BIOB11 Final Exam Review
Table of Contents
RNA Structure
RNA Processing
RNA Structure
RNA transcribed (primary RNA) → Pre-mRNA is processed (incl. splicing) → Exported from nucleus → translated by ribosomes.
This process is followed through with by non-coding RNA (snRNA, rRNA, snoRNA, tRNA).
RNA splicing in eukaryotic cells
Different exons are included in mRNA (alternative splicing) and leads to isoforms all coming from same gene with different functions, sizes, and structures.
Introns are much larger than exons and the genes can vary in size.
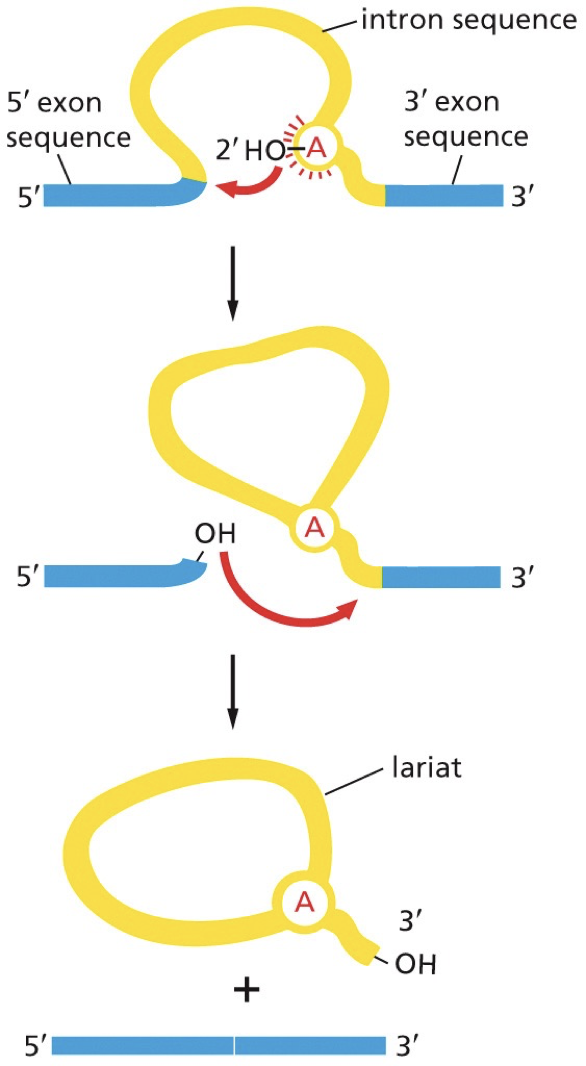
How splicing happens
Lariat: each intron removed, it forms this “lasso”
Remove one intron between two exons, which are joined together to one continuous protein coding sequence. Complex process carried out by 5 snRNAs and 100s of proteins.
snRNA base pairs with: 5’ splice site, 3’ splice site, and a branch point in intron. Splicing and binding is coordinated with consensus sequences.
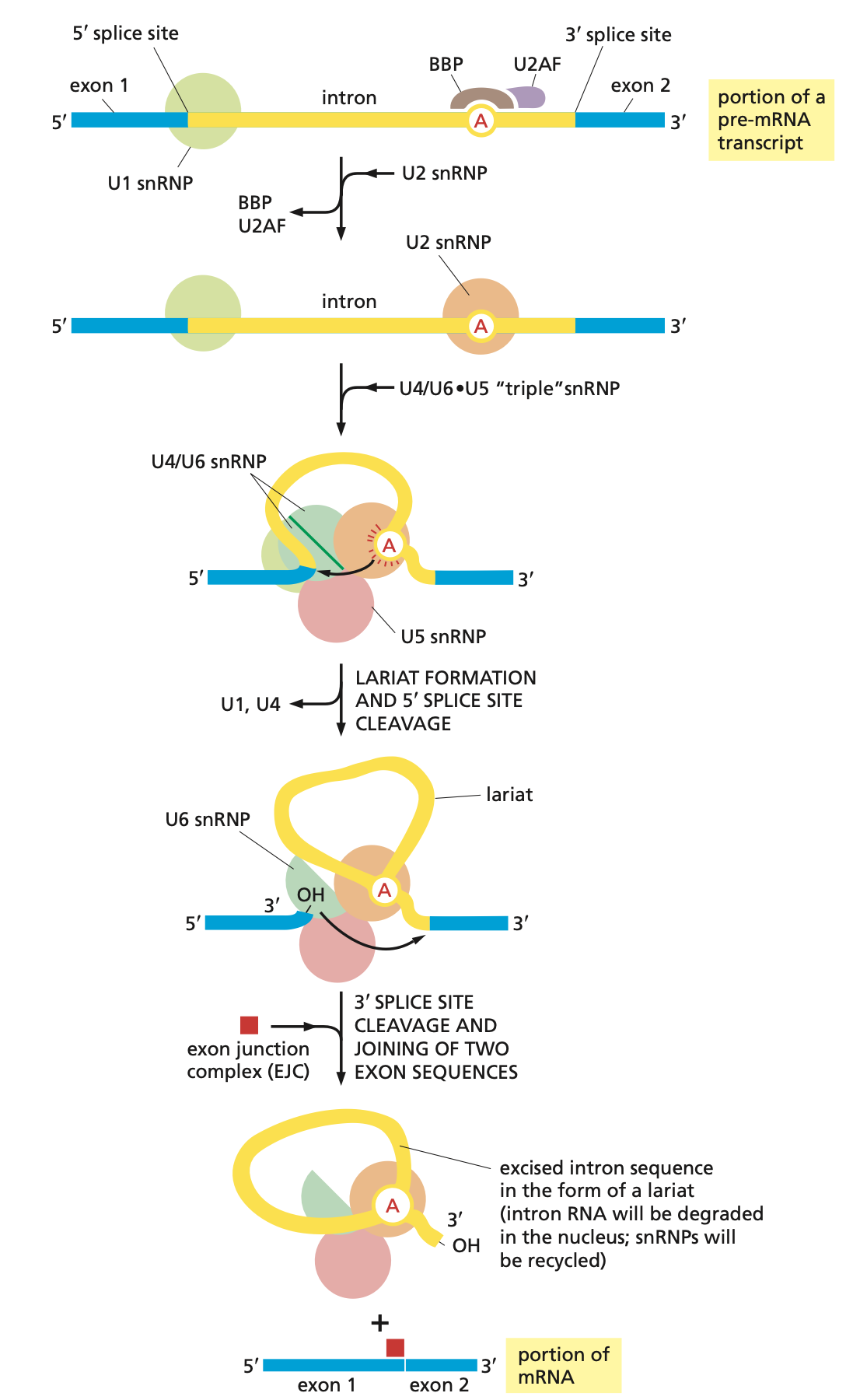
snRNPs: a complex of the 5 snRNAs (U1, U2, U4, U5, U6) with the proteins, and together they form a spliceosome. They assemble on mRNA molecules (mRNA required). snRNAs base pair.
Steps
U1 snRNP binds to 5’ splice site
U2 snRNP binds to branch point site, and adenosine bulges out.
U4/U6 pair and U5 snRNPs enter and U6 replaces U1 at 5’ splice site. U4 and U1 are released.
Branch point adenosine attacks 5’ splice site
3’OH from the 5’ splice site reacts with 3’ splice site
Exon junction complex (EJC): marks newly completed splicing event.
Splicing errors
Changing sequences included in mRNA:
Skipping an exon: particular RNA has an exon skipped, driven by splice sites being read incorrectly. Exon skipping because of a cryptic splice site (a slightly off consensus sequence). snRNPs need proteins to mark recruitment to decrease frequency of errors. A point mutation could impact because it could add to consensus sequence splicing and alter splicing and protein sequence in that way.
Splicing errors may cause disease. Could end in a single point mutation or an entire exon being lost. Severe anemia condition is caused by altered splicing. Spliceosome system will just search for next available site.
10% of inherited human diseases are associated with mutations that cause splicing errors
Consequences of errors in splicing
Mutations prevent excision or alter splicing
Change stability of RNA
RNA degradation
Accumulation of unspliced intermediates
Frameshift mutations
Missense mutations
Impaired mRNA transport
Impairing mRNA export and processing
Pre-mRNA is mostly not matured mRNA
mRNA is rapidly degraded and turned over (half life varies, 1 minute-10 hours).
Huge amount of RNA is not translated, so there has to be ways to know what RNA is processed and ready to export.
Cells need a process to get rid of unprocessed pre-mRNA
How mRNA is selected for export
To allow for unimpaired mRNA transport, we need a default fate of RNA to be degraded. RNA exonuclease cleaves it all up.
To save it from this fate, have proteins bind to it, recognize it as a good RNA molecule (processing is complete or ongoing), or have completed molecules travel through nuclear pore complex.
Nuclear pore complex: Get help out of nucleus into cytoplasm. Only occur if right set of proteins are attached to the RNA molecule.
The proteins still attached to pre-mRNA:
RNA polymerase
Guanyl transferase
Poly(A) polymerase
U1 snRNP
Transcription of rRNA
rRNA is made of rDNA, and it is a needed machinery to process mRNA. rRNA coordinates translation. rDNA is found in clusters: nucleoli, ribosomal biosynthesis. and other non-coding RNA.
During growth, rRNA increases and there is a huge amount of nucleoli. Amphibian eggs are a good study.
Nucleoli refer to the the christmas tree structure, the black dots on the end are RNA polymerase, and the branches are RNA transcripts.
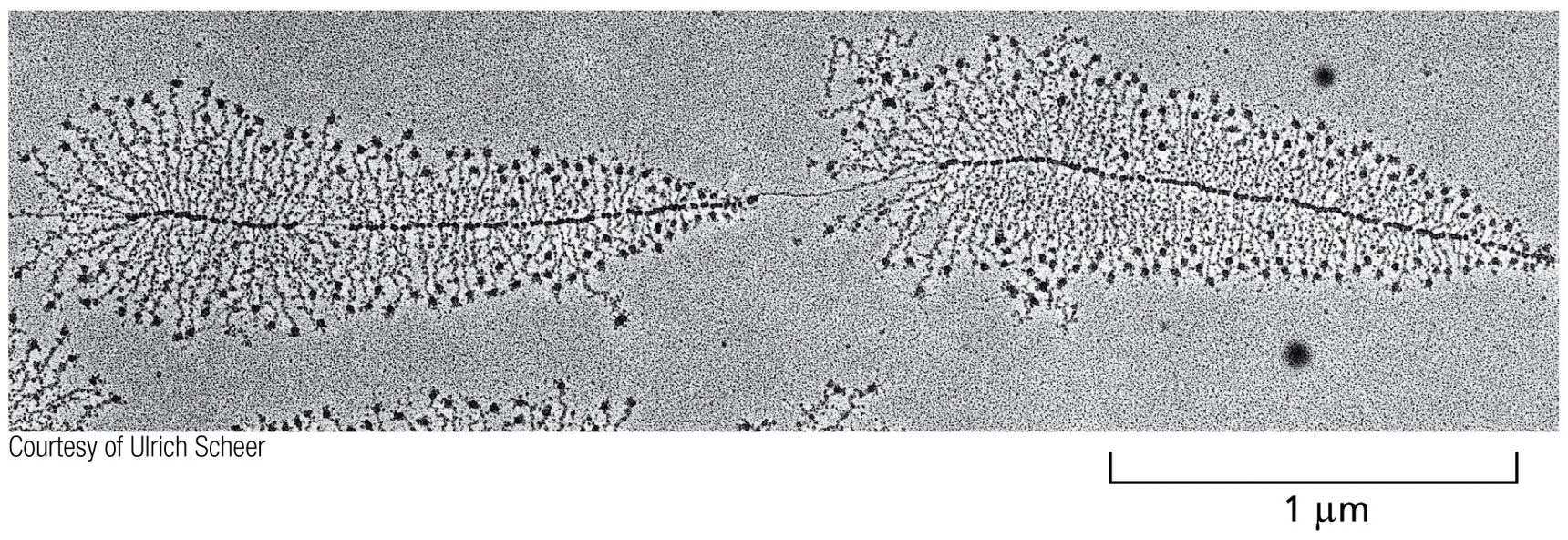
S-value: measurement of sedimentation rate during centrifugation, affected by both mass and shape.
The larger the S-values, the larger the molecule, the faster the sedimentation.
rRNA in eukaryotes is depicted by different S-values:
Large centrifuges: 28S, 5S
Small centrigues: 18S - 40s
rRNA in bacteria is depicted by:
50s large, 30s small, and 70s overall
rRNA makes up 80% of RNA in most cells. Multiple copies of rRNA genes in the genome.
rRNA is not translated, there is no RNA to protein amplification step. It is advantageous for cell to make more ribosomes.
Ribosomes are protein synthesizing machines. RNA-protein complexes are where they are most abundant. Overall structure is a very similar complex. Small and large subunits exist. Prokaryotes have slightly different numbers, but they both function same way. Use GTP hydrolysis.
Larger rRNAs are made up of RNA polymerase I, while the 5s is made up of RNA polymerase III.
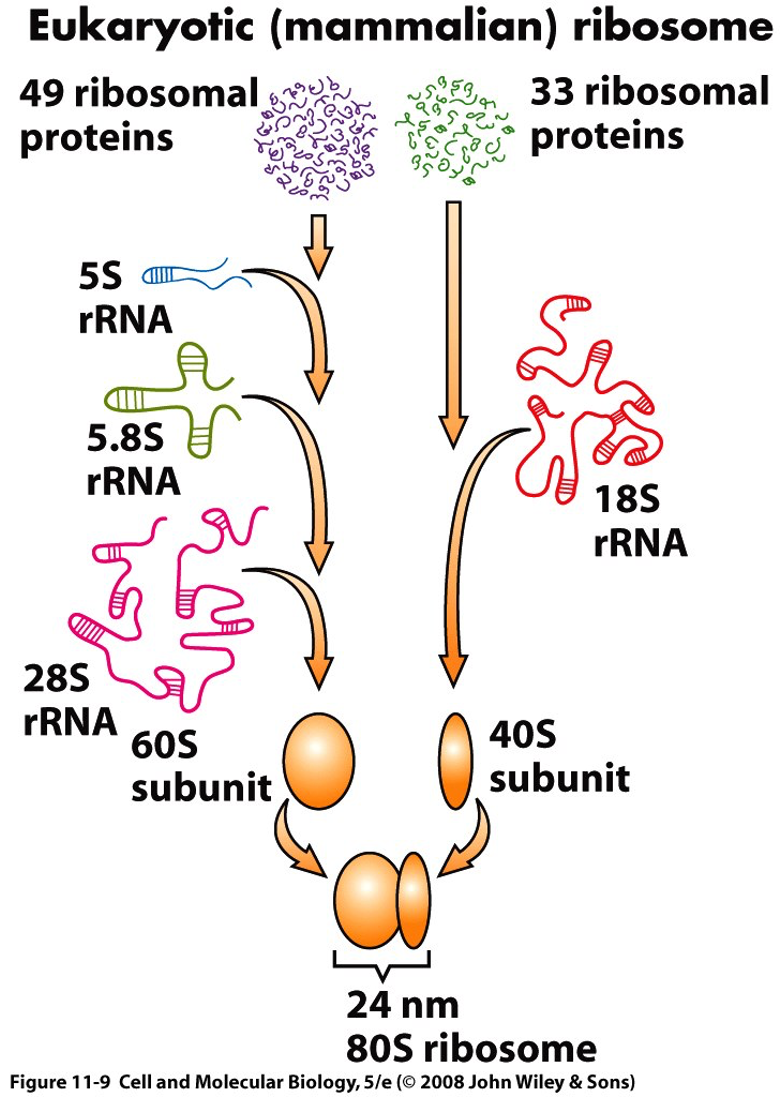
Precursor rRNA is chemically modified (methylation) and cleaved (into smaller molecules) during transcription. 5s is made up separate cluster and does not need chemical modification.
Modifications of rRNA
Processing happens for non-coding RNA.
Isomerization: some uridines are processed and modified to become pseudouridine.
Methylation: methyl group is attached to the 2’OH of the ribose
snoRNAs: small nucleolar RNAs made by RNA poly 3, is associated with snoRNP proteins.
Ribosomes are produced and all of this is coordinated in the nucleolus. Stuff moving in and out results in small and large subunits.
Transcription of tRNA
tRNA = transfer RNA. Is encoded by tDNA. Exists in different species, about 80 nucleotides in length and marked by which amino acid they carry. They are transcribed by RNA pol III. tRNA pre-cursor must be trimmed and modified.
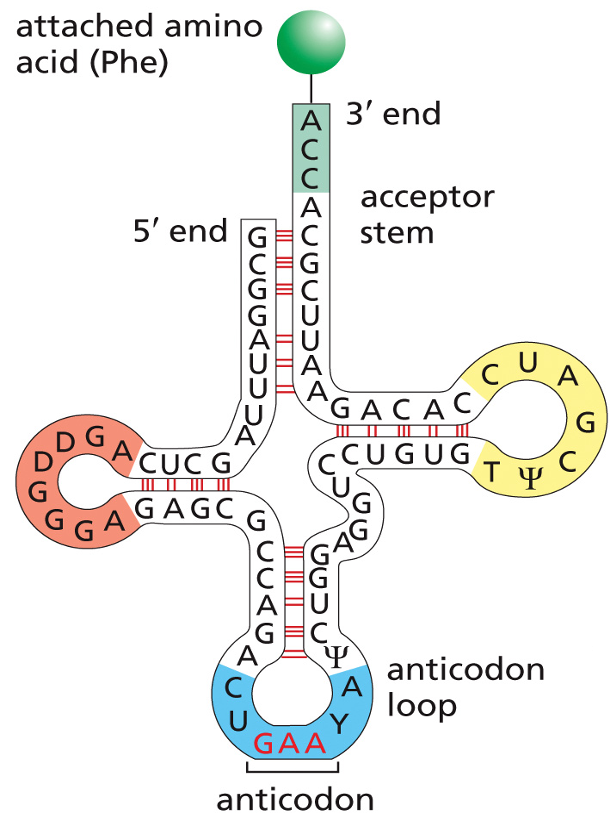
A lot of modifications in tRNA. tRNA are modified to carry different amino acids based on their anticodon sequence to base pair with codon and match up with proper amino acids. Proteins made of polypeptides and amino acids are connected by polypeptides.
The tertiary structure twists into and L-shape. Anticodon loop is at one end, and the 3’ amino acid acceptor arm is on the opposite end. There is a variable number of tRNAs. 30-40 different tRNAs in bacteria, under 50 in plants. Only 20 amino acids are coded by multiple codons.
Ribosomes (with rRNA) and tRNA translate mRNA into polypeptide chain. A codon in the sequence of the mRNA is 1 amino acid. Some amino acids are coded by multiple codons.
How codons are read: non-overlapping code. Ribosome reads three and shift forward thre.
This was tested by mutating one C and see if it affected three amino acids or only one. A single nucleotide change results in a change in one amino acid (is non-overlapping).
There are three possible reading frames based on where you start.
Use AUG - Methionine - Met.
Degenerate code: more than one code can code the same amino acid, known as redundance.
Codons with same amino acids have same first (sometimes second) letter, and differ mostly in third letter.
They’re grouped with having similar molecular properties.
Point-mutations less severe, codon is similar and one nucleotide is switched. Pretty good odds that amino acids with similar properties will be okay.
Silent mutations alters the nucleotide sequence, but encodes for same amino acid.
Missense mutation changes one amino acid
Nonsense mutation is a mutation that leads to a stop codon
Removal or addition in coding sequence leads to frameshift mutation
Wobble base-pairing: U can pair with A or G. G can pair with U or C. I can pair with U, A, or C. Same tRNA has two that are set, and the third base-pairing can differ using same RNA.
61 codons with as few as 31 different tRNA. Compatibility of how they’re read is based on if there are enough tRNA.
How correct amino acid is added
Charging a tRNA: the appropriate tRNA must be covalently linked to the free OH on the adenosine at the 3’ end of the tRNA (amino acid acceptor stem).
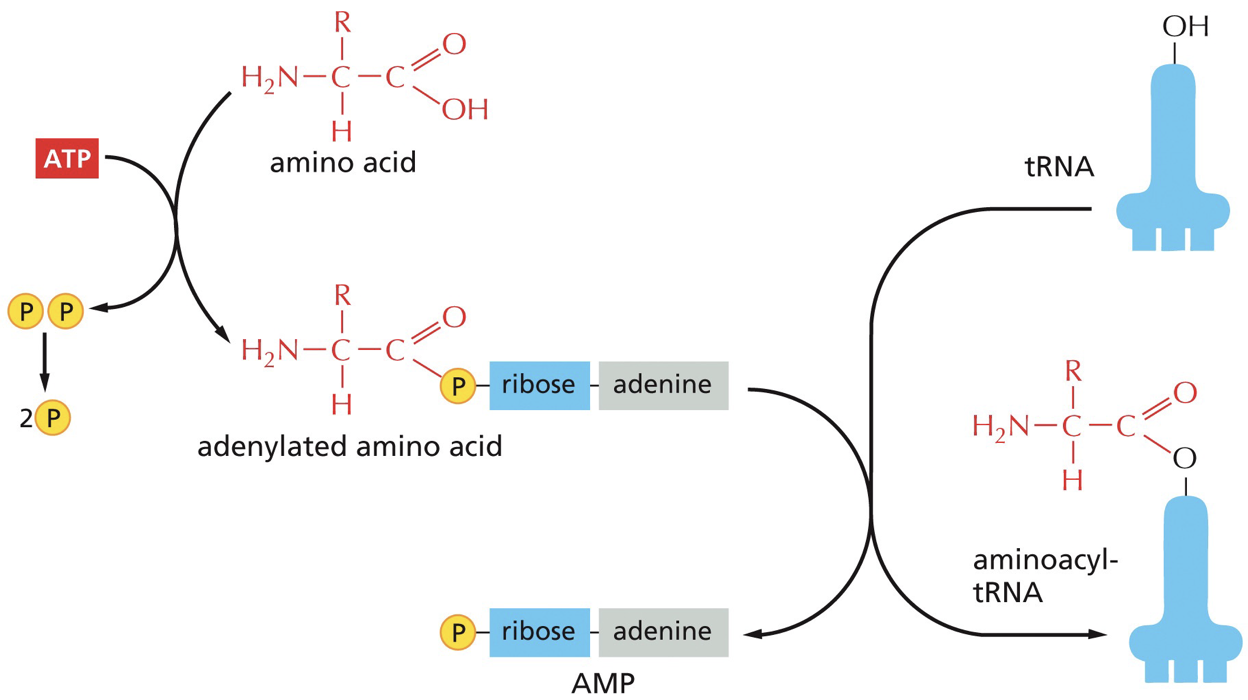
The anticodon loop determines the appropriate amino acid.
