same.
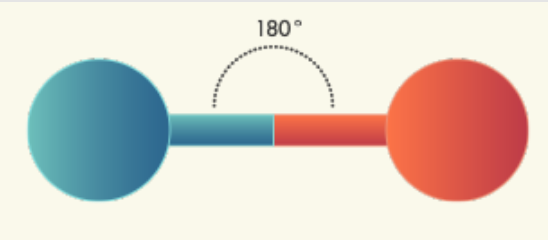
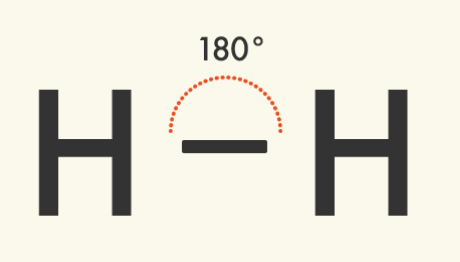
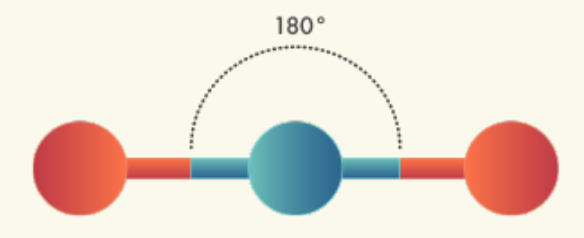
Linear Molecules – One Domain | Central atom has: 1 bond; 0 lone pairs VSEPR: Linear Molecular shape: Linear
| |
Examples of linear molecules with one domain: | ||
|
| |
Linear Molecules – Two Domains | Central atom has: 2 bonded atoms; 0 lone pairs VSEPR: Linear
| |
Examples of linear molecules with two domains:
| ||
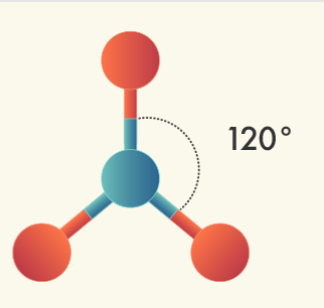
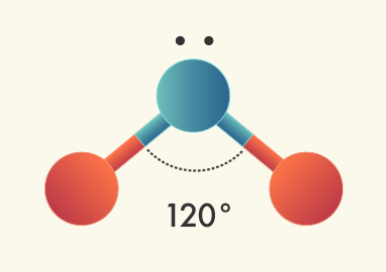
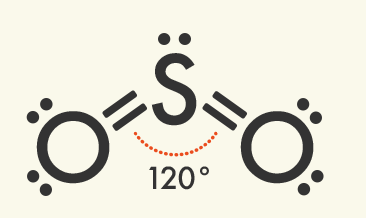
Triagonal Planar Molecules – Three Domains | Central atom has: VSEPR: trigonal planar
| Central atom has: 2 bonded atoms; 1 lone pairs VSEPR: trigonal planar
|
Examples of linear molecules with three domains: | ||
|
| |
03.06 Forces and Bonds:
Dipole Movements:
Intermolecular forces: the forces of attraction that occur between individual molecules
Electronegativity: the measure of attraction of an atom for the electrons in a chemical bond
Dipole: a separation of positive and negative electric charges
Dipole moment: the measurement of the polarity of a chemical bond
Before heat is applied, the molecules in the liquid are attracted to one another by something called van der Waals forces. These forces keep liquids together, and they work because of dipoles. An electric dipole is a pair of opposite charges separated by distance.
The strength of a dipole is called its dipole moment, the electrical charge multiplied by the distance between the charge centers.
A dipole is caused by the separation of electrical charges due to an asymmetry in the locations of the electrons in the molecule. This happens when atoms in the molecules have substantially different electronegativities.
Permanent | Instantaneous | Induced |
These occur when atoms in a molecule have substantially different electronegativity and form polar bonds. If the polar bonds are arranged asymmetrically, the molecule has a permanent dipole moment and is a polar molecule.
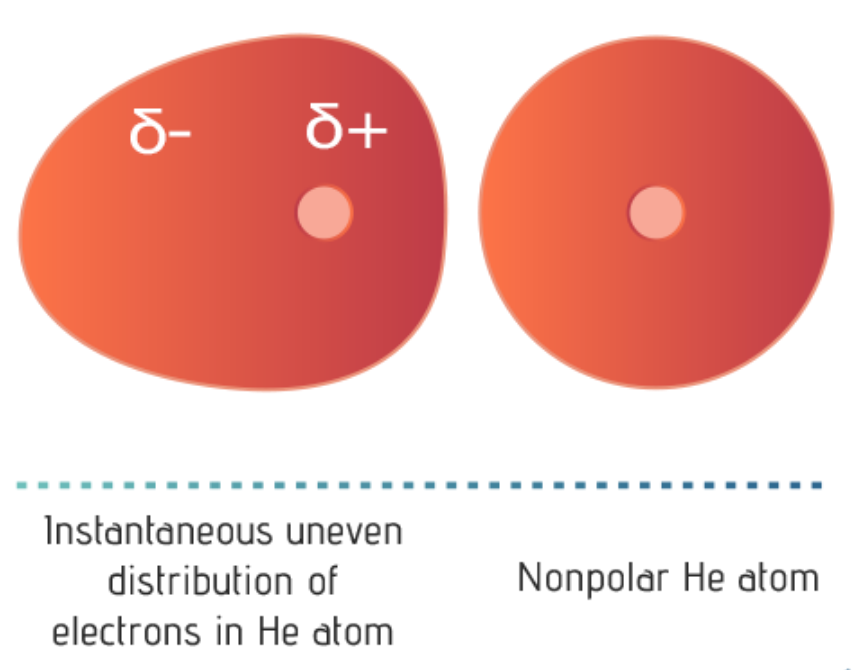
| These chance occurrences happen when moving electrons are briefly more concentrated in one place than another within a molecule, creating a temporary dipole. A molecule is polarized when it carries an instantaneous or an induced dipole.
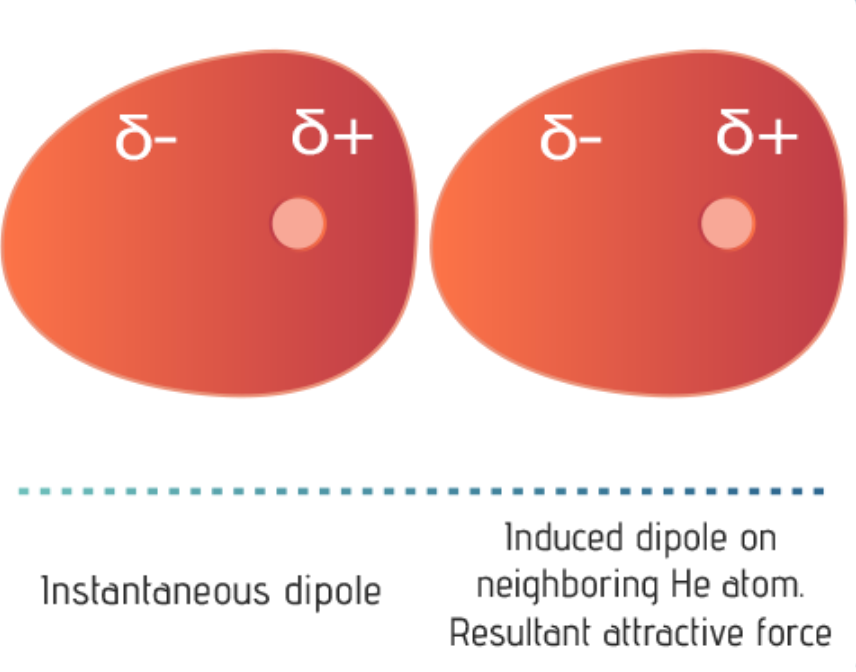
| These temporary dipoles can occur when one molecule with a permanent dipole repels another molecule's electrons, "inducing" a dipole moment in that molecule temporarily.
|
Most molecules have differences in electronegativity between their atoms that draw electrons closer to the most electronegative element. However, a molecule will not have a permanent dipole moment if the molecular arrangement is symmetrical. This is why it is important to consider the VSEPR model of an atom when determining the polarity of a molecule.
Intermolecular Forces:
Intermolecular forces are attractive forces between molecules. These forces exist between molecules when they are close to each other. They are responsible for properties of matter such as boiling point and melting point.