Aminoacyl-tRNA synthetase: catalyzes the charging of tRNA with amino acid. There are about 20 different, which means there is one per amino acid. It is going to add it to tRNA. Interacts with anticodon loop and amino acid acceptor arm. Modifications in both regions. The ability is the heart of everything.
tRNA is binded to tryptophan and the addition of amino acid is catalyzed. There is a proofreading and editing function. Editing function on AA tRNA has the amino acid pushed into editing site that breaks bonds and removes the amino acid.
1 in 40 000 error rate
There is 1 aminoacyl tRNA synthetase gene per each different tRNA. There are more different codons than there are different aminoacyl tRNA synthetase.
Amino acids are added to the C-terminal end of growing polypeptide chain. This decoding of the mRNA message into protein occurs in ribosomes
Each ribosome has three sites: A(minoacyl), P(eptidyl), E(xit). Small ribosomal subunit aids in decoding mRNA by interacting with anticodon. Large ribosome aids in peptide synthesis by interacting with aminoacyl-tRNA and catalyzing bond formation.
mRNA threaded through small subunit. Tunnel out of large subunit, growing chain leaving.
Ribosomes are ribozymes that have catalytic activity. Spliceosomes are also ribozymes.
*****
Protein, Translation, and Regulation
Polypeptide structure
Polypeptides are linear sequences of amino acids linked by peptide bonds, which join the carboxyl end of one and amino end of another (N-terminus, C-terminus). Each amino acid differs by the side chain/r-group.
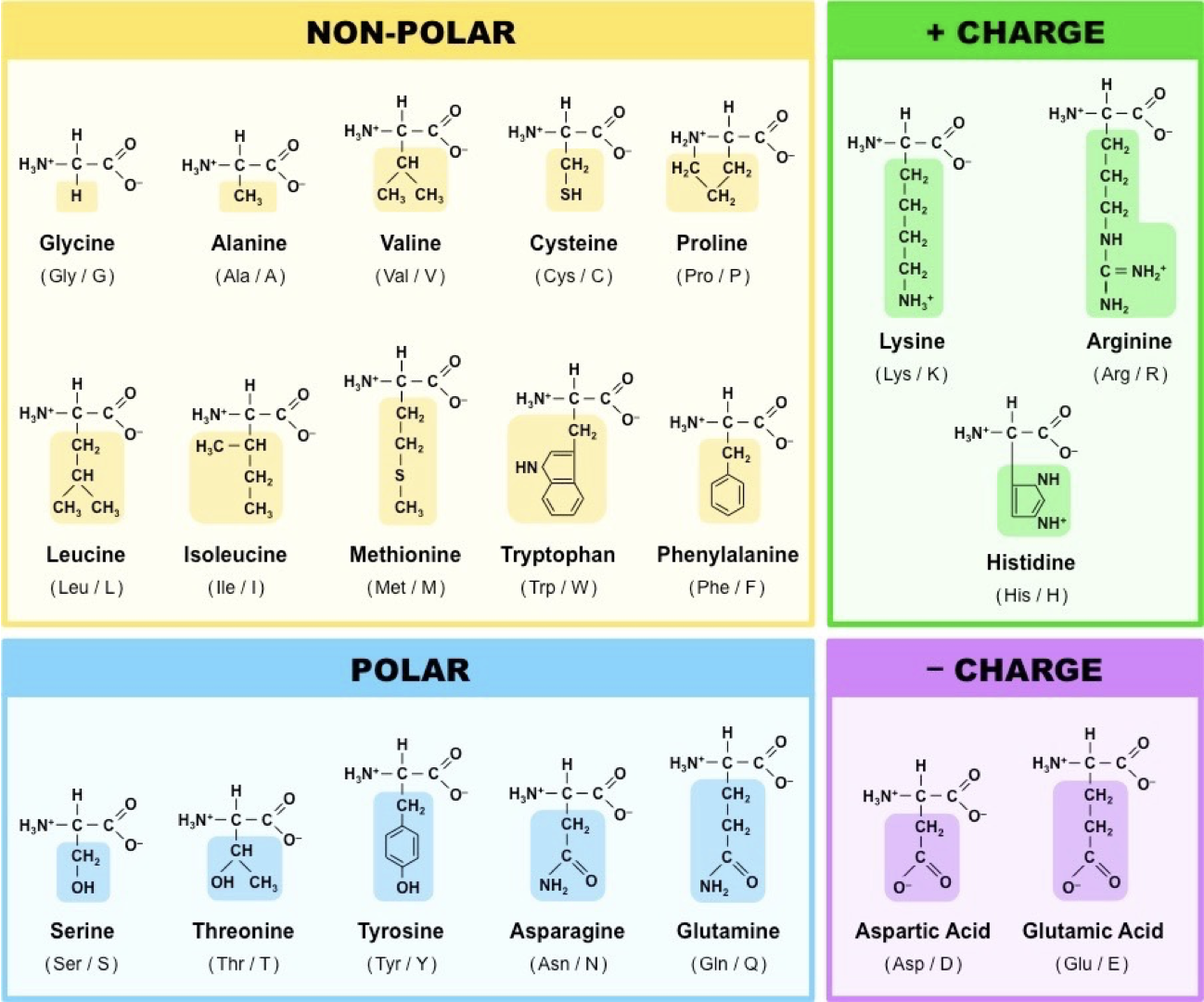
Protein structure and functionality (folding)
Polypeptide becomes functional once protein is folded. Proteins are composed of 1+ polypeptide subunit, making up a functional protein. To be functional it has to fold into correct cluster. Folding is promoted by non-covalent interactions. Most fold as they’re leaving the ribosome, but as they leave, chaperones guide folding.
Functional domains: Proteins fold into these, and they have a particular function. The domains are the structural units of the cell.
A domain usually folds independently, and have a particular function.
Different proteins may have the same domain.
Domains are shuffled and used in different cells.
It is the functional unit and structural unit.
Same domain doesn’t mark difference in sequence.
In one polypeptide you could have multiple domains
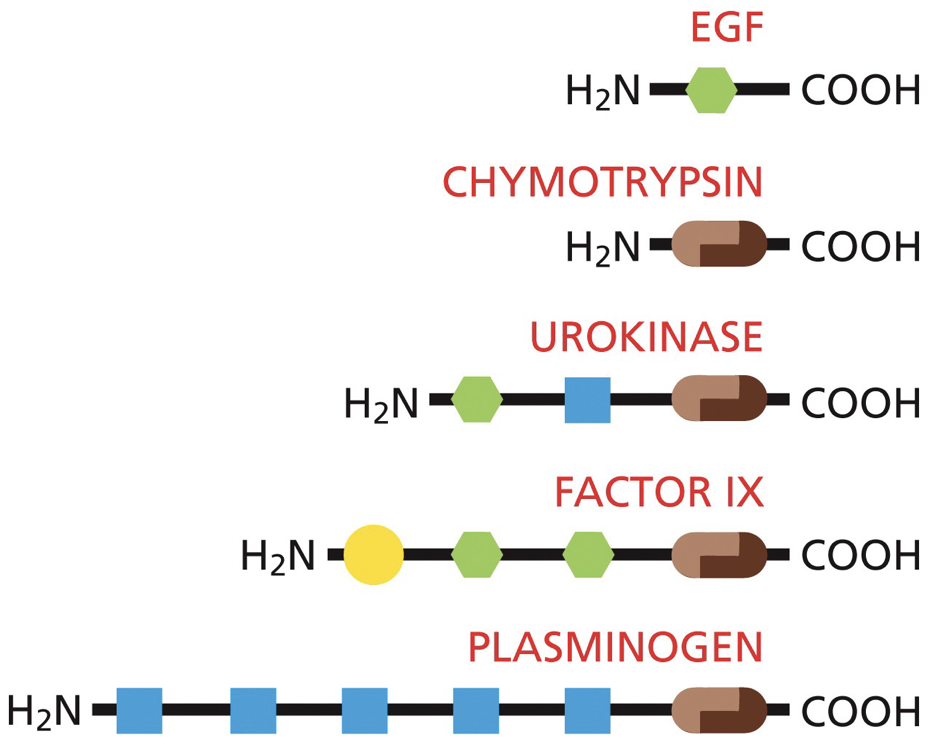
DNA binding proteins: contain a common set of domains, and this is a limited set of domains.
We can move sequences like with exon shuffling, where stuff is multiplied or deleted into other areas. They’re used or reused. This is how the same DNA-binding domain can be found in multiple different proteins.
Different proteins can differ greatly in size and number of polypeptide subunits.
Translation
Translation is a highly conserved mechanism
Initiation
Assembly at start codon, which determines reading frame.
Met is the first amino acid added with “AUG” (which is the 5’ end), and we start building N-terminus of polypeptide. Amino group of incoming binds to C-terminus of growing chain.
You can have multiple ribosomes at once.
Initiation factors (IF, eIF) are required for beginning initiation.
In Prokaryotes
The small subunit binds to AUG.
Shine-Dalgarno sequence: AUG guided by a specific sequence of nucleotides upstream (5’-AGGAGG-3’). It is complementary to a sequence near the 3’ end of 16s rRNA, which positions the ribosome in the correct spot.
Initiation factors: attach to mRNA, and GTP-binding protein that is required for attachment of first AA-tRNA is there. They prevent premature attachment of large subunit.
IF1: Attachment of mRNA
IF2: GTP-binding protein required for attachment of first AA-tRNA.
IF3: Prevents premature attachment of large subunit.
N-formylmethionine (fMet) is the first trna that gets position and bind in P site of ribosome. Once initiator tRNA is found, we release initiator proteins and GTP hydrolysis occurs so large subunit can bind.
In Eukaryotes
Export out is the first step, and this can happen when the ends are matured.
Initiation factors: require more proteins (12eIFs). eIFs bind to 40s small subunit along with Met-tRNA entering the P site.
eIF1 and 1A: conformational change to allow for binding of mRNA.
eIF3: will interact with eIF4G on mRNA complex
eIF4E: binds to 5’ cap
eIF4A: has helicase activity that uses ATP hydrolysis to unwind any double stranded regions in mRNA
eIF4G: links 5’ cap and 3’ poly A tail, which converts mRNA into a circular message.
Kozak sequence: small ribosomal subunit + eIFs find 5’ end of mRNA scans along until reaches 5’-CCACCAUGC-3’). The actual sequence may vary and the most efficient will look most like the ideal. Differing sequence may effect the proteins produced because they will have different amino acid sequences at the N-terminus.
GTP bound to eIF2 is hydrolyzed and is release with rest of eIFs. Dissociation of IFs allows for 60s large subunit to attach.
Elongation
Series of steps created over and over. A new peptide bond forms between amino group of incoming amino acid and C-terminus of growing chain.
Steps
AA-tRNA binds to empty A site
Peptidyl transferase catalyzes new peptide bond between growing chain and new amino acid.
Once peptide bond is formed, the large subunit moves first, then the small subunit translocates.
Oldest tRNA (P→E) is ejected.
Transcription elongation factors: We need a certain level of accuracy. These hydrolyze GTP to provide energy for conformation changes. EF-Tu is part of an accuracy check that corrects tRNA base pairing.
Accuracy checks: Small subunit hydrogen bonds with codon-anticodon. A correct pairing will trigger conformational changes in ribosome. Stuff is moved away until proofreading function moves it close enough to bind.
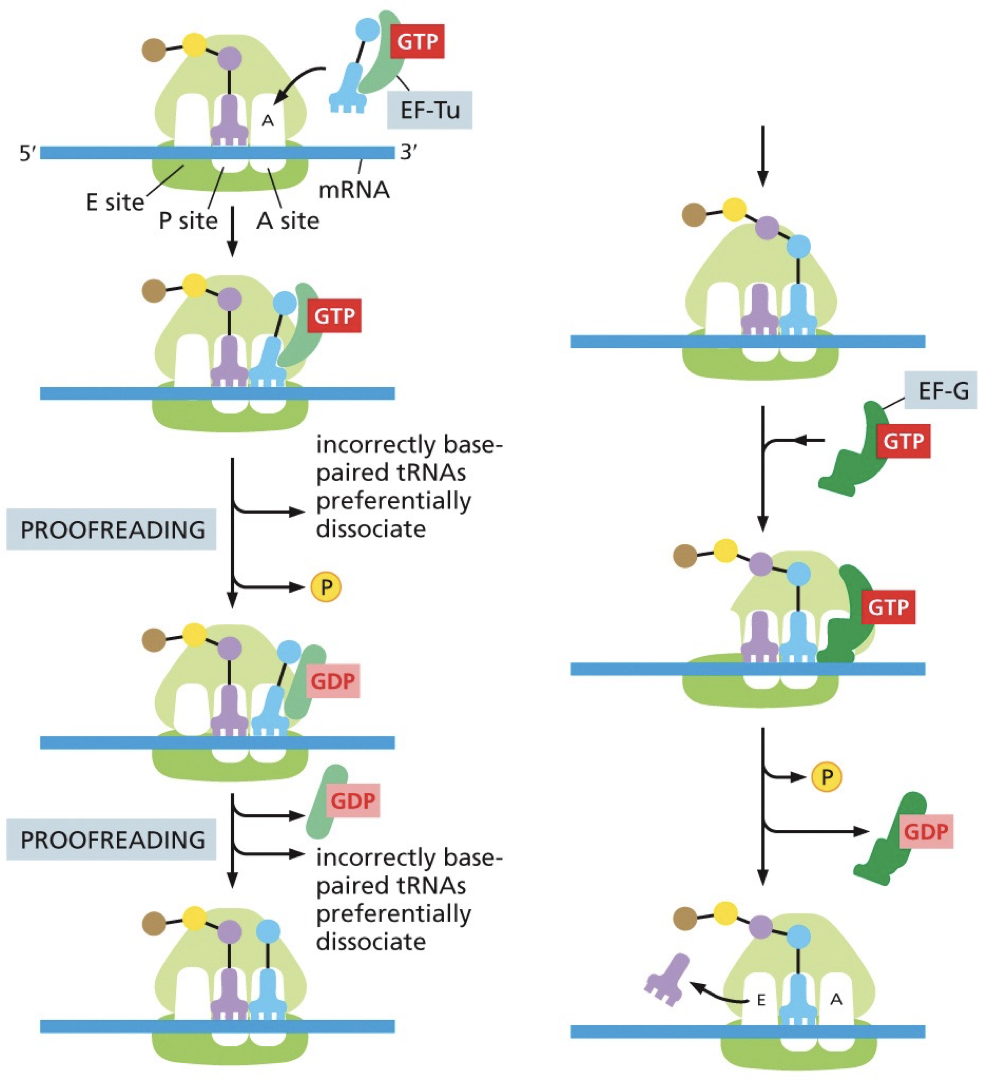
Termination
Stop codon signals the end. N to C direction. Once it reaches stop codon in A site, there is no corresponding tRNA. What enters is a release factor which resembles tRNA. Instead of a polypeptide bond, it adds a water molecule to the end (-CO-OH). Catalyzes release of polypeptide.
Polyribosomes can have a new one scan before the other one is terminated.
Protein Translation Regulation
Regulation makes different cell type/function. Produces a phenotype from the genotype.
DNA-RNA-Protein all have differences in structural organization and linear sequences.
In making organisms, we have processes of commitment (gradual process of restricting cell fate) and cell renewal. A stem cell retains the ability to divide and re-create itself and make progeny capable. Once you have a liver tissue you need to do constant cell renewal.
Commitment (gradual process of restricting cell fate) levels:
Pluripotent: produce all cell types in body (embryonic stem cells)
Multipotent: produce a related group of cells (hematopoietic stem cells)
Unipotent: only produce cells of their own type but have property of self renewal required to be labelled as a stem cell.
In situ hybridization: mRNA is detected through base-pairing with a labeled probe, complementary to a specific sequence in the RNA of interest.
Techniques allowing the detection of specific mRNA transcripts confirmed that expression can be restricted only to specific sites (or times) in an organism.
Differential gene expression is necessary to make each cell type unique.
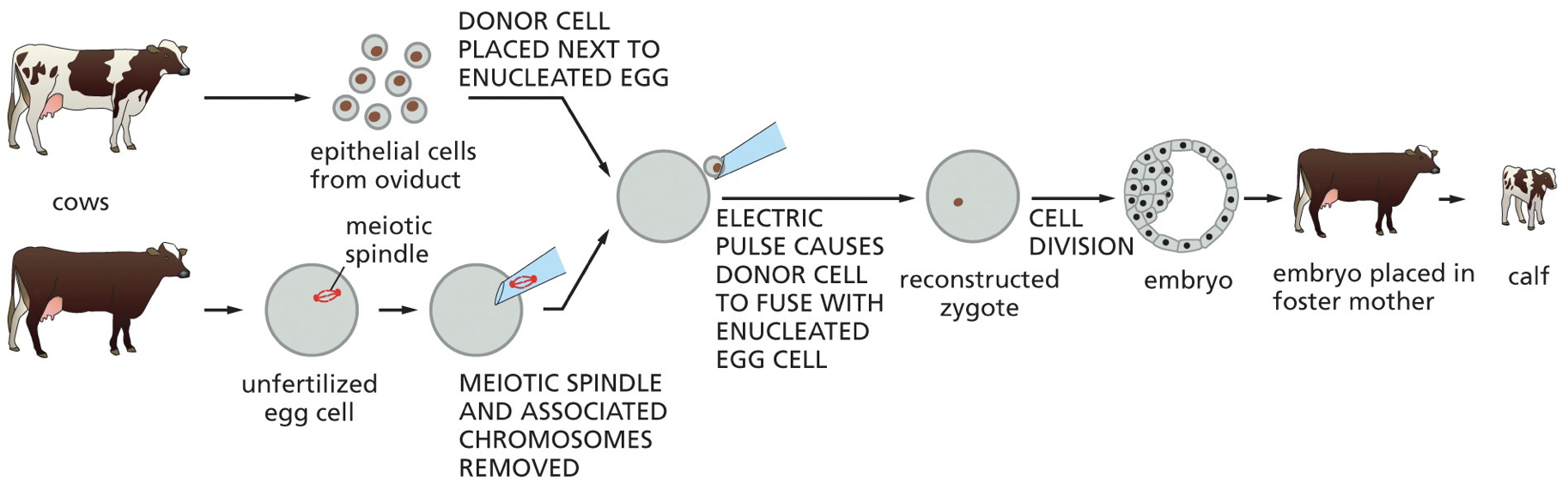
Genomic equivalence: How it is possible that every somatic cell contains the same DNA. This was proven in animals, when we take an egg and take out the nucleus and replace it of the nucleus of another cell, and the blueprint is all still there. The idea that the cell does not throw away its DNA.
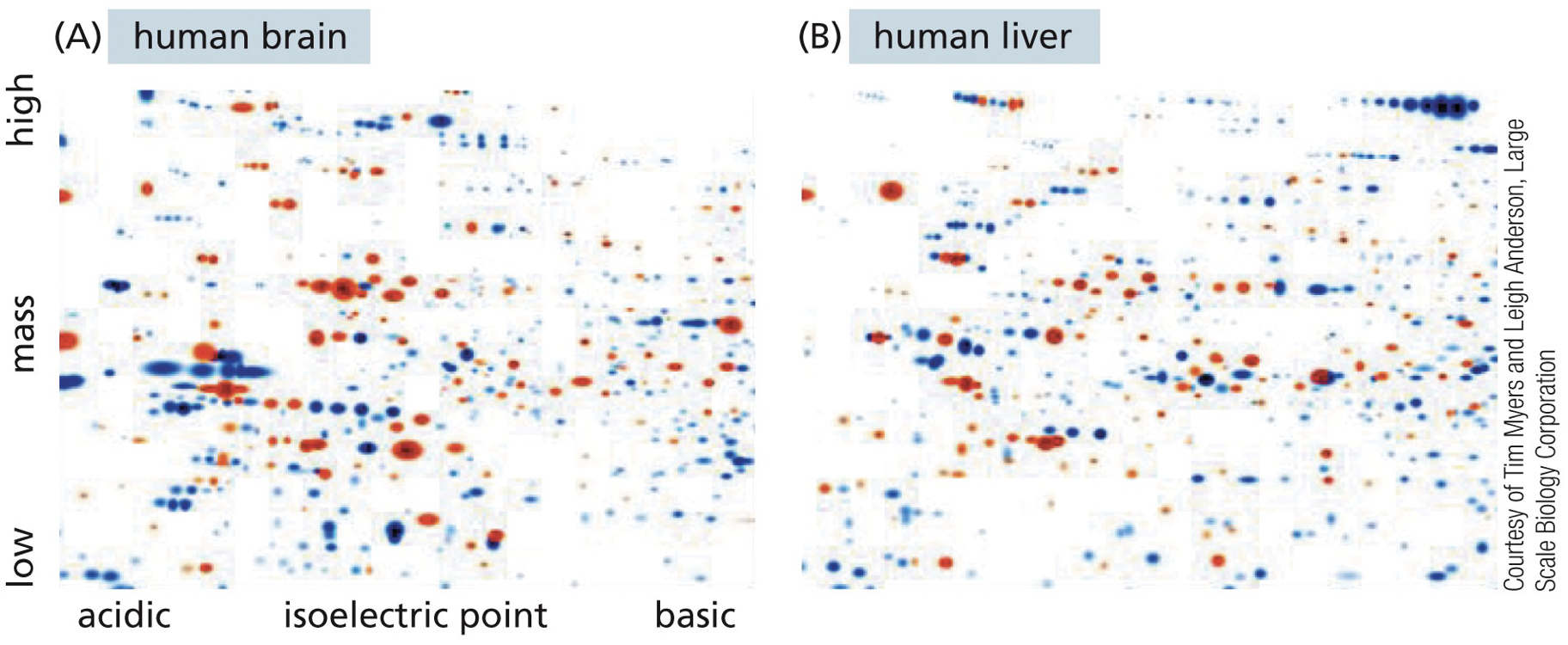
2D Gel Electrophoresis: Used to separate proteins by charge and by mass. Brain v. liver have a lot of different sections of their genes light up. It is used to get a general look.
RNA sequencing: Get a whole bunch of RNA sequencing in the cell. All the mRNA is analyzed. Can study transcriptome (all transcripts of the RNA), and find genes that differ in different samples. Tells the sequence of nucleotides scooped out of the cell and matches it up with genes in the genome to see what is highly transcribed.
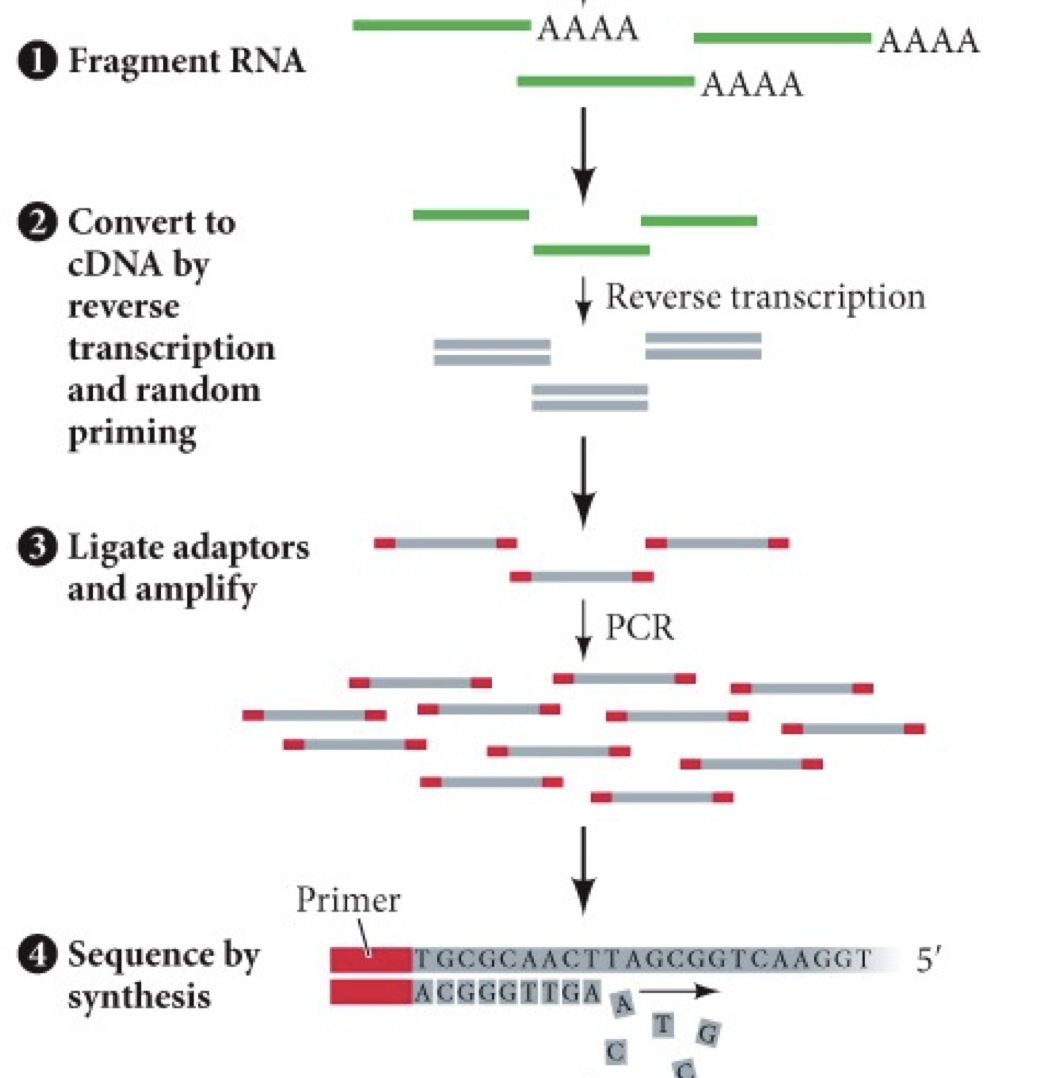
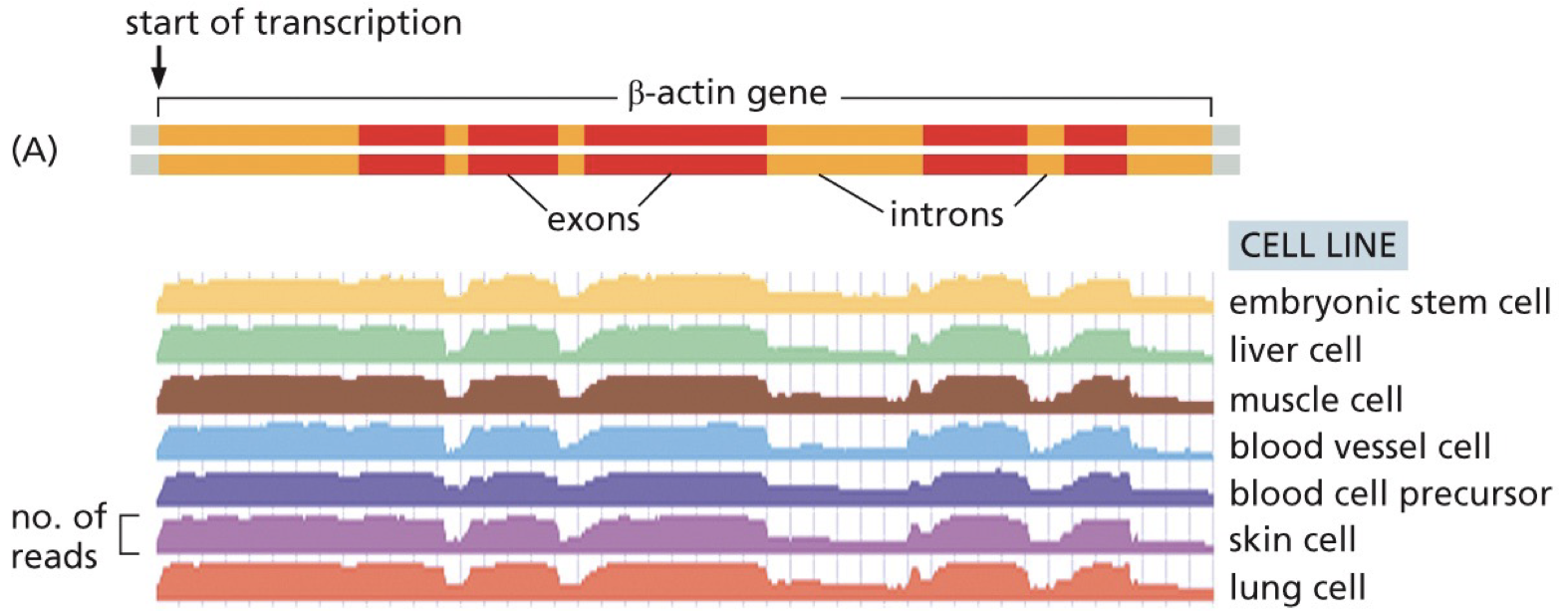
Sequence reads tell you how much of transcript was present. Show how dramatically a gene’s expression can differ.
Constitutive expression: all cell types, all the time.
Beta-actin gene: exhibits constitutive expression. Cell function requires right genes to be expressed.
Cell function: requires genes to be in the right cells, at the right time, in the right amount.
Exons have more reads than introns.
It is possible to tell which exons are more often included/excluded by alternative splicing.
RNA reads are detected for introns sequences because they are transcribed and some are still there in that snapshot.
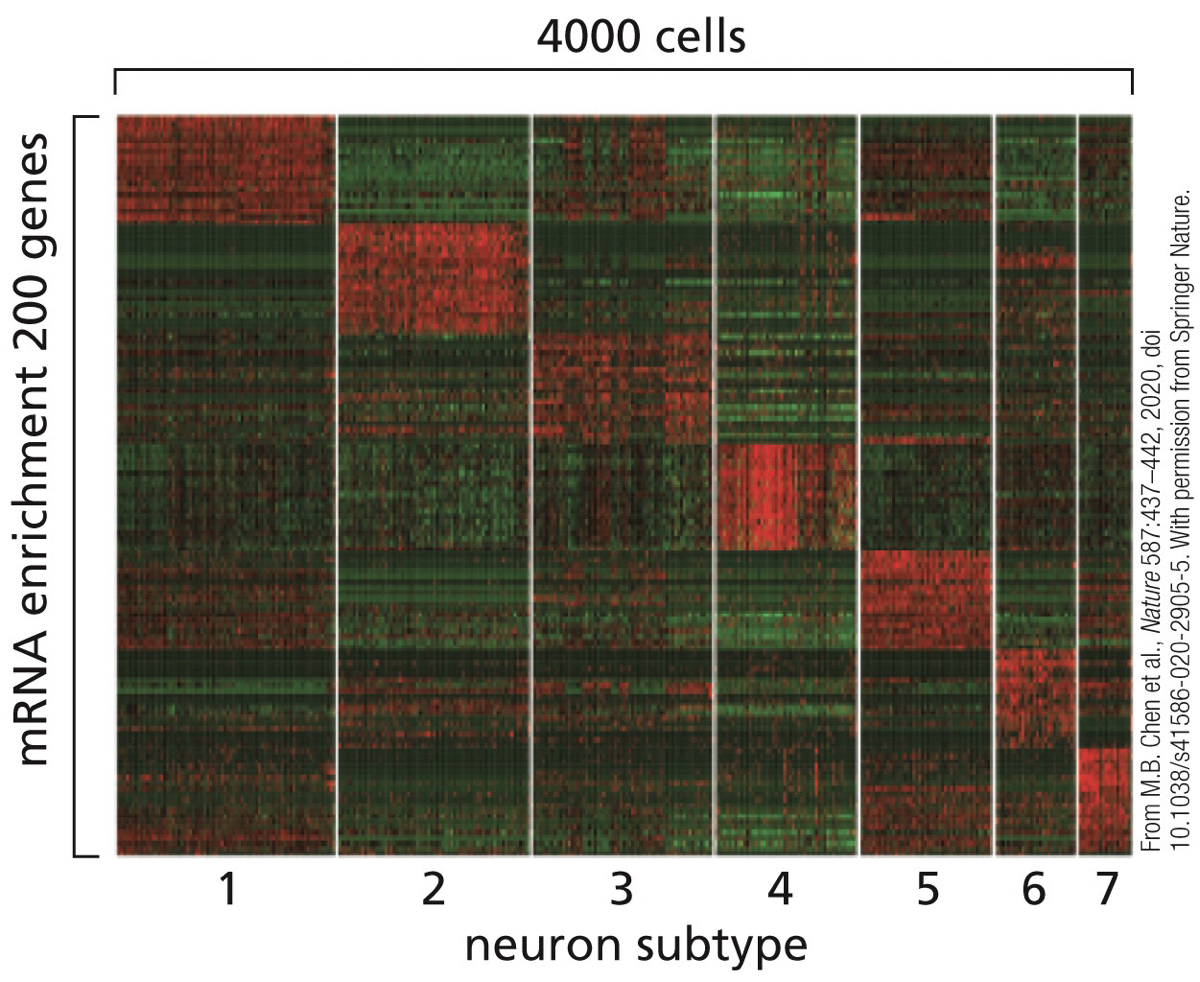
Heatmaps: displaying groupings (treatments being compared or clusters/subtypes discovered based on similar expressions of genes) and a colour scheme is used to reflect the level of expression/detection. Each line represents a different gene that was expressed. Easier to handle than RNA-Seq.
Types of transcriptional regulators
DNA can be recognized for specific binding
Cis-regulatory elements: act on same strand of DNA they are found. Sequences encoded on the same chromosome as the gene they affect.
Recruit proteins or help with repression maybe by silencing
DNA consensus-sequences
Trans-regulatory elements: can be expressed from any chromosomes. They contain structural motifs that recognize/bind to cis-regulatory elements.
Many different trans-regulatory elements work with many cis-regulatory elements.
Transcription factors
ChIP-seq: Chromatin immunoprecipitation and sequencing. Cross-linking, break up, antibody binds and pulls away from everything else in the cell, sequencing.
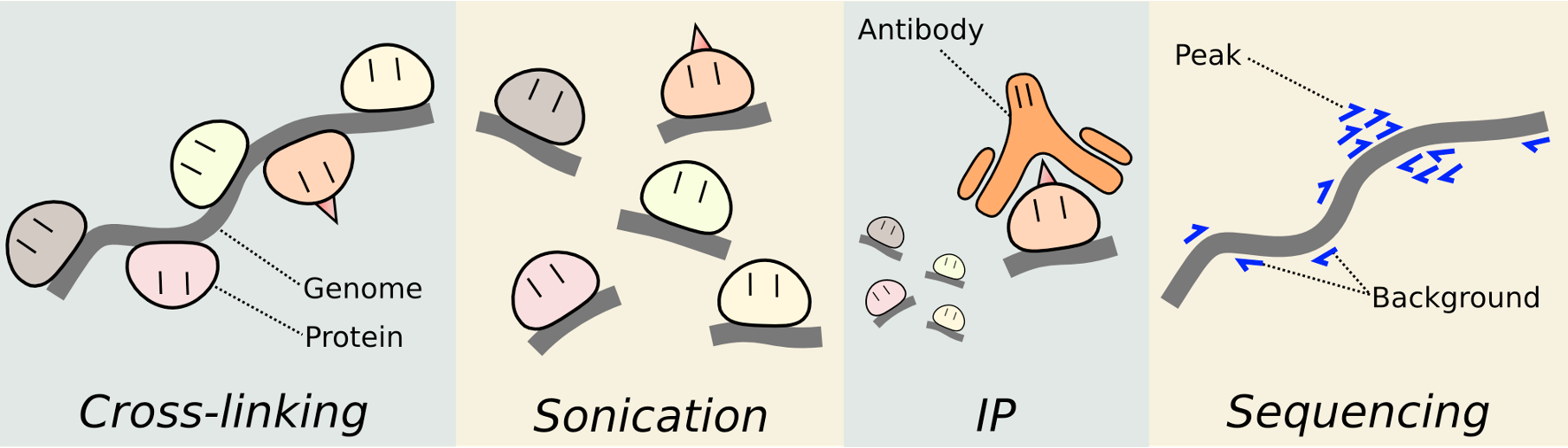
Transcription factors (TFs)
TFs are proteins that bind specific DNA sequences and influence transcription. Binding to DNA is dependent on forming complexes with other trans-regulatory elements. Not only proteins, but everything that binds to and is the cis regulatory elements.

A single gene can be regulated by multiple cis-regulatory elements
Binding domains: stuff often binds as dimer, and they can now recognize adjacent groups and double how much they can check out. Sometimes homodimers, sometimes heterodimers (and working with greater numbers).
****
Transcriptional Regulation
Cis-regulatory elements
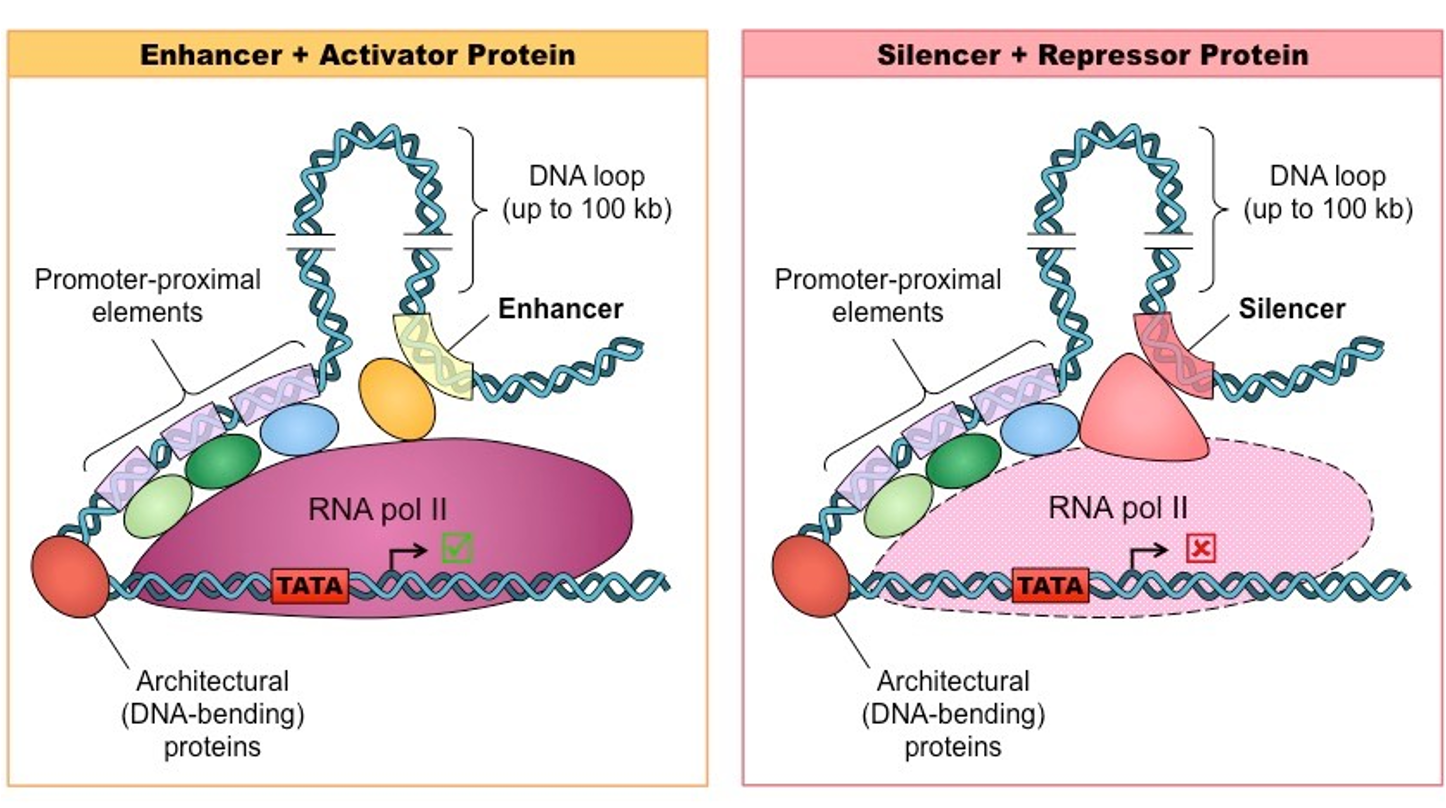
Promoters: sequences of DNA where RNA polymerase can be recruited to initiate transcription
Enhancers: tell how much gene product is made from a promoter. One gene may have multiple (the one being used is what changes a situation).
Silencers: DNA regulatory sequence that prevent promoter use and inhibit transcription. Restricts gene expression to its proper cell and time. Tests so certain genes are not expressed.
You can delete or add the three above without affect amino acid sequence.
DNA may loop around so cis-regulatory elements are further or closer.
Cis-regulatory elements are the same in each cell type, the combination of TFs is what differs in which one is picked or used.
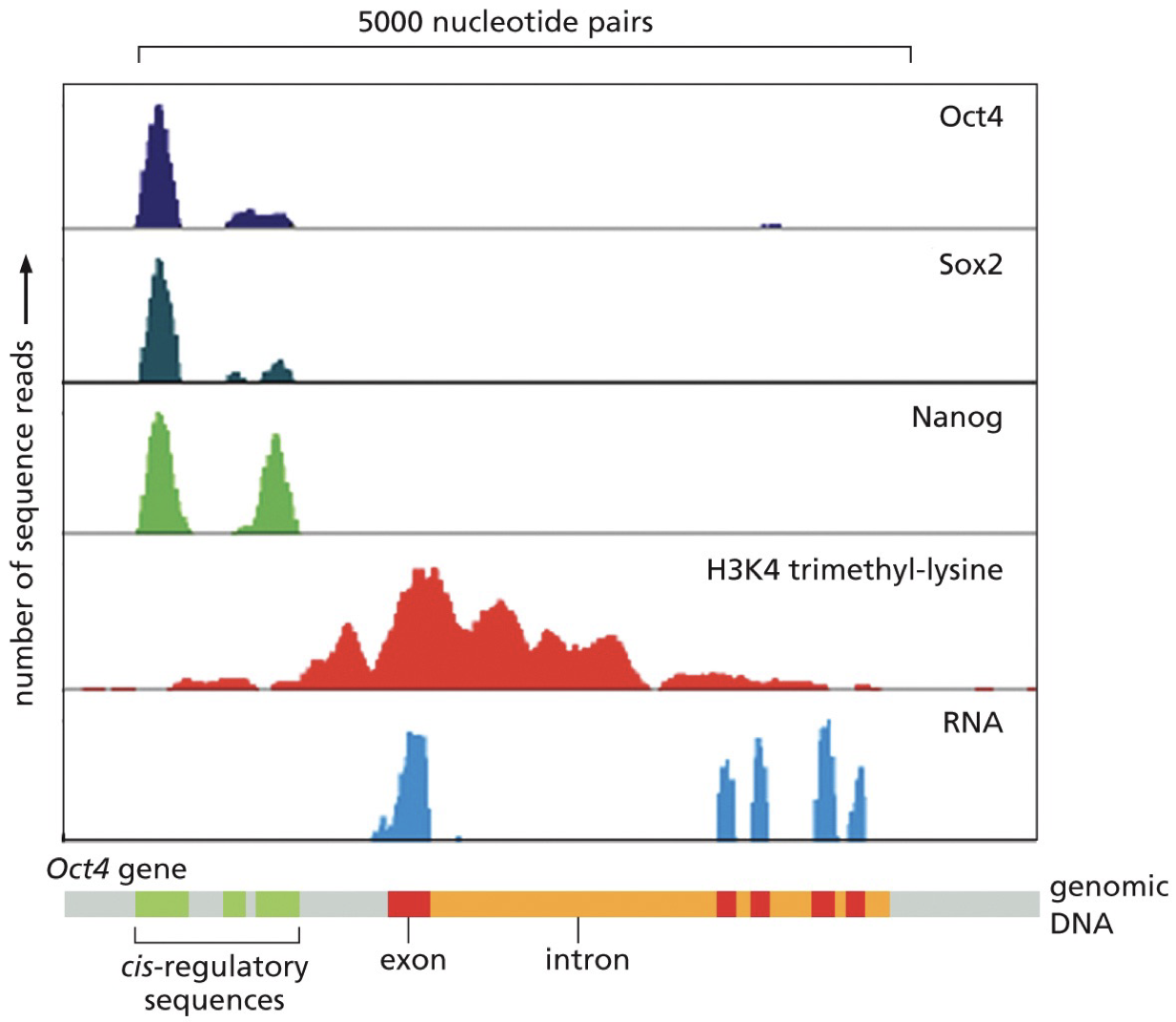
How enhancer sequences are studied
Reporter genes (transgenes): we can track and see when a particular gene comes on.