London Dispersion Forces | Dipole-Dipole Forces |
London dispersion forces occur between all molecules and particles but are the only force of attraction between nonpolar molecules or noble gas atoms. These forces are the weakest of the intermolecular forces. London dispersion forces are temporary attractive forces that result when adjacent molecules form instantaneous, or temporary, dipoles.
| Dipole-dipole forces are electrostatic interactions of permanent dipoles in polar molecules. Since opposites attract, the positive end of one polar molecule and the negative end of another polar molecule tend to align the molecules to increase the attraction.
|
Hydrogen Bonding | Ion-Dipole Forces |
Hydrogen bonds are strong dipole-dipole interactions, not actual bonds. They only occur between molecules containing N–H, O–H, or F–H bonds. Hydrogen forms highly polar covalent bonds with the highly electronegative elements N, O, and F. Hydrogen's small atomic radius allows two molecules containing these polar bonds to come in very close contact with each other, increasing the attraction between the dipoles of each molecule.
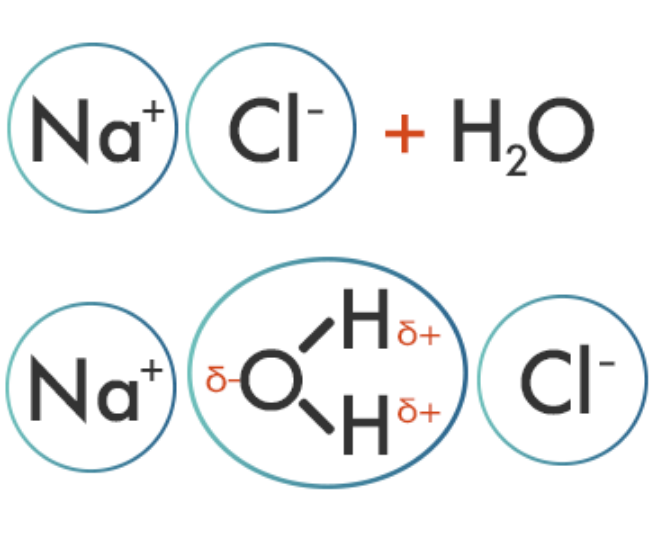
| Ion-dipole forces are attractive forces that result from the electrostatic attraction between an ionic compound and a polar molecule, commonly found in solutions. Because there is a full charge on the ions and a partial charge on the dipole molecules, ion-dipole forces are stronger than dipole-dipole forces, but not as strong as ionic bonds. For example, when NaCl is added to water, the cation (Na+) is attracted to the partially negative end of the water polar molecule, while the anion (Cl−) is attracted to the partially positive end.
|
Force vs. Force:
Molecules contain equal numbers of protons and electrons. Therefore, they have no net charge. Molecular substances are poor conductors of electricity, and there is no strong attraction between molecules. Many covalent substances are found as liquids or gases at room temperature.
Molecular substances are sometimes found as solids, indicating some force of attraction between molecules.
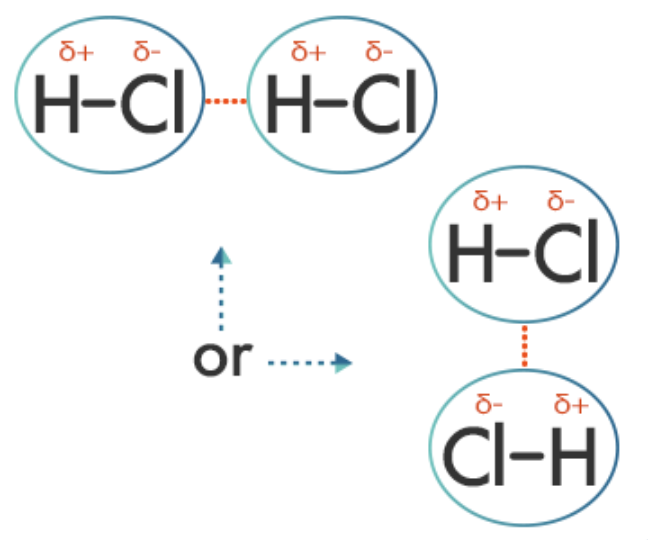
Regarding hydrogen fluoride, HF:
The fluorine atom is far more electronegative than the hydrogen atom and draws the shared electrons of the bond nearer to itself. This makes that side of the molecule slightly negatively charged, leaving the hydrogen end of the molecule slightly positively charged. The hydrogen-fluorine bond is a polar covalent bond. The molecule is said to have a permanent dipole. The delta symbols indicate that the charges are weak compared to those charges carried by ions. The forces and attractions between polar molecules cause adjacent molecules to align with one another. This is an example of a dipole interaction.