Green fluorescent protein (GFP): beta-galactosidase; fix and stain embryo for galactosidase activity. DNA sequences are fused to reporter gene.
Deletion mapping: deleting various segments, you may delete an enhancer. Seeing what sequences affect transcription.
Reporter gene fusion occurs when we fuse one strip of genes with reporter genes and track what cis-regulatory elements make the strip important.
Transcription Factors (TFs) or Trans-Regulatory Elements
Activator: Binds enhancer DNA elements
Repressor: Binds silencer DNA elements
Co-activators and co-repressors are recruited to help influence transcription.
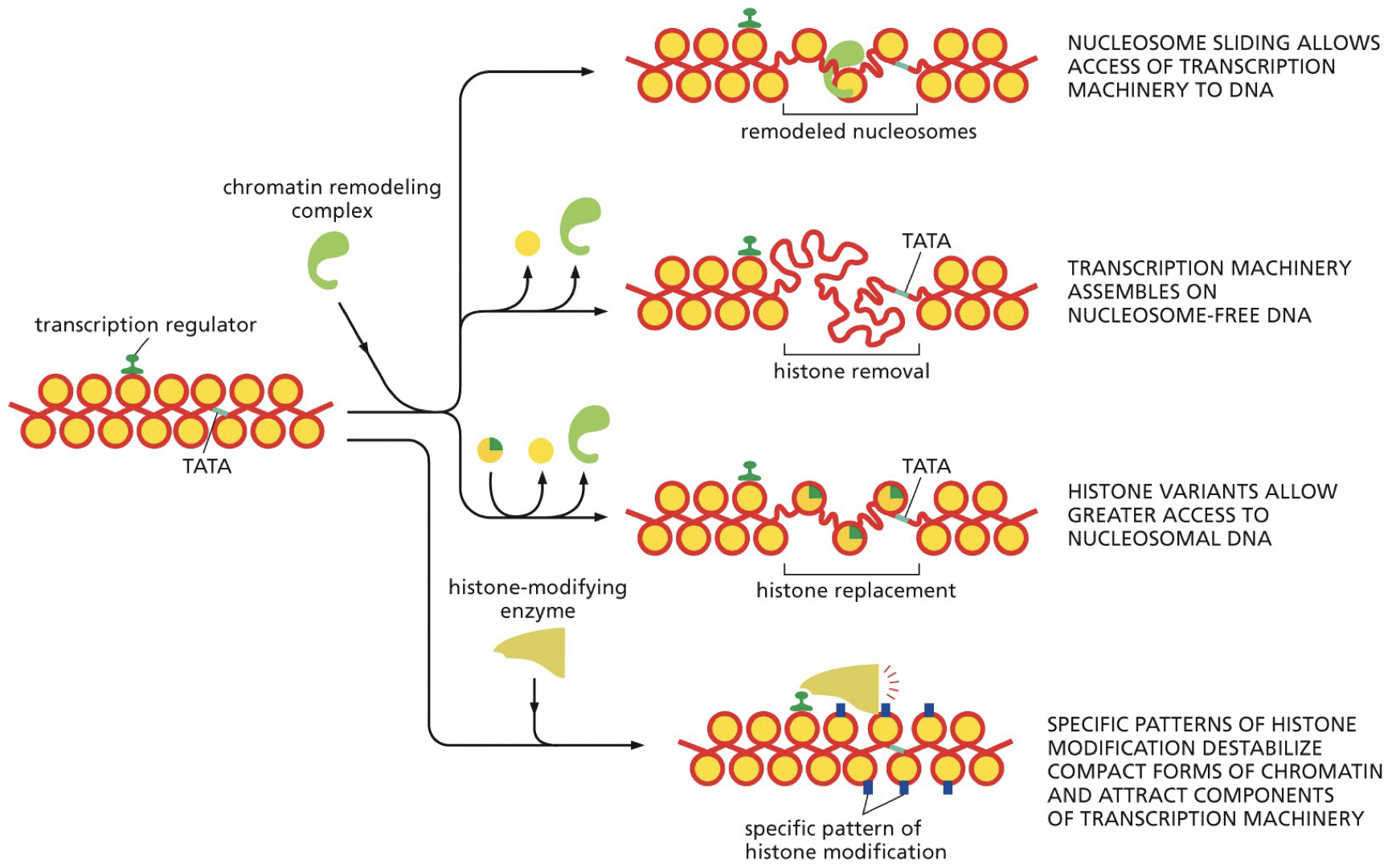
Nucleosomes affect TF binding. Some TFs need to destabilize nucleosomes, as their binding sections are facing towards histones. Major groove is used as a binding site.
Activators can affect chromatin structure. Different options for how to get to the promoter.
Sliding machinery
Transcription machinery
Histone variants
Patterns of histone modification destabilizes compact forms
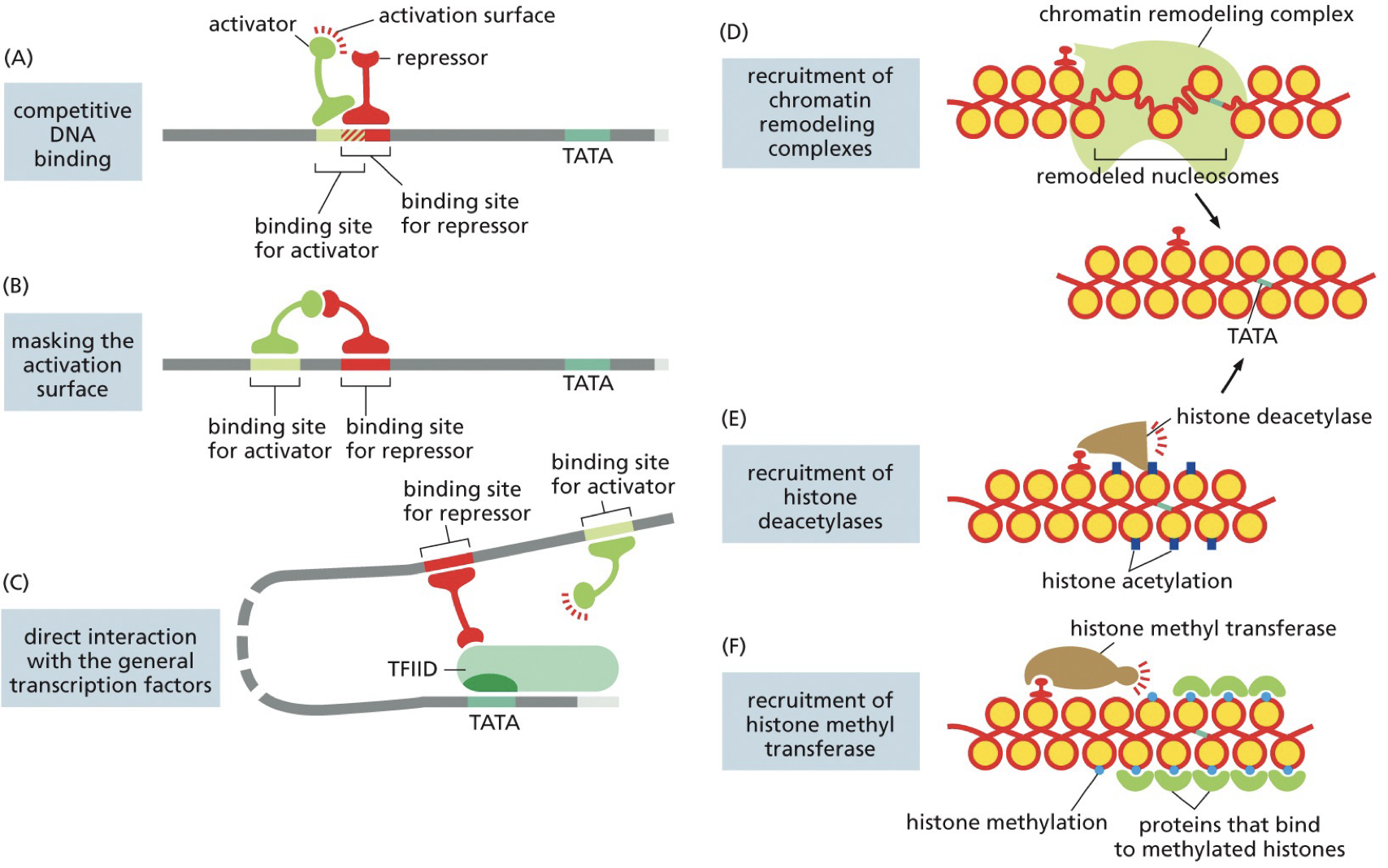
Opposing mechanisms to repress transcription
Activator binds but overlaps with silencer (blocked)
Repressors bind and interact
Direct interaction between repressors and GTFs
Chromatin remodeling complex
Histone deacetylase
Histone methyltransferase
Long non-coding RNA (lncRNA) is an example of a regulator not being a protein
They play a wide variety of regulatory roles, mostly in repression. Have to be transcribed to be active, sometimes in introns. Can act as cis or trans (only expressed regulator that acts in cis).
Histone reader-writer complexes and DNA methylation
They are passed on during cell division. TFs could come and repress or turn on, and then others can come and make different changes.
Insulators and Barriers: Stop enhancers and silencers from acting on the wrong gene as they are far from the start site. Insulators divide DNA into looped regions.
Insulators limit how far a cis element can interact from.
Create loop of DNA and things can interact with the loop and only that loop.
CTCF is the most well known example.
TFs act in complexes. The combination that interacts and causes what happens. TFs may differ in whether they turn genes on and off based on the co-activator or co-repressor present.
A transcriptional regulator can act on multiple different genes. Allows for cordinated regulation of whole sets of genes. This allows us to wuickly have everything needed for a program.
Eukaryotic networks
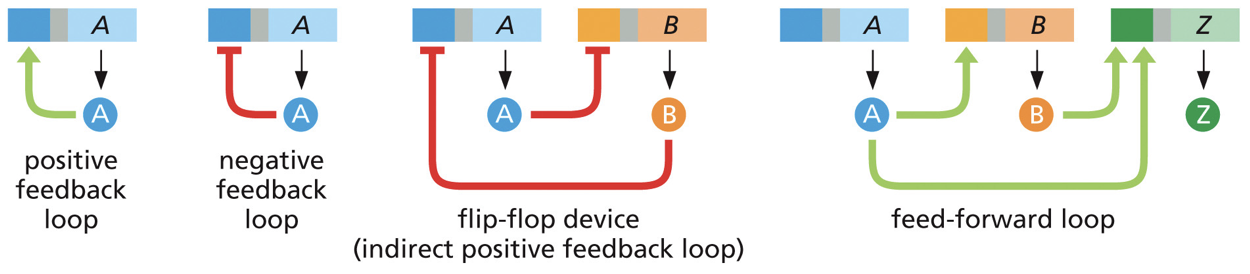 Once we turn on transcription, we could potentially turn on something to regulate.
Once we turn on transcription, we could potentially turn on something to regulate.
Negative feedback loops: thing we start transcribing feeds back to turn off
Positive feedback loops: turns on other genes involved in the same process
Activity of TFs depends on proteins present, and differentiated cell types depend on genes expressed.
Prokaryotic regulation
Has cis and trans regulatory elements, as well as benefits of polycistronic DNA.
There are multiple genes from a single promoter, multiple points for initiation or translation. Changes in gene expression depends on environmental factors.
Operon: operator
Tryptophan repressor
Binds to operon in presence of tryptophan.
When tryptophan operon is transcribed, everything comes together.
With high levels, it is a waste of energy. Operon is blinded by a repressor and turned off and then RNA polymerase cannot be recruited.
Lac operon
Has 2 regulators because if glucose is present, it is used as a preferred energy source, and if glucose is absent, the cells can use lactose as an alternative source of energy.
High glucose → low cAMP
Low glucose → high cAMP
High cAMP is CAP (catabolite activator protein) binding, and positively binding regulates transcription by enhancing attachment for RNA pol 1
cyclic AMP is a secondary messenger, and lets them know glucose status.
Post-Transcriptional Regulation
Everything at RNA level. Processing, stability, degradation, localization, alternative splicing.
Alternative splicing
One gene produces multiple different proteins. Those RNA transcripts can give different amino acid sequences.
Proteome complexity exceeds number of genes in eukaryotic genome. Constitutive alternative splicing has RNA itself regulated.
If spliceosome cannot recognize consensus sequence, it keeps moving.
Constitutively splice: might be 80% somewhere, 20% somewhere else.
ESE, ESS (exonic splicing enhancer/suppressor): control recruitment and how often something is included or excluded.
Negative control: prevention of access to splice site by a repressor
Positive control: where an exon is inefficiently read and left in and leads to recruitment of activator to splice sequence out. Removing sequence from final mRNA.
Splicing goes wrong
Once splicing is complete, we get EJCs (exon junction complexes) that mark completed splices. There may be an EJC with an extra stop codon, and it is recognized and targeted for nonsense-mediated decay.
Control over which mRNA are exported from nucleus and proteins connect 5’ cap and poly A tail to initiate translation.
Other editing issues
If C→U, we may end up creating a stop codon.
Issues may influence: secondary structure, stability of RNA, recruitment of translation machinery.
And in RNA editing to fix these issues, we may end up over correcting to get the C→U or A→I. Regulated by whether the enzyme involved in editing is present.
Alternate cleavage and polyadenylation
Two different cleavage sites, a weak and a strong. CstF jumps on poly a, and when there is not very much we are less likely to bind to the weak poly a site. With abundance we will have a higher likelihood of CstF binding different sequences.
This alters the 3’ end/C-terminus
Leaky scanning of kozak sequences can create version of proteins that differ at N-terminus. Alternative splicing can create splice isoforms with different amino acid sequences. Post-transcriptional RNA editing can create versions of the protein with different amino acid sequences.
3’ untranslated region on the end and 5’ promoter with N-terminus.
5’ and 3’ untranslated regions: sequences that do not code for proteins but are on mRNAs and interact with regulators and machinery.
Control: mRNA stability, mRNA translation, mRNA localization.
mRNA stability: capping a polyadenylation is in charge of stability and stopping degradation.
mRNA degradation: through recruitment of endonucleases. If consensus sequence is available, it can degrade mRNA and prevent it from being translated. Cis-acting sequences recognized by specific protein of whether we get cleaved of a message.
Protein binding to block translation.
Proteins bind to cis-acting sequences often found in 3’ UTR.
More mRNA regulation
Blocking the Shine-Dalgarno sequence in bacteria. It is temperature sensitive and increasing the temperature causes chain in hairpin, and pathogens change.
mRNA localization: specific localization of RNA through multiple mechanisms. Sequences in 5’ and 3’ UTR come into contact with a specific thing and you can have a localized anchor, protection, and transport.
It is useful: localized translation where protein is in high demand, allows for unequal distribution into daughter cell. One stays a stem cell, one differentiates.
Axons on neurons are long and contribute to active transport. Cell migration with leading edge that requires a lot of actin proteins.
A particular cell that is going to involve localizing certain RNAs to one end in order to guide development and set up body plans.
In development: patterns of division is carried out by proteins and RNA stored in egg before fertilization. All cells went directly to location without any DNA of their own.
Bicoid protein: head and thorax
Oskar protein: germ cell in posterior
Regulation of mRNA in P-bodies
P-bodies: sites of mRNA degradation or storage of translationally repressed RNA. Abundant in decapping enzymes. Dots light up, not membrane bound. Regulate whether RNA is localized or not.
Stress granules: Accumulates under stressful situations, work similar to P-bodies. Move stuff into stress granules until stress has passed.
Regulation using non-coding RNA: lncRNA can bind complementary sequences and recruit proteins to act on those genes. They can recruit and have functions in regulation, can complementary base pair to target particular RNA or DNA.
Exertin effect while attached to RNA polymerase (in cis)
X-inactivation: lncRNA Xist acts on chromosome and starts producing Xist RNA. When one starts to work, it is going to work to recruit mediators of epigenetic silencing.
Insulators might help separate out the few loops of DNA that remain transcriptionally active by creating looped domains.
RNA interference (RNAi): More non-coding RNA. Superpower of complementary base pairing. A number of genes are regulated by this. Small pieces of RNA is made and it recognizes targets by complementary base-pairing and regulating a large number of eukaryotic genes.
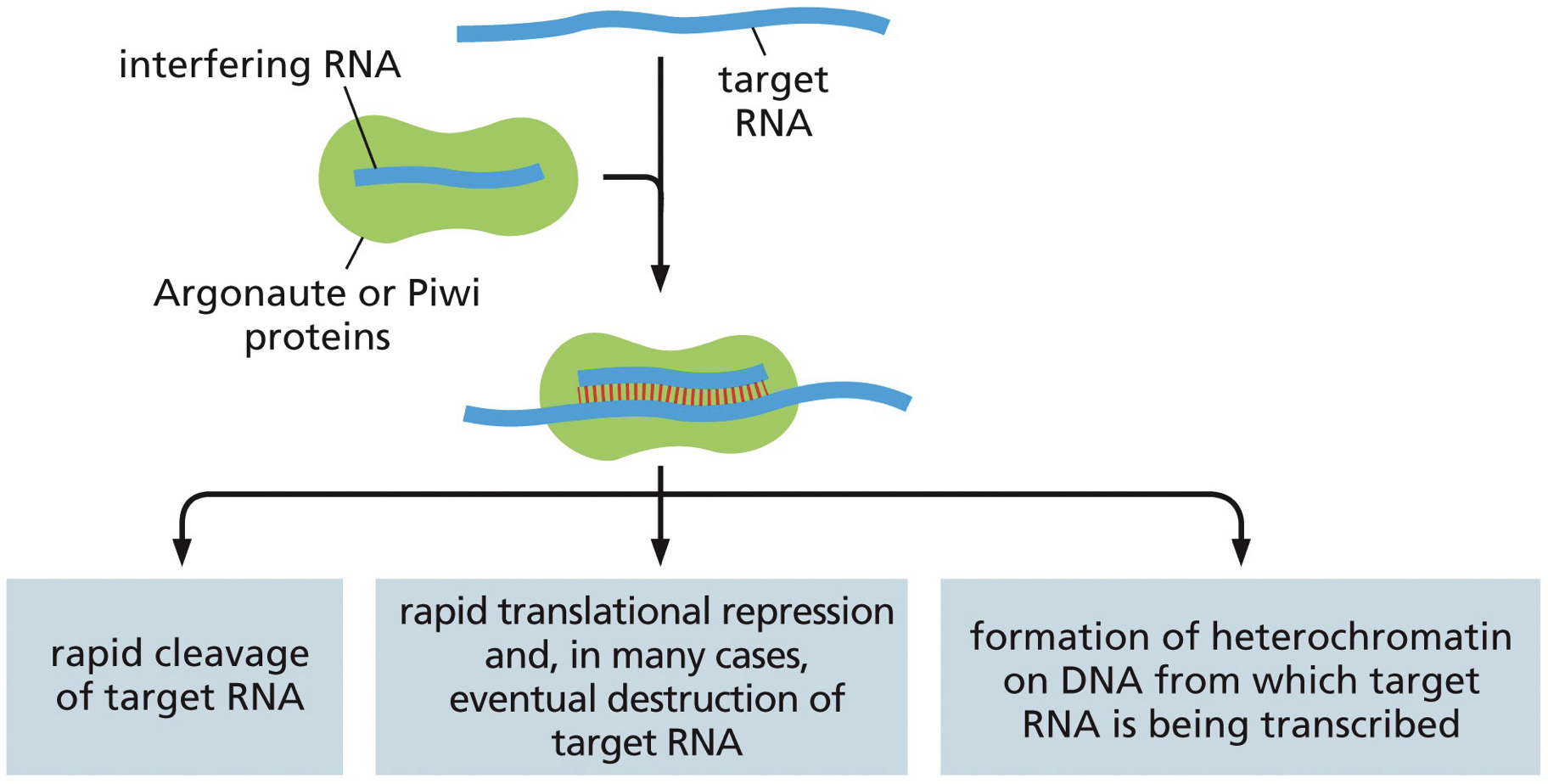
Process
Single stranded RNA binds to a complementary RNA sequence and directs protein to the site
ssRNA is loaded onto a risc complex
Rapid degradation or rapid translational repression
miRNA → processed
siRNA → not?
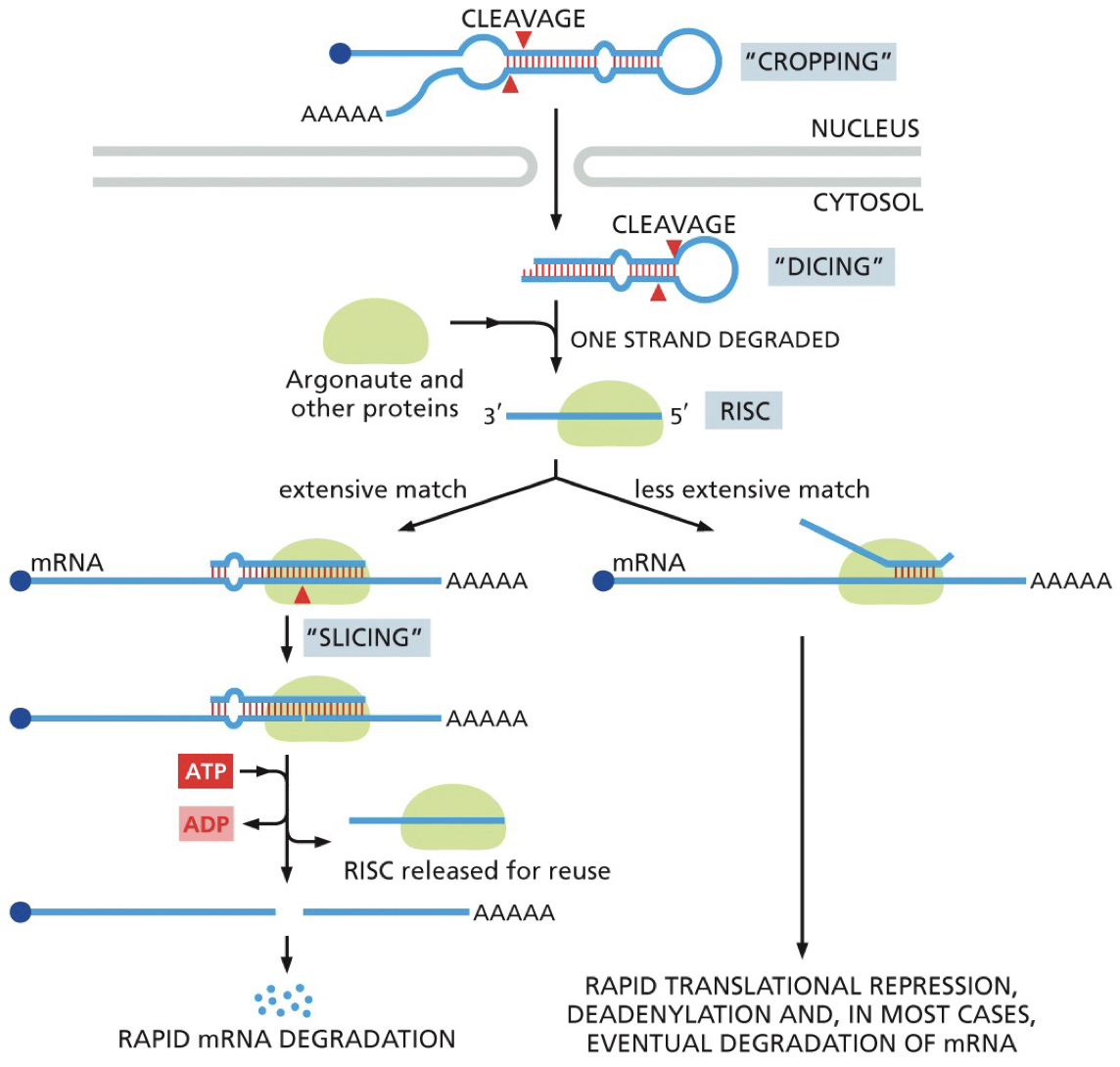 Processing miRNA
Processing miRNA
More than 1000 miRNA, half of genome is regulated by them.
Form hair pin and then are processed to ssRNA.
Dicer enzyme: cleaves dsRNA
RISC complex where 1 strand is degrade leaving miRNA
Argonaute protein (of RISC complex) is the key.
Once released, it will work again.
A single miRNA regulating many different mRNA is useful during development: space saving. Using all maternal contribution to do everything before using new stuff.
Processing siRNA
Evolves from miRNA
Does basically the same process (recognize, cut it up, targets genes based on complementary base pairing)
dsRNA comes from outside the cell.
The ds siRNA can be transfected into cells to knockdown expression of a target mRNA
RNAi is through to have evolved as a cell defense system. Protects again dsRNA of viruses.
Prokaryotes have a similar system where small non-coding RNA will target/clean up.
Short viral DNA sequence is integrated into CRISPR locus.
Immunological memory: it can take viral DNA and express viral sequences and use it as RNA template to make an RNA template for the immune system
Cas protein does the cuts. CRISPR locus is where stuff is stored.
CRISPR - clustered regularly interspaced short palindromic repeats
Has viral and repeated sequences, helps with loading. Directs Cas if there is a reintroduction of a virus.
Bacteria doesn’t have extra junk DNA, but keeps that stuff to fight against other viruses.
CRISPR/Cas9 genome editing
Swap out guide sequence and target double strand breaks. For permanent deletion, minimal change could still cause issues.
To avoid frameshift, an edited gene in place is added so nothing changes about regulation of transcription or leads to cancer.
****
Regenerative Medicine and Gene Therapy
Gene therapies: therapies where we alter or introduce DNA sequences to create a therapeutic benefit.
CRISPR/Cas9 - it is now easier to make a disease in a mouse to see how it is shared. Different versions are being produced at a growing rate.
A defense mechanism in bacteria using small noncoding RNA molecules (crRNAs) to seek out and destroy invading viral genomes through complementary base-pairing and targeted nuclease digestion.
For sickle cell disease therapy, CRISPR gene editing involves fixing a problematic gene in the patient’s own hematopoietic stem cells and then reintroducing those cells back into the patient
In mammalian cells: NHEJ over Homology-Directed Recombination due to it’s efficiency and how many mammalian cells there are.
In experimentation HDR may be chosen however it likely will not be due to its errors and off-target effects
In germline cell editing, there is a greater ethical debate, because in somatic cells, the alteration will die with the individual, however with germline cell editing, you could potentially add the alteration into the gene pool forever.
However some disease must be edited at a germline stage
Diseases we can ethically edit out or which make for good editing are diseases that negatively impact quality of life and/or lifespan, are caused by a mutation in a single gene, and the cells causing the disease are easy to get rid of (good for regenerative medicine).
Regenerative medicine: Seeks to restore tissue structure and function, like a skin graft for burn victim, transplant, and letting tissues self renew.
This does not involve altering germline cells. It is taking cells out, modifying, and putting them back in.
This is cell therapy and can be combined with gene therapy and tissue bioengineering.
Yamanaka factors: a somatic cell can be reprogrammed to a pluripotent state by the expression of these.
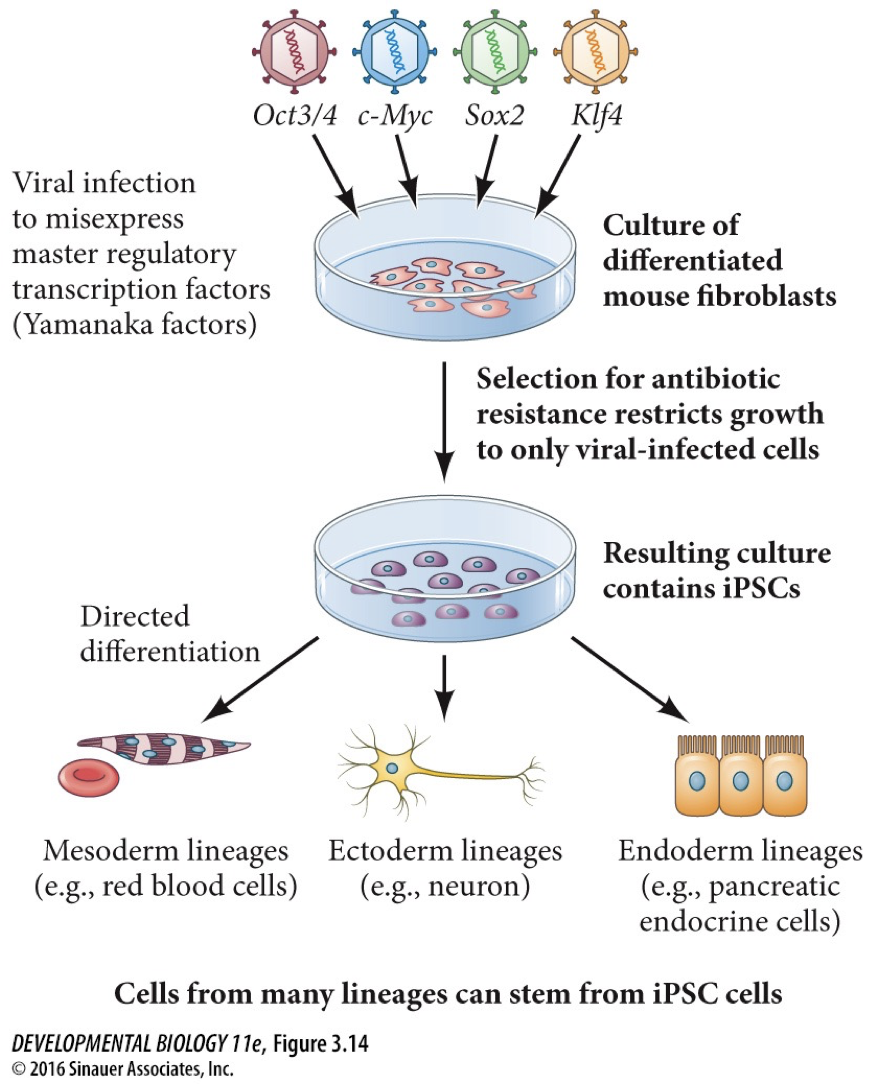
Induced pluripotent seme cells (iPSCs): you are inducing them by adding master regulators
This discovery was important because the source of pluripotent cells that is genetically identical to the patient’s cells can be introduce easily.
FDA approved stem cell therapies
Leukemia and sickle cell diseases
No CRISPR yet, only bone marrow or hematopoietic stem cell therapy has been approved so far.
Are cancers good targets
No, cancer is caused by multiple mutation and there is too many for this to make sure. Most of our approaches to cancer therapy is not about editing the cancer cells themselves
Post-translational regulation
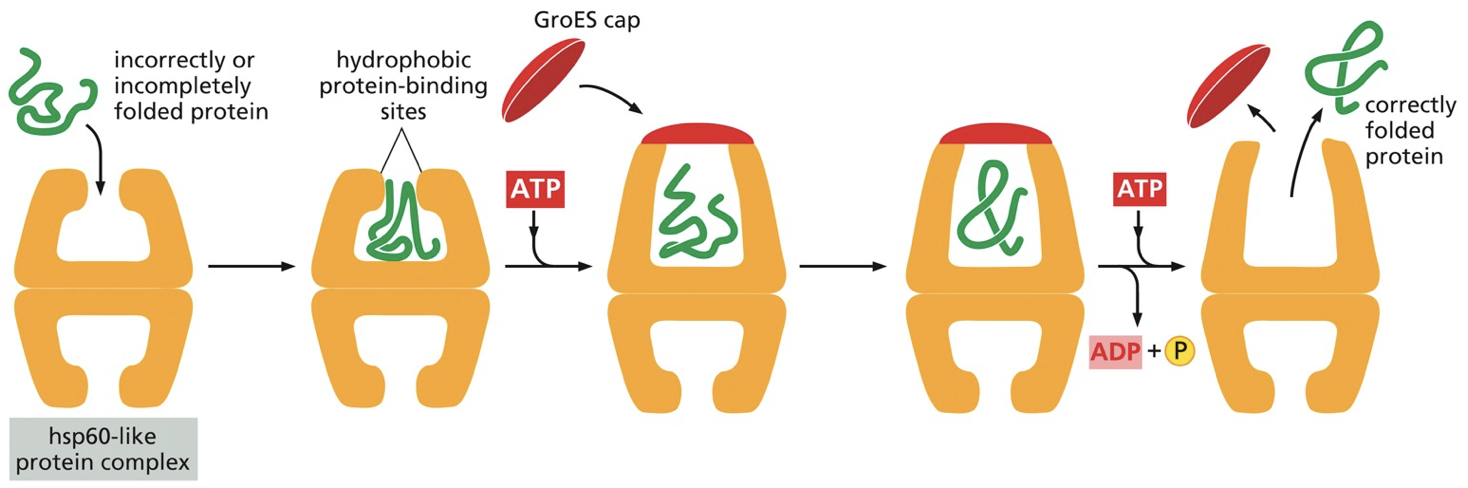
Molecular chaperones help with and lead to correct folding of proteins by repeatedly binding to hydrophobic and partially folded patches and releasing them.
Hydrophobic regions hold a protein together. Heat shock proteins (hsps) (a molecular chaperone) increases expression in response to heat. Hsp60 creates a hydrophobic barrel region for folding to occur.
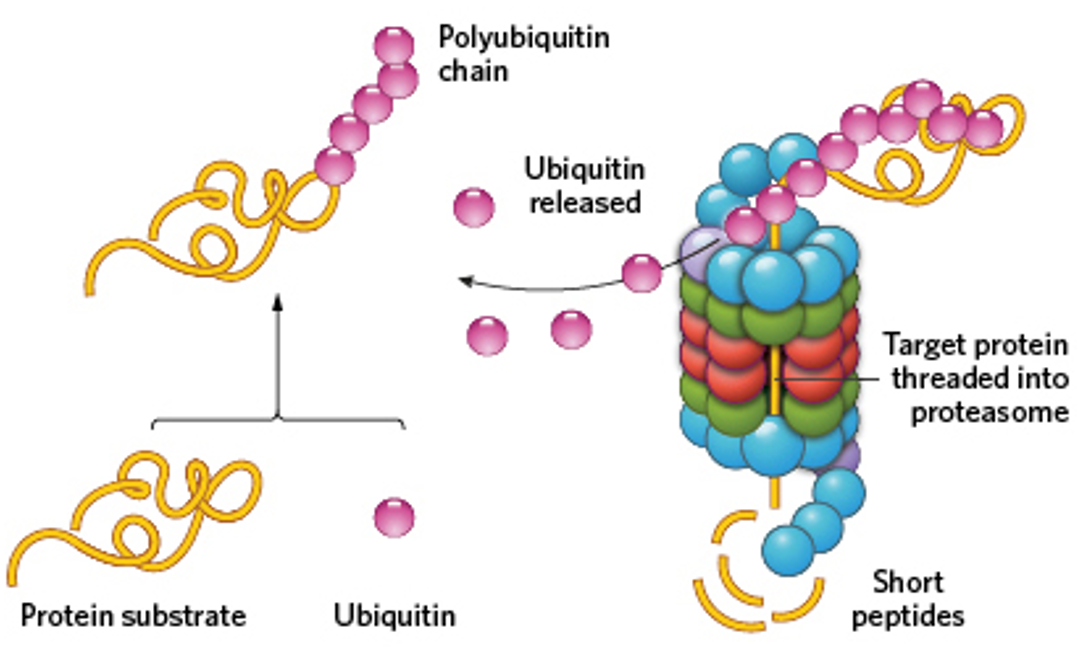
Proteasomes are abundant sites for protein degradation. They cleave polypeptide chains and release peptides. Misfolded regions are marked with polyubiquitin chain, and the polyubiquitin codes protein. We can tell a region needs to be degraded based on its hydrophobic regions.
Ubiquitin ligase adds polyubiquitin chains on, but can be regulated. Could have binding of a specific ligand, phosphorylation or other subunit to control. Covalently added.
Ubiquitin ligase complex is activated by a particular signal for a given amino acid.
Destabilizing the N-terminus, recognition site is created.
Post-translational modification: Allows for rapid changes in protein activity, localization and stability.
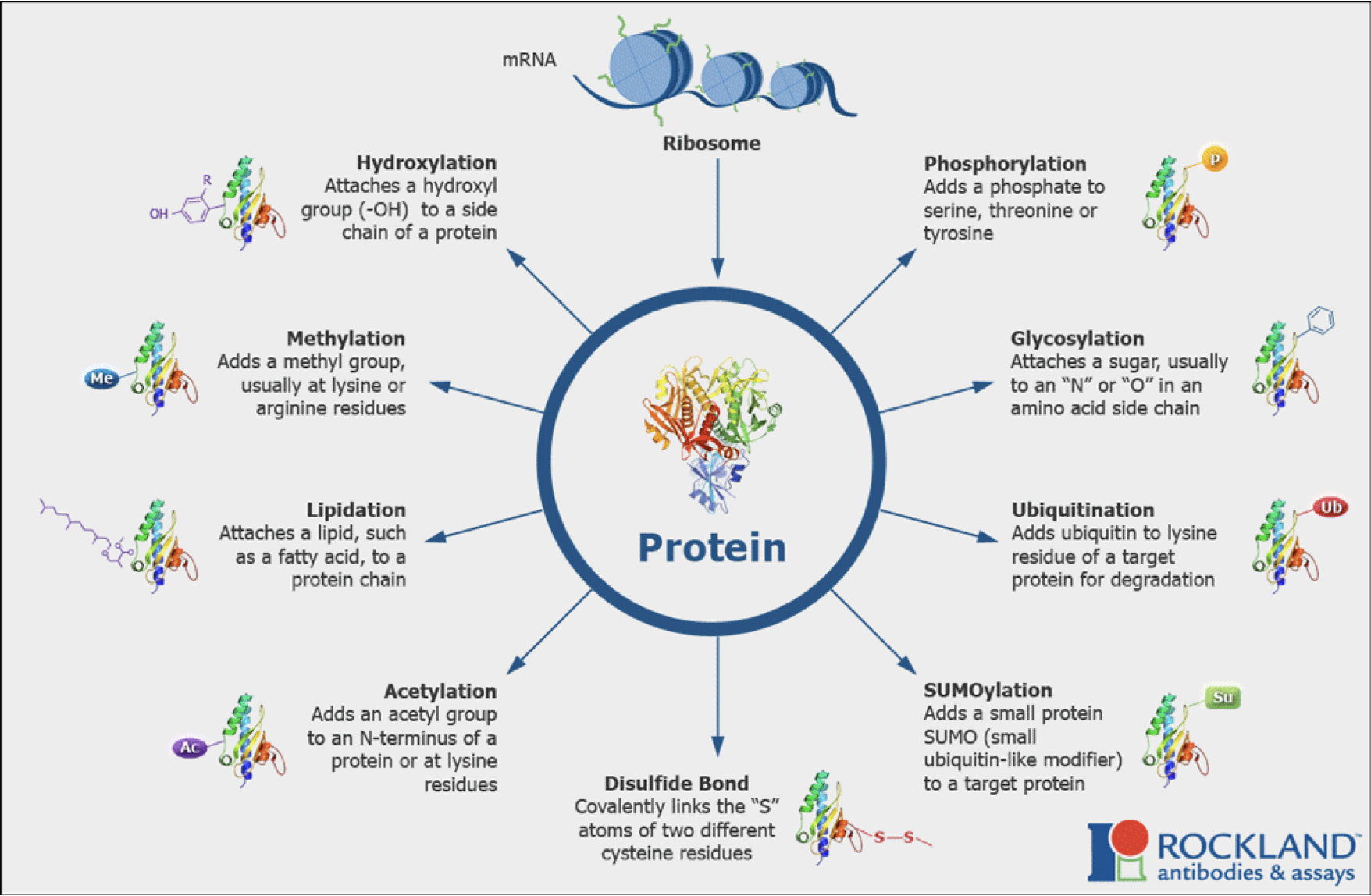 Ubiquitination is one way to modify. There are many covalent addition onto proteins which may have facilitated the process. Acetyl/methylation - Histone tails are an example of a protein being acetylated and methylated.
Ubiquitination is one way to modify. There are many covalent addition onto proteins which may have facilitated the process. Acetyl/methylation - Histone tails are an example of a protein being acetylated and methylated.
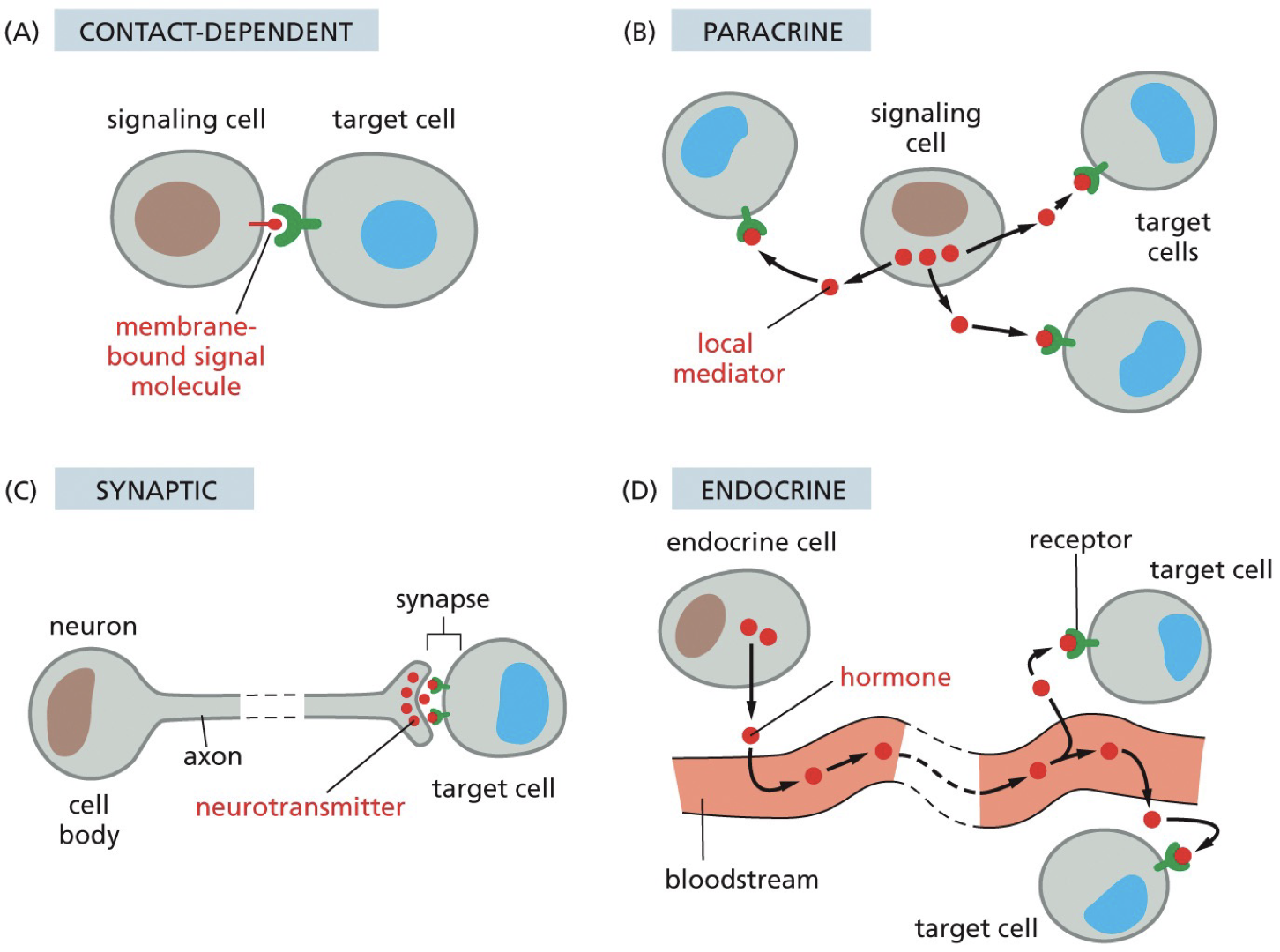
Signal transduction pathways
Transducing a signal in cytoplasm. Receptor and ligand binding, passed around and it is amplified. This is the key to cells communicating with each other. To make a functional tissue or to fix a broken bone or sickness, cell-cell communication is imperative.
Endocrine pathway: signalling factors that can travel long distances
Paracrine pathway: signalling involves secreted factors with a limited range
Autocine pathway: signalling involves secreted factors with a limited range, in bacteria.
Juxtacrine pathway: signalling mediated by contact, not secreted. Homophilic and heterophilic binding.
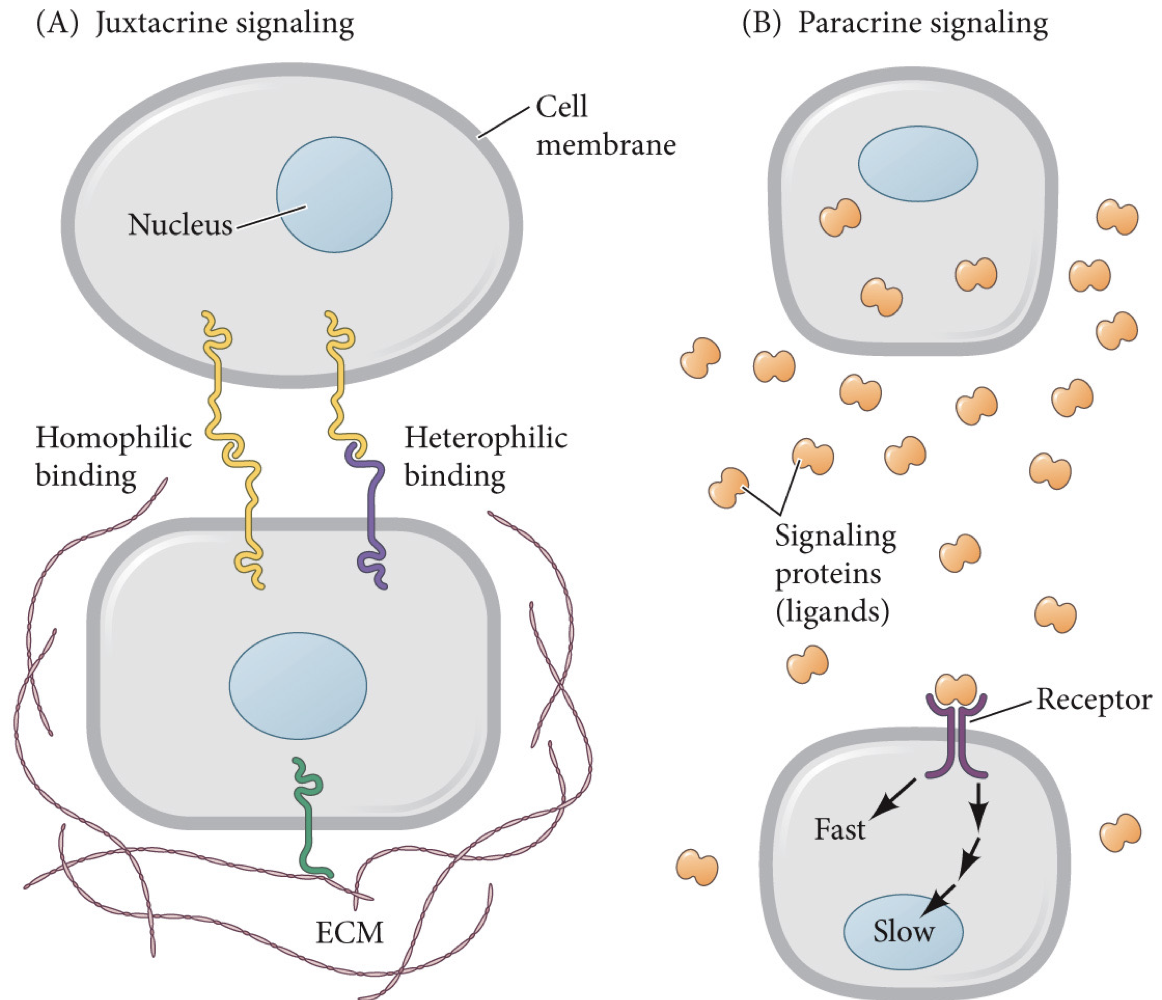
Signaling factors
Nuclear receptors: different from above. Signaling receptors can be in the cell. They are usually transcription factors, Ligand binding results in a change in control of transcription.
There are a limited number of signaling factors (like transcription factors)
We have a different effect on different cell types. Combination gives a different overall response.
Some signals are required for survival (maintenance)
Integrins are proteins that help cells adhere to extracellular matrix and interact with actin.
Part of cells sense the environment.
Anoikis: A term for apoptosis that occurs in response to loss of adhesion (lack of integrin signaling).
Example: Mouse mammary epithelial cells were grown in tissue culture plates made of plastic coated with the specializing extracellular matrix they usually interact with in the body (called basal lamina). In lab, they started turning off genes and turning on milk proteins. You can get the proper shape if you have correct ECM, and the phenotypes differ.
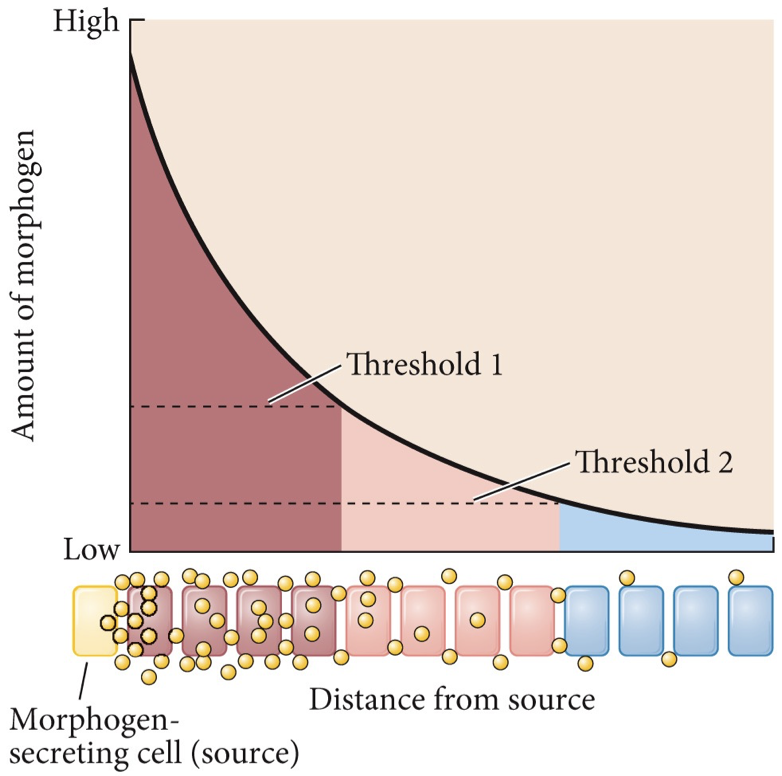
Morphogens: Guides phenotype and development of cell fate. Cell fate is concentration-dependent of cell signaling, based on how far or close they are to morphogen-secreting cells. Our tissues are often composed of different cell types, it is not about what cells only but also where they are.
Signal responses can be fast or slow, may need processing. Fastest responses are going to include direct modification of existing proteins to rapidly alter cellular processes.
Molecular switches
Turning proteins from active to inactive (vice versa) quickly.
Based on if they are bound to GTP or GDP.
GAP - ATP hydrolysis
GEF - stimulate release of GDP so that GTP can bind
Oncogenes have uncontrolled signaling.
Deletion of a GAP and a mutation that impaired or slowed down the ability of KRas to hydrolyze GTP could promote aberrant increase signalling of a KRas GTPase.
Phosphorylation can change protein conformation, catalytic activity, and can create/destroy binding sites for other interactions. Kinases add P from ATP to amino acid. Phosphatase removes P.
Influences signal transduction pathway. If we cannot bind, it will mess stuff up, and the P can make it active for binding.
Different specificity in kinases and phosphatases to act on different proteins - other molecular switches.
Tyrosine kinases - phosphorylates tyrosine amino acids
Serine/threonine kinases - phosphorylate serine and threonine amino acids.
****
Cell signaling
Phosphorylation can create molecular switches that regulate protein binding and catalytic activity. Can change protein conformation, catalytic activity, and create or destroy binding sites for different interaction. Phosphorylation can activate signaling protein or pathway.
Phosphatases are more commonly destroyed by cancer, which means they turn off proliferation, and they do not limit signaling.
What can help identify changes in expression
RNA-Seq - taking all RNA and sequencing will show us the expression
ChIP-Seq - Finding out where you protein of interest binds may be able to tell about mutations.
We can make signaling pathways turn on or off.
Tyrosine-kinase is autoinhibited into a state with a phosphorylation site. The whole complex is turned off until growth is activated, or other things like cell mobility. Maximally ready to go when Tyr416 is phosphorylated (normally by Src)
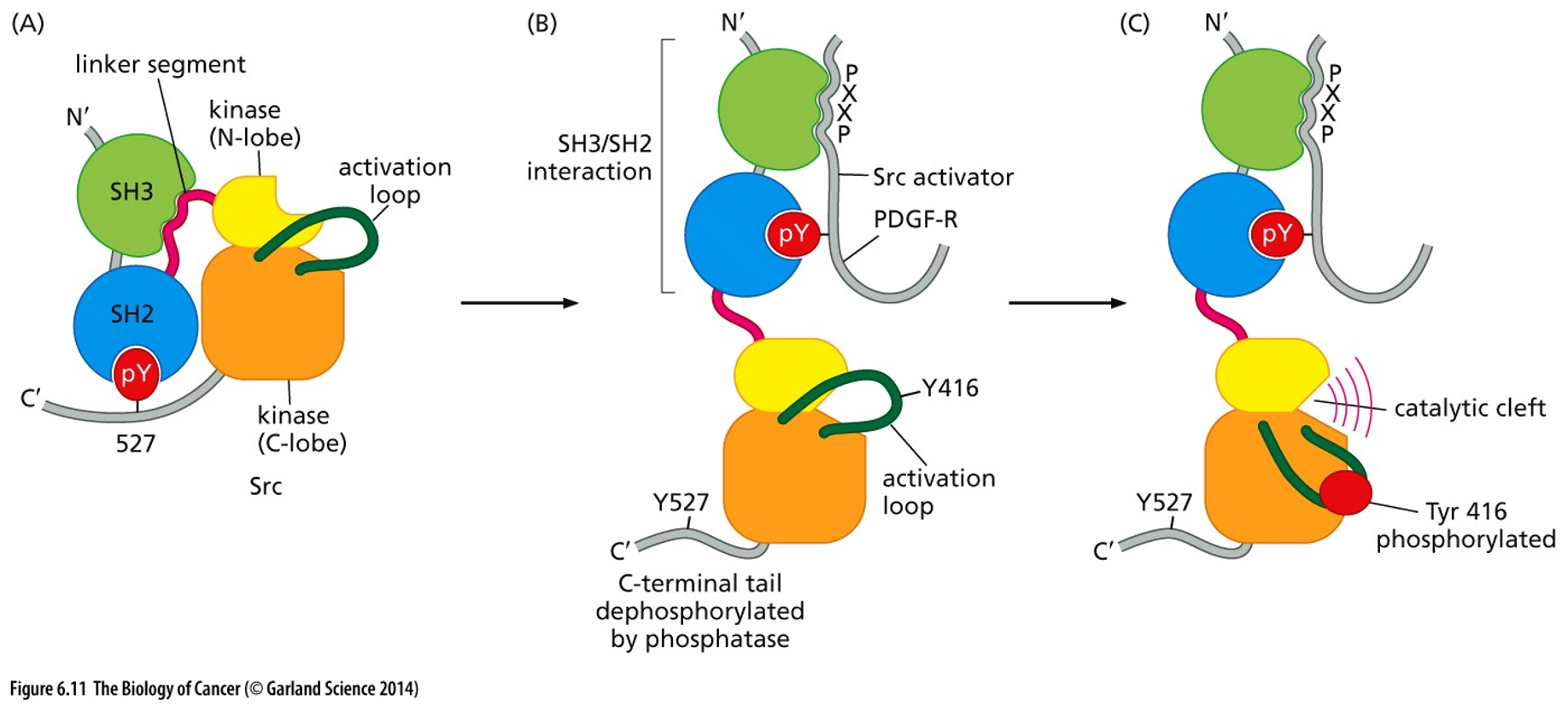
Src mutations that make cause an increase in Src kinase activity
A point mutation that changes tyrosine 527 (loses inhibitory interaction)
A mutation to SH3 that prevents linker binding (loses inhibitory interaction)
A mutation that creates an overabundance of Tyr-phosphorylated Src binding proteins.
Nonsense mutation shortly after C-terminal loses inhibitor pY
V-Src
Src is first seen as the V-Src oncogene.
People tried finding tumour causing viruses, and there aren’t many but this is one.
V-Src has a small genome, easy to pick out
Sequences of very similar gene to the gene in the genome. Increases the expression, virus goes and effects the cell, bringing normal genes with it.
C-Src is the gene already found, and V-Src is the corresponding virus.
Western blot: Detects specific protein(s) using specific antibodies.
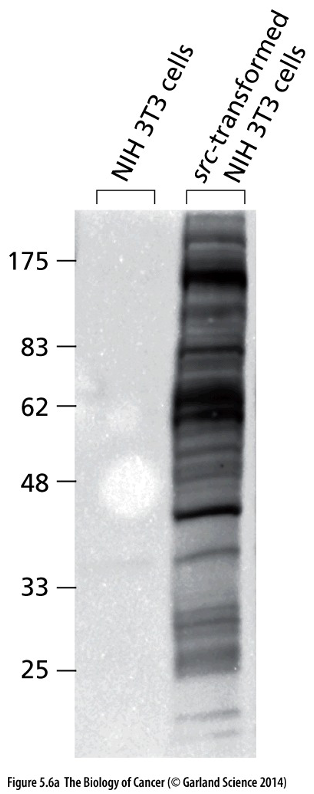
When comparing Src gene sequences in virus and Src genes in chickens, viral oncogenes had increased activity because it was truncated/ended early, cannot phosphorylate to inhibitory point.
Many proteins involved in early cell division and cell growth are connected to oncogenes.
Mechanisms related to oncogenes and cancer progression
Cell growth and survival
Cell motility (metastasis: to travel around the body and make tumours at other sites)
Angiogenesis (making tumour blood supply)
Oncogenes are a gain-of-function mutation: turn on pathways promoting processes that drive growth and survival of cancer cells. Mutations in KRas and Src.
Tumour suppressors: genes typically contain a loss-of-function that remove a protein that usually functions to limit one of these cancer-promoting processes.
P53 - most common. Normally would stop cell division by causing apoptosis. Has an important sensor that detects and stops.
Src activation is going to activate cancer pathways, and that could allow for upstream receptors being activated. Ligand stimulation.
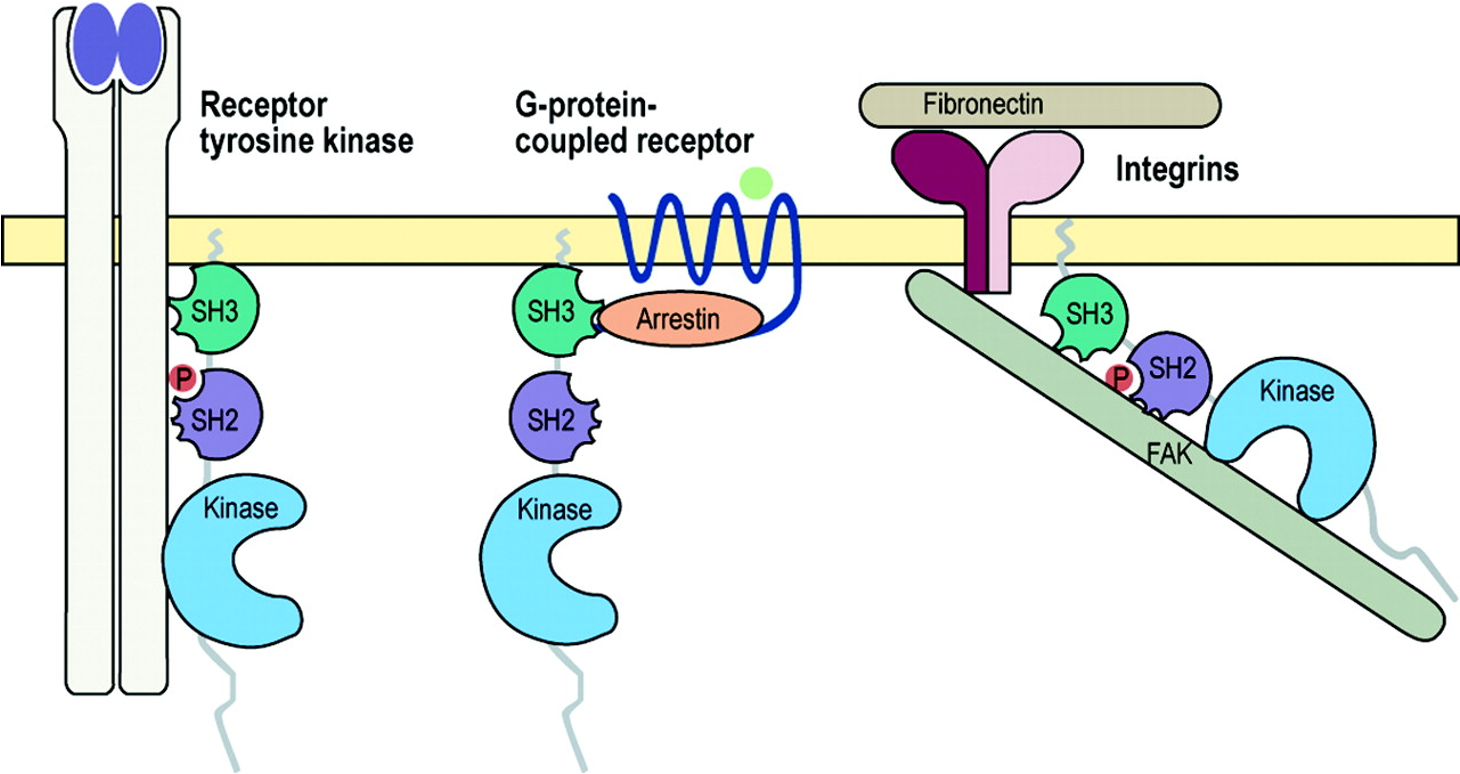
Surface receptors
Enzyme coupled receptors: ligands binds and turns on signaling
RTK (receptor tyrosine kinase) - action of one on the other turns on activity of other. Siblings.
Downstream and upstream based on where it starts
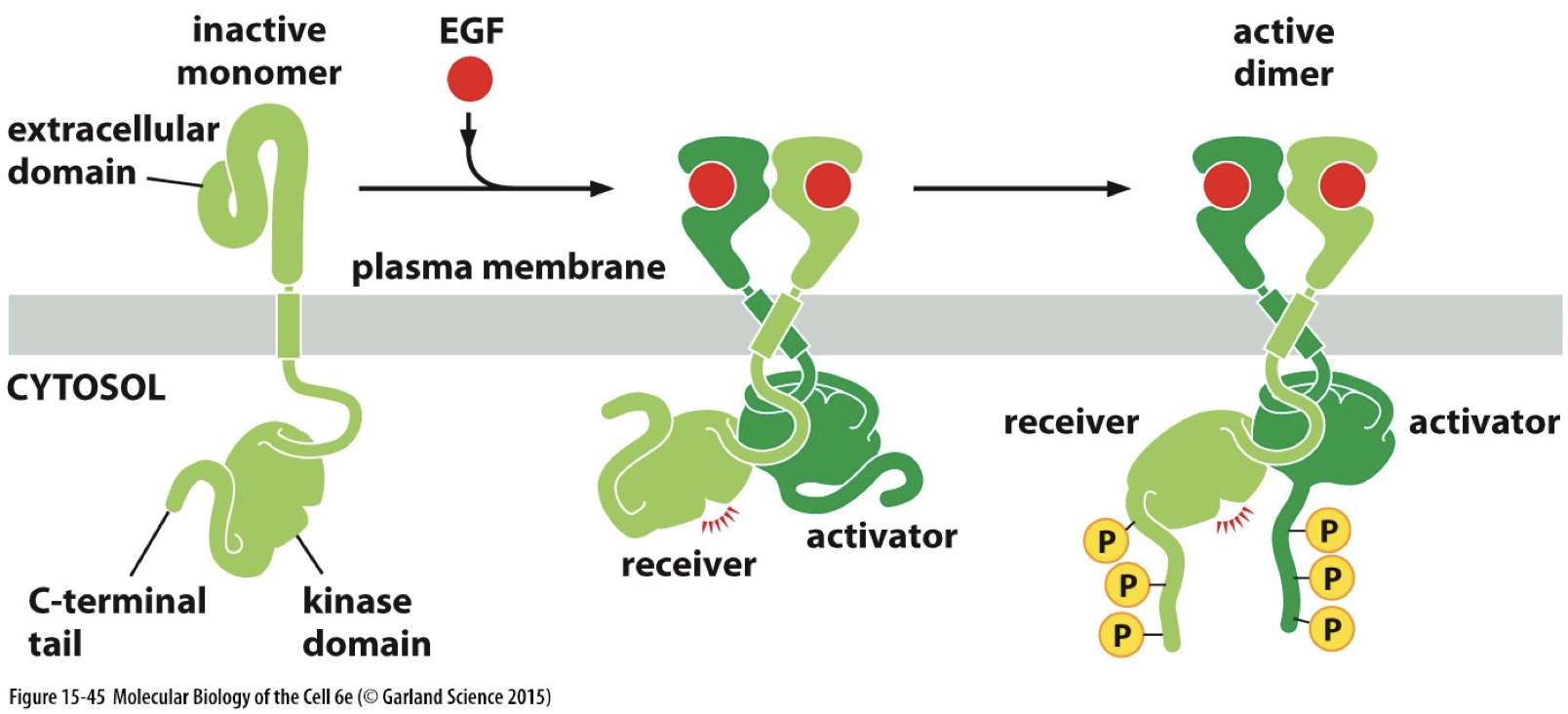
Ligand binding - dimerization activity. Results in conformation change that activates the kinase domain to phosphorylate targets in cytosol.
After growth factor binding, the RTK will begin to phosphorylate tyrosine residues in target proteins.
Likely shows sequence similarity to Src
Extracellular domains differ and bind to different signaling molecules from inside the cell.
EGFR (example of RTK)
Epidermal growth factor receptor
Does not have extracellular receptor
Has a tyrosine kinase domain homologous to Src (which is like an RTK without receptor activity)
Has receptor function and tyrosine kinase activity
The EGFR family is related to epithelial growth
Variety of signaling molecules
Regulates cell behaviour
Pathway contains many potential oncogenes
HER1, 2, 3, 4 are the receptor family
EGFR signaling → apoptosis, migration, growth, adhesion or differentiation.
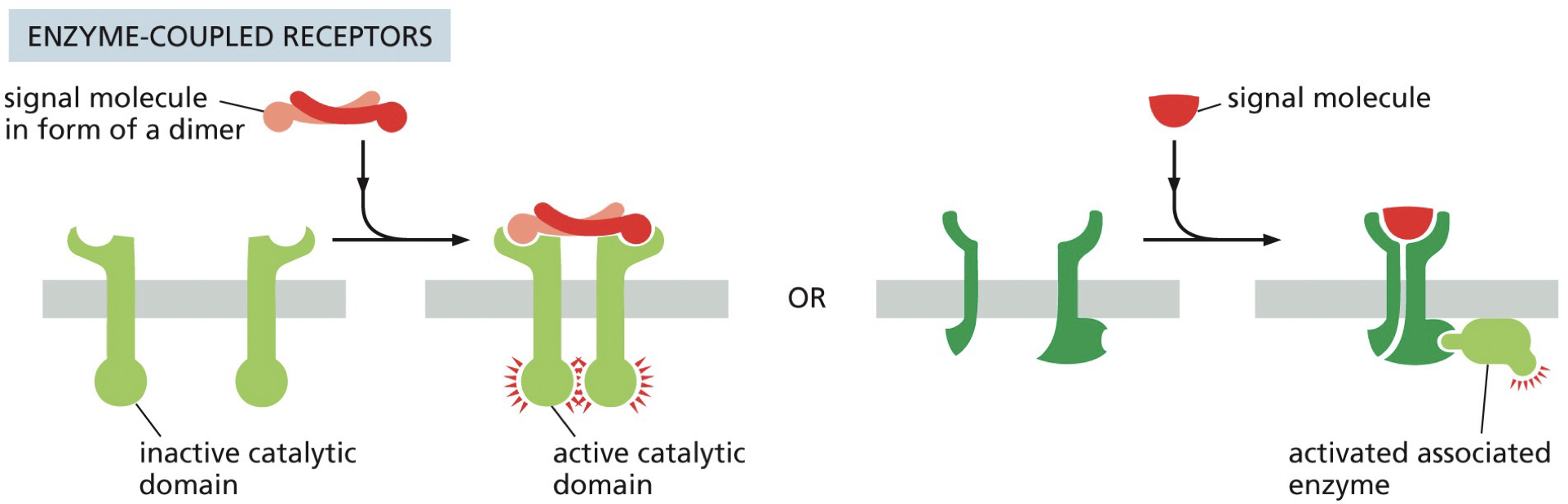
Truncanation: A tumour virus helped to identify one of the oncogenic mechanisms of activation. Allows cytoplasmic domains to come together and not just get ligand bonded, can be mimicked by truncating the receptor. Removes extracellular domain, leading to cancer.
Normal cells turn on for a limited time, but cancer cell is continuous due to confirmation change or increase in expression of receptor leads to more signaling (density, accidental activation)
Autocrine signaling may happen in cancer cells: cancer starts to make its own ligand. Cancer cell can stimulate their own growth.
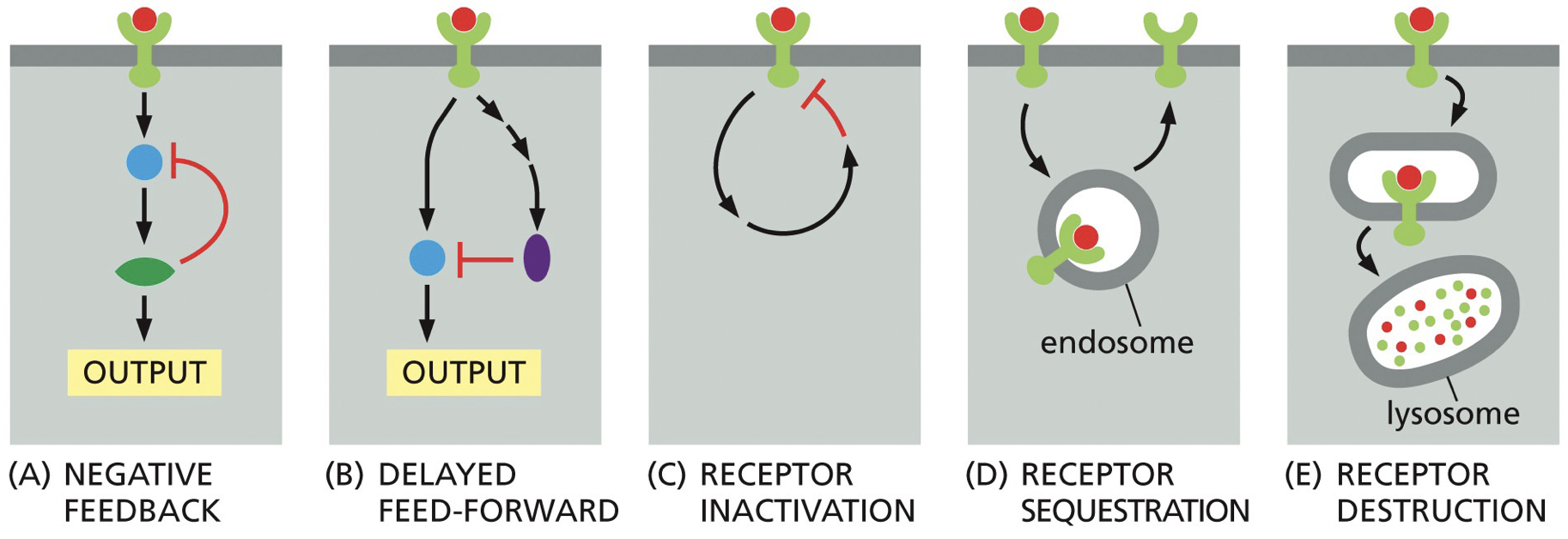
Ligand-gated channels: where binding with ligand allows movement across membrane (ions)
Ion-channel receptor: signal molecule works and there’s binding. However, they can be released and have channel be flooded by cell.
Neuroreceptors.
G-protein coupled receptors: crosses the membrane 7 times
Also called GPCR
Crosses 7 times. They bind ligands on extracellular side, turns the receptor into a GPCR. GDP gets removed, allowing GTP to bind and signaling pathway to be activated.
Similarities and differences to RTK
Both are cell surface receptors
Both can receive secreted signals to participate in cell-to-cell communication
Both undergo conformational changes upon binding to a signaling molecule
GPCR cross membrane 7 times, and do not have a cytoplasmic kinase domain
RTK is not a GEF
Growth factors are signaling molecules that stimulate cellular processes like survival, growth, repair, and differentiaition. There are often oncogenic mutations in growth factor signaling pathways.
Involves paracrine signaling (secretion of signaling molecules and diffusion of cells)
Oncogenic mutations drive uncontrolled, increased signaling
Src and Kras are intracellular signaling proteins
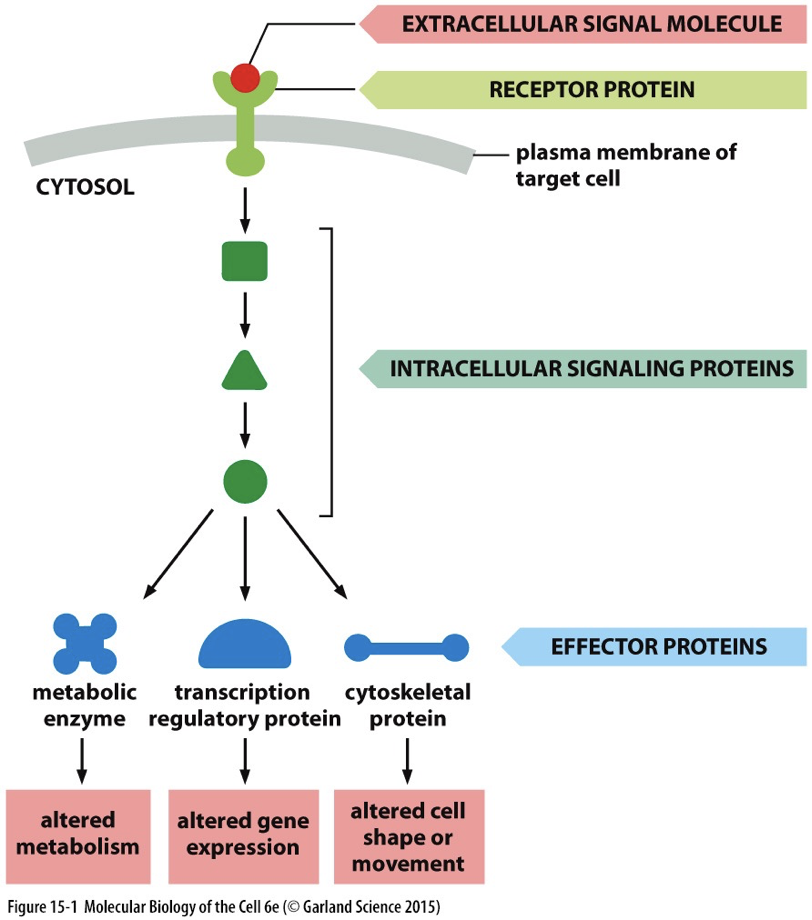
Major ways to assemble intracellular signaling complexes
Protein formation and complex formation help create efficiency and precision in signaling cascades
Assembly on an activated receptor (phosphorylation)
Proteins bind to specific amino acids with phosphorylated site. (GEF and Ras GTPase)
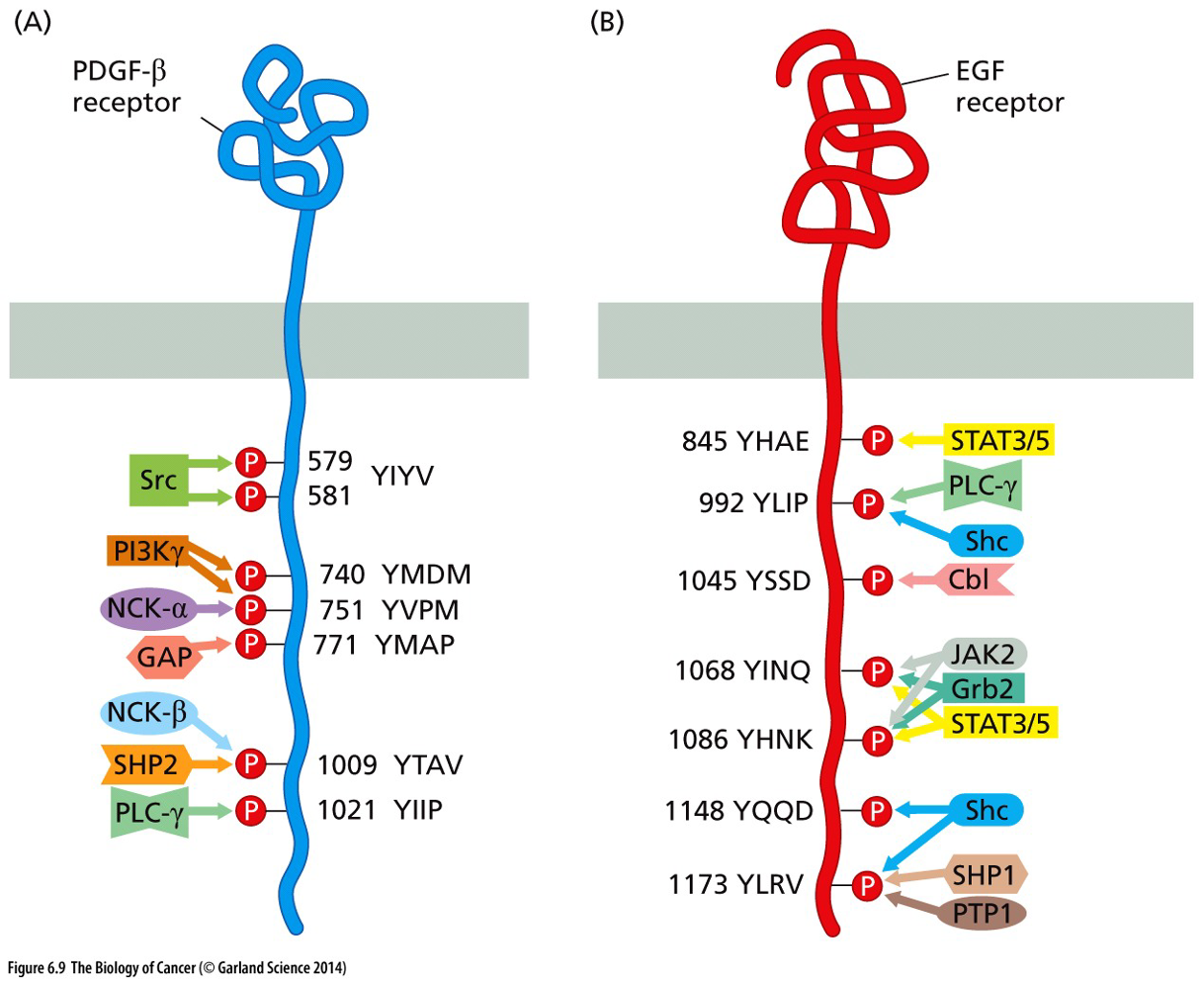
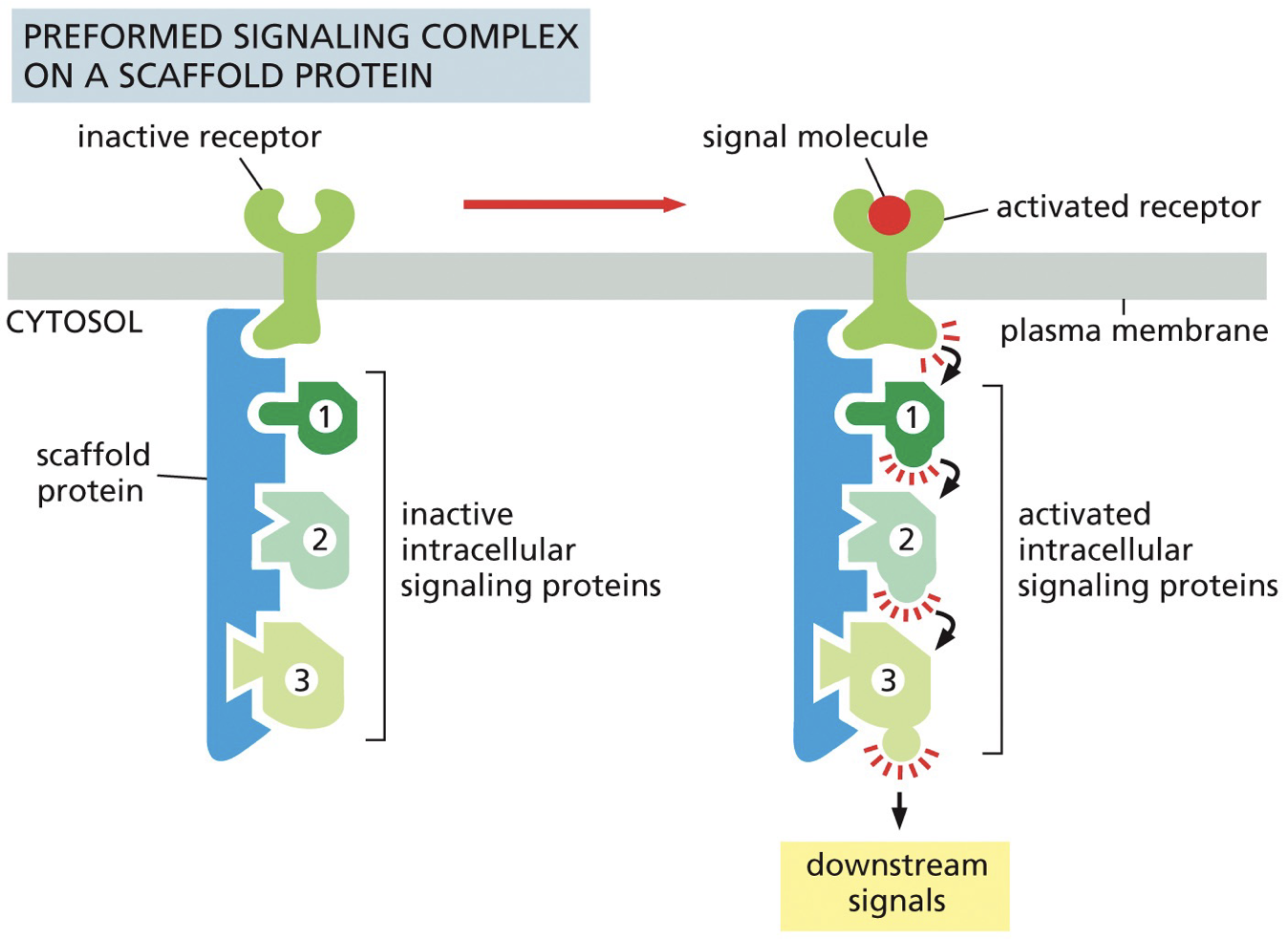
Assembly on scaffold proteins
Large scaffold proteins serve to assemble groups of multiple downstream signaling components components
Smaller adaptor protein can link proteins together
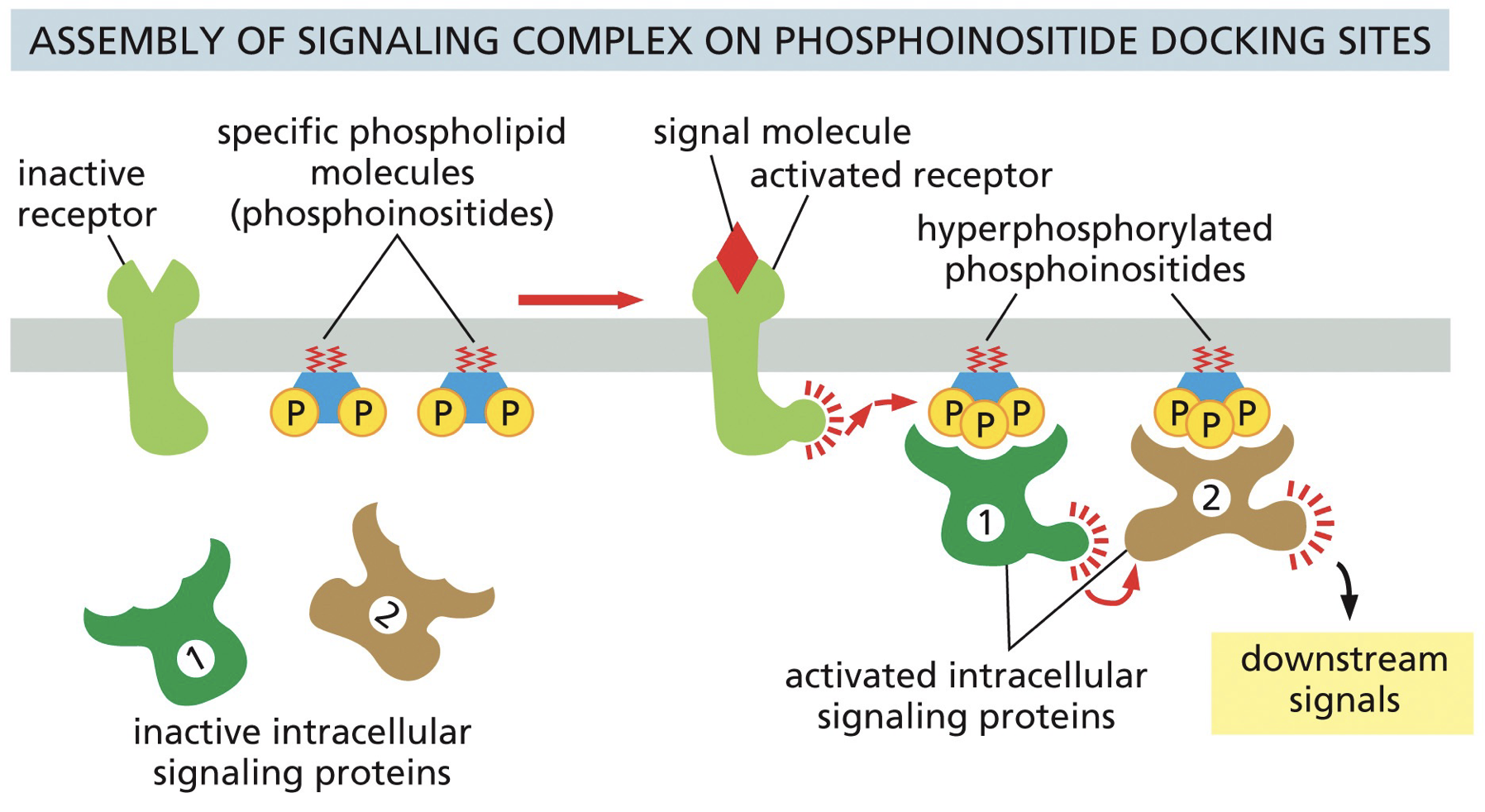
Assembly on phosphoinositide docking site
Facilitated activation creates a binding site for assembly
Docking is based on a phosphorylated site
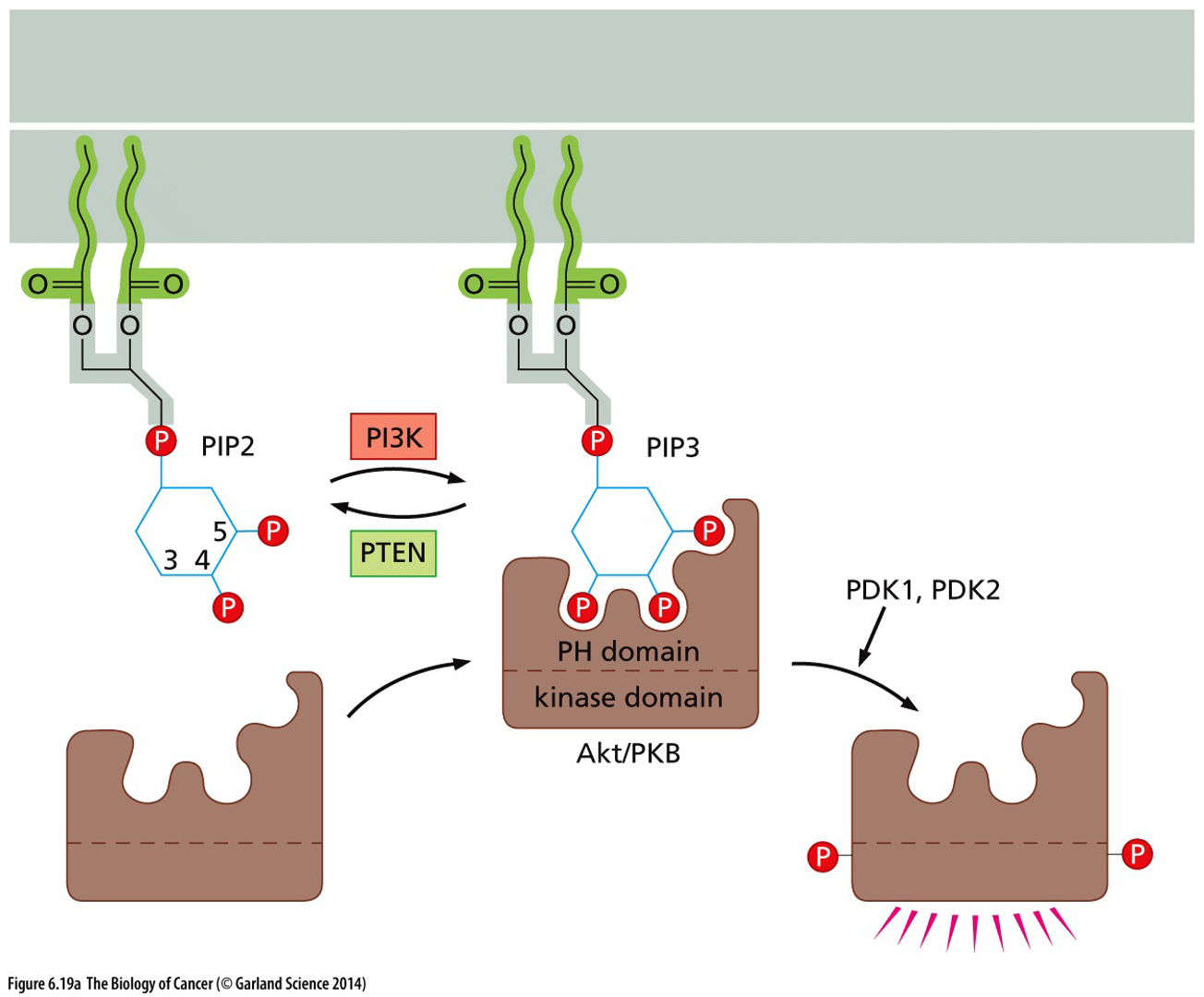
PI3K/Akt pathway
(related to PI docking site) PI3K is activated downstream of growth factor signaling. P13K converts PIP2 → PIP3
PIP3 results in recruitment and activation of signaling molecules such that Akt serine/threonine kinase, which influences key processes such as proliferation and cell metabolism.