Dispersion forces are the weakest of all molecular interactions. Dispersion forces are also called van der waals forces.
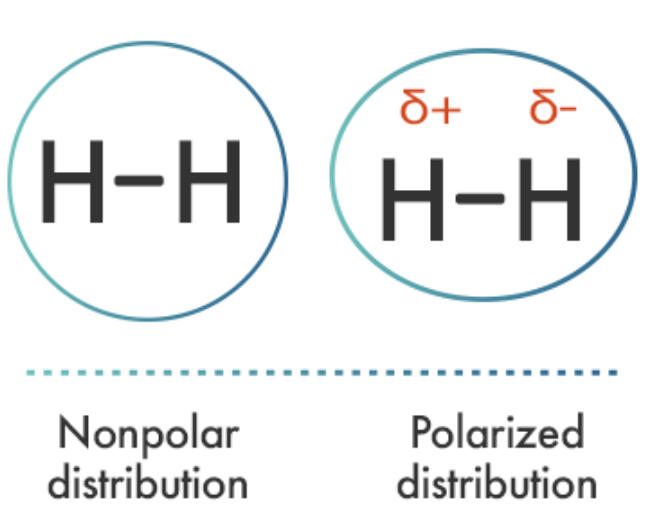
Although the bond within a hydrogen molecule is nonpolar, its electrons are very mobile. At any one instant, both electrons may find themselves on one end of the molecule, making that end slightly more negative in charge than the other. This slight imbalance of charge is called a temporary dipole.
Predicting Properties:
Polymers are used in many industries, such as manufacturing, construction, and agriculture.
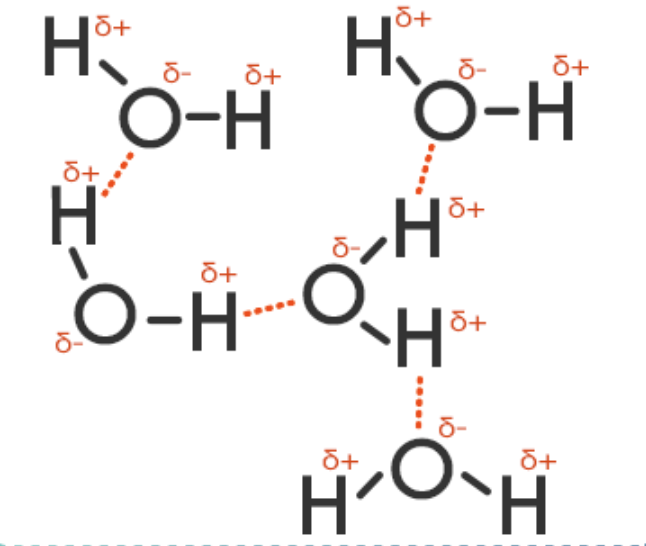
Water is considered a universal solvent because of its ability to bond with other substances in solution. This ability is due to its polar nature and hydrogen bonding.
Water's molecular structure are held together by hydrogen bonds. The weakly or partially positive hydrogen end of one molecule is attracted to the weakly or partially negative oxygen of another molecule, and hydrogen bonds form.
The solid phase is the coolest of any of the three states of matter. The lower the temperature, the less energy the molecules have. Raise the temperature, or in this case heat ice until it melts, and it is the intermolecular forces that are broken. The molecules themselves are unchanged.
The fact the water has a melting point of zero degrees Celsius and a boiling point of 100 degrees Celsius means that the intermolecular forces must be much stronger in water molecules. These stronger forces are due to the permanent dipoles within the H2O molecule itself.
Heating ice to the point where the molecular lattice structure breaks up and heating liquid water to the point where enough hydrogen bonds are broken for it to vaporize requires a lot of energy.
Regarding Trends in Melting and Boiling Points—Dispersion Forces:
In general, ionic compounds have the highest melting and boiling points. Polar covalent compounds have lower melting and boiling points than ionic compounds but higher melting and boiling points than nonpolar compounds. However, all molecules experience weak dispersion forces, which also affect their melting and boiling points.