PTEN is a phosphatase that acts on phosphoinositide and opposes the activity of PI3K and shuts everything down.
 RTKs stimulate Ras GTPase signaling. Ras promote tumour development and progression. MAPK cascade activation and PI3K/Akt pathways are common downstream of RTKs. Ras activate PI3K.
RTKs stimulate Ras GTPase signaling. Ras promote tumour development and progression. MAPK cascade activation and PI3K/Akt pathways are common downstream of RTKs. Ras activate PI3K.
Second messengers in intracellular signal transduction: small chemical that are signaling molecules, and their presence influences activation or inhibition of proteins-pathways. Generated in large amounts in response to signal activation.
Signaling molecules
Amino acids and derivatives
Gases
Steroids (from cholesterol)
Eicosanoids (from fatty acids)
Variety of polypeptides/proteins
Protein/pathway | Promote or inhibit tumors | Upstream (5’) /downstream (3’) |
|---|---|---|
Growth factor | Promote | Either |
RTK (EGFR) | Promote | Either |
Ras GTPase (Kras) | Promote (oncogene) | Downstream |
PI3K | Promote | Downstream |
Akt | Promote | Downstream |
Src | Promote (oncogene) | Upstream |
PTEN | Inhibit | ??? |
MAPK cascade | Both | Downstream |
*****
Mutation, Signaling, and Cancer
Cancer: A group of diseases of abnormal, uncontrolled cell division
Benign: Abnormal growth without invasion
Malignant: Cells invade nearby tissue and can spread to other parts of the body
Transformation: Process of a normal cell becoming cancerous. Beginning with abnormal cell growth and uncontrolled cell division.
Metastasis: Cancer cells spread and form tumours at additional sites in the body
Stage: How large a tumour is and how has it spread to other tissues
Grade: How abnormal do the cells look and how fast they are growing
Knowing stage and grade allows form pathologists to understand origin, diagnosis, prognosis and treatment options.
Cancer characteristics in cells: loss of adherence (in tissue), loss of contact inhibition, altered cell morphology (weird cell shape), enlarged and variable nucleus, overcrowded.
Cancer is promoted by a few different things
Genomic instability
DNA ends up having at least one mutation every time it divides and these accumulate into cancer.
Pathways involved in DNA damage response and repair are among the most frequently altered in cancer.
Cell division
Everytime cell division occurs, new mutation occur, and thus the more cell division, the more likely a tumor is to form.
Heterogeneity
Tumour suppressors are recessive, and if you lose both genes coding for it you won’t have them.
Acquisition of driver mutations
Arise and cause expansion of unique subpopulations of mutations in cells that already have mutations.
Occur in somatic cells.
Philadelphia Chromosome: From 7 patients with chronic myelogenous leukemia (CML), all had an abnormally small Chr 22, and large Chr 9. This occurred via translocation.
Bcr-Abl is an in frame fusion of the coding regions of the genes caused by a reciprocal translocation between Chr 9 and Chr 22.
The abl tyrosine kinase domain becomes hyperactive in Bcr-Abl, which comes from Chr 22.
“Bcr”: breakpoint cluster region
Bcr-Abl was a good cancer for drug treatment, as Gleevec in early stages of CML leads to remission in most patient. This is because Gleevec binds to the oncogenic kinase and inactivates it, blocking the ATP from making it hyperactive.
Bcr-Abl is an oncogene
Mutation patterns
A missense mutation occuring in a non-coding non-regulatory area isn’t a big problem so there aren’t as many tumour suppressor genes. A truncating mutation is more harmful and can affect protein all over regardless of area, hence importance of tumour suppressor genes.
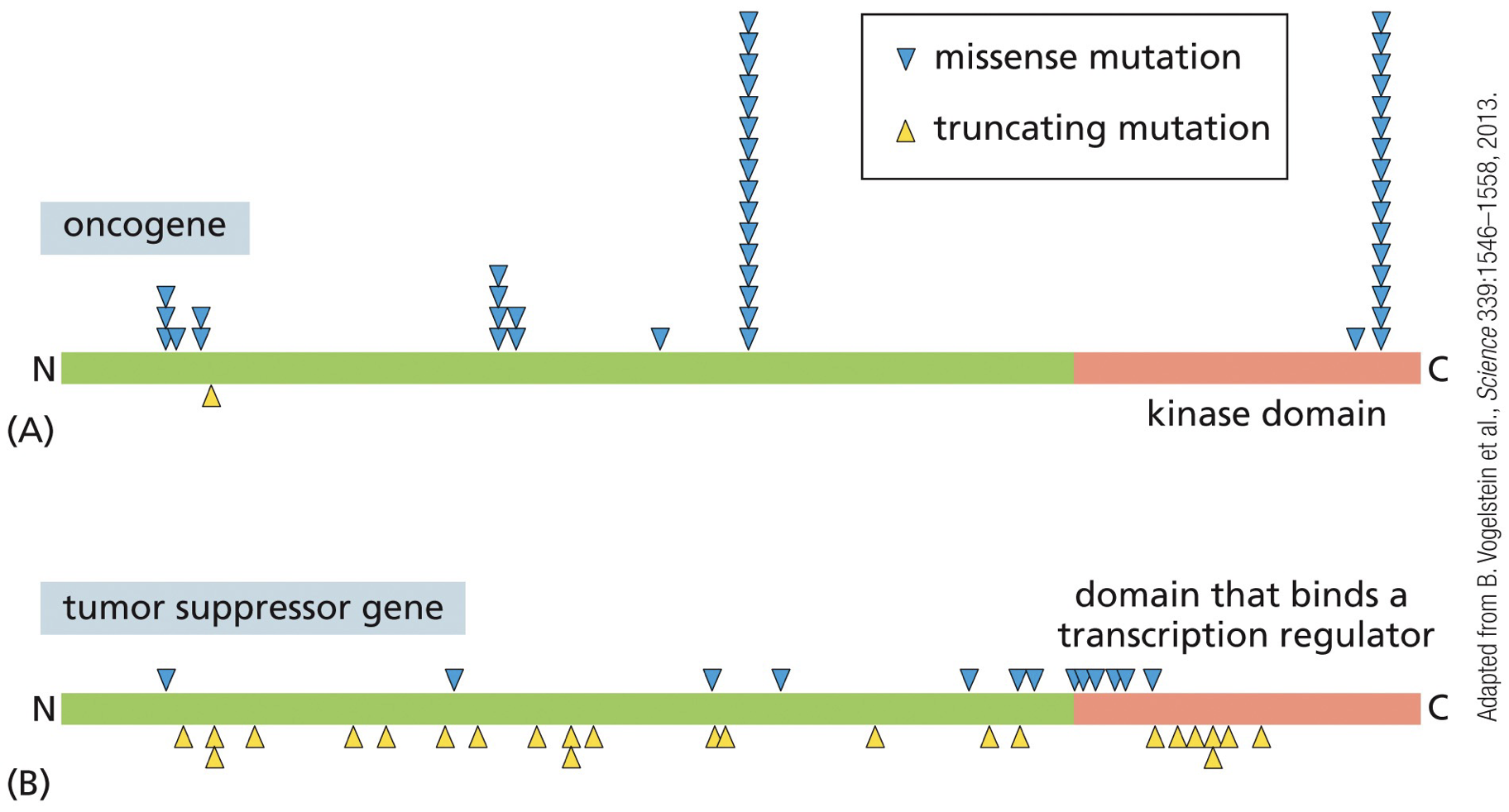
Oncogenes: promote tumour growth, survival, migration, angiogenesis, etc., while tumour suppressors are genes normally involved in promoting cell death or negatively regulating growth/survival pathways. Gain-of-function, and are dominant (switch on signaling even if only one copy of gene is modified)
Mutations
Activating mutation enables oncogene to promote cell transformation
Tumour suppressors: gene alterations in cancer are quite different from the types of changes seen in oncogenes. Alterations in them are recessive (both copies of the genes must be eliminated/ downregulated to allow cancer progression).
Mutations
Two inactivating mutations functionally eliminate tumour suppressor gene.
Alterations in the Rb (tumour suppressor)
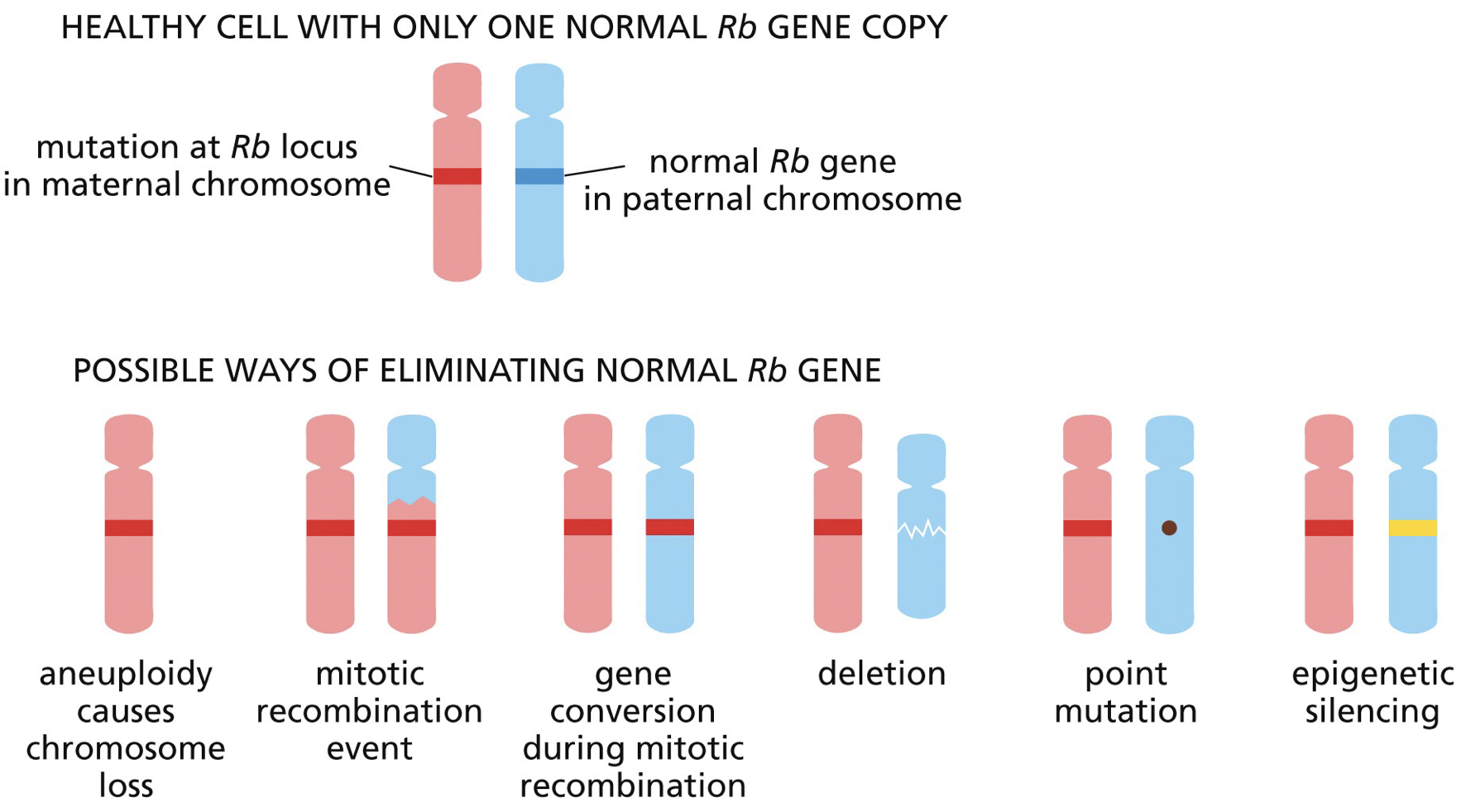
With a loss of both Rb we get retinoblastoma (can be hereditary which gets in both eyes due to excessive cell proliferation leading to retinoblastoma earlier in life, or nonhereditary which is likely only one eye).
Tumours are majority monoclonal growths - arise from a single normal cell-of-origin
Clonal evolution of cancer: early stages of disease progression, genetic changes promoting cancer arise and are inherited by daughter cells, leading to clonal expansion (outcompete slower neighbours).
More cell division = greater cancer risk
External factors that promote cell proliferation can promote cancer promote cancer without altering DNA. A driver mutation is a rare event, but with many cells and one will make many more cells.
Tumour microenvironment: The surrounding network of ECM, signaling molecules, immune cells, fibroblasts, blood vessels, and resident normal tissue.
Referred to as tumour stroma (supportive connective tissue and blood vessels in an epithelial organ.
May have tumor promoting growth factors in environment, which optimize the environment for the tumour.
Homologous recombination or homology directed repair leads to loss-of-heterozygosity (LOH = gene conversion = nonreciprocal exchange of genetic information)
NHEJ causes inversions and translocations without the need for homology, however also loses functional copies.
Signaling pathways can be additive, redundant, or both. Inhibiting a negative regulator can result in activating a signaling pathway (and vice versa for activating a negative regulator).
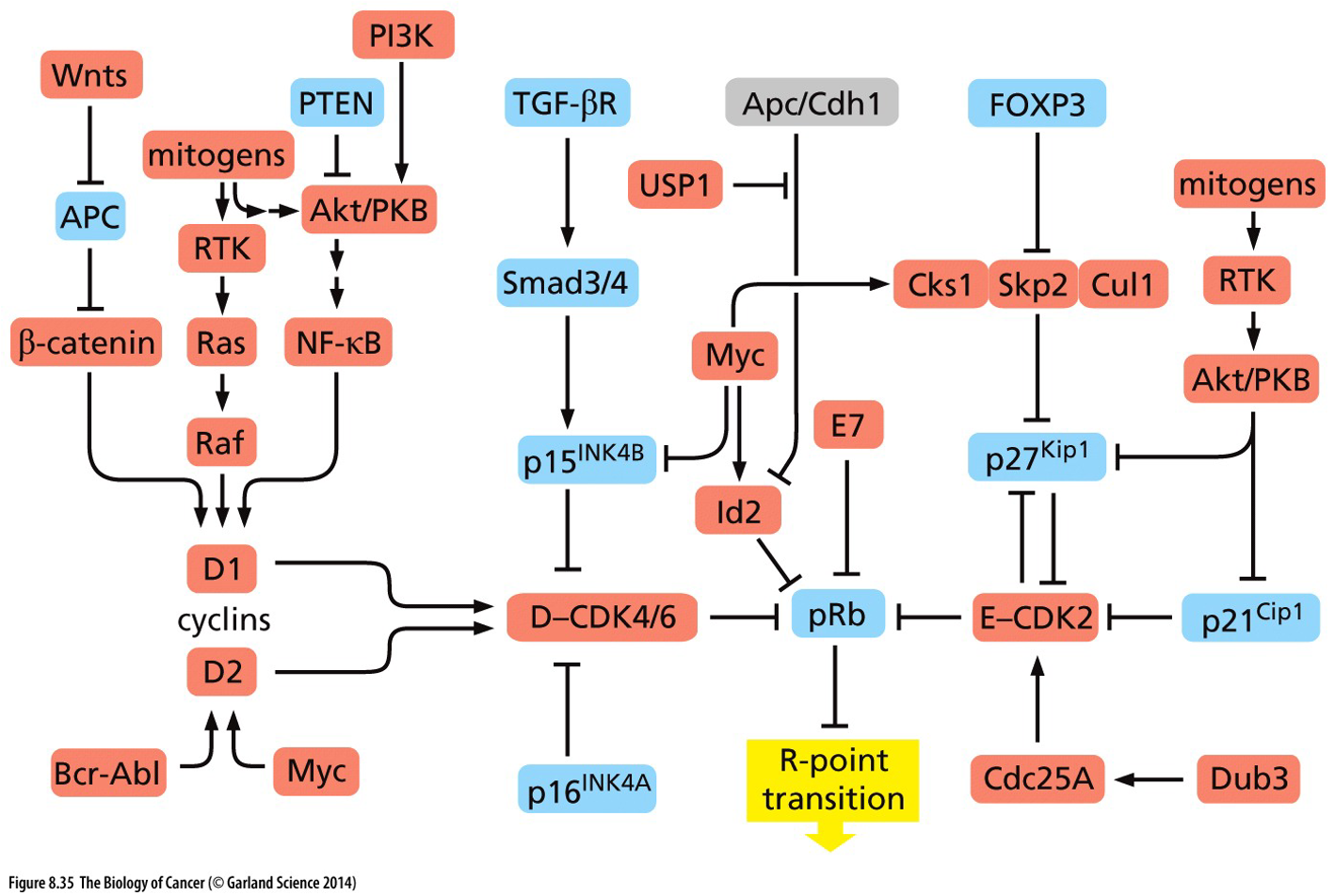
Cell cycle
The cell cycle is in charge of stopping itself and only going when necessary. Growth signals reach cell at G1 phase until decision to be made has been made, which we call the “R point” / “Restriction point”.
Checkpoints throughout the cell cycle ensure division only proceeds if everything is in order. Ensure only healthy cells divide. Cancer cells create their own constitutively active growth factors that will grow and bypass checkpoints.
Abnormal chromosomal arrangements are common features of cancer cell mitosis (caused by translocation, and altered number of centromeres).
Rb regulates R point by restricting entry into S phase. Hypophosphorylation of Rb results in binding the E2F transcription factor and preventing transcription. Results in release of E2F at R point and then drives transcription of a cyclin (initiation of S phase).
p53
p53 tumour suppressor is the most commonly mutated gene in cancer
Has prevalence near a tumour site.
Elephants don’t get cancer because despite being big with a lot of cell division, they have 40 TP53 genes to out 2
Stresses activate p53 in normal cells
In non-stressed cells, MDM2 ubiquitin ligase targets p53 for rapid degradation. Stress results in phosphorylation of p53 at the MDM2 binding site.
Phosphorylated p53 is not degraded and can stimulate transcription of a variety of genes.
Apoptosis is programmed cell death; it is an important part of both development and tissue maintenance