The strength of dispersion forces increases with the number of electrons within molecules. The halogen group consists of four elements that all naturally take the form of diatomic molecules held together by a single nonpolar covalent bond.
03.07 Organic Chemistry (HONORS):
The Carbon Atom:
Sources of Carbon in the Environment:
Atmosphere
Fossil Fuels
Rocks
Ocean Life
Surface Ocean
Animals
Plants and Trees
Soil
Carbon atoms have the unique ability to bond with other carbon atoms in long chains, rings, and other formations. This property allows carbon to be the base of a variety of large molecules.
Organic Chemistry: the chemistry of carbon atoms
The Carbon Properties:
Carbon is a nonmetal that is found in nature both as a pure element and in various compounds.
A carbon atom has four valence electrons in its outermost energy level, and it has a strong tendency to share those electrons in four bonds (four single, two double, or a single and a triple bond) to have a full octet in its valence shell. Carbon atoms often naturally bond with each other in chains, rings, plates, or networks, and they also bond readily with hydrogen, oxygen, nitrogen, sulfur, phosphorus, and the halogens.
Organic Compounds:
In organic chemistry, functional groups are covalently bonded atoms within molecules that are responsible for the chemical characteristics of those molecules. They have their own unique molecular structures and undergo similar chemical reactions no matter what molecule the functional group is bonded to.
Hydrocarbons: Organic compounds that contain only carbon and hydrogen are called hydrocarbons.
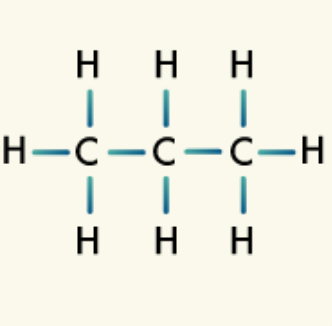
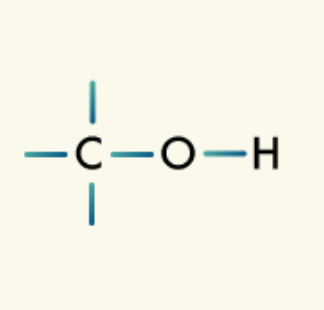
Alcohols: Alcohols are organic compounds in which a hydroxyl group, an oxygen atom bonded to a hydrogen atom, is bonded to a carbon atom.
Ethers: Ethers are organic compounds that have an oxygen atom bonded between two carbon atoms in the carbon chain.
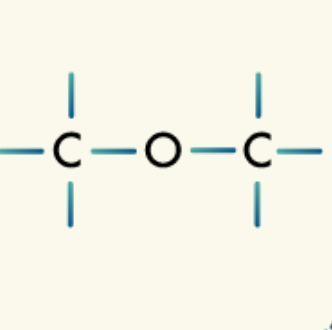
Amines: Amines are organic compounds that contain the amine group, a nitrogen atom bonded to one, two, or three carbons.
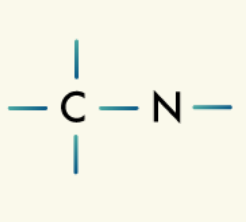
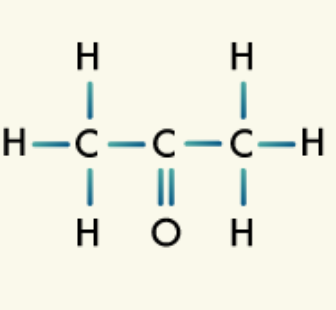
Carbonyl group: The carbonyl group consists of a carbon atom double-bonded to an oxygen atom. Depending on where the carbonyl group appears on the organic compound and what else is bonded to it, the compound may fall into the category of ketone, aldehyde, amide, carboxylic acid, or ester.
Carbohydrates: Carbohydrates are the most abundant biomolecules on Earth made up of carbon, hydrogen, and oxygen atoms.
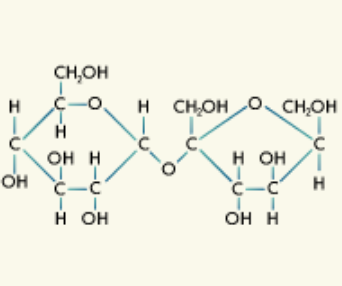
Polymers: Polymers are extremely long molecule chains that consist of repeated molecular units called monomers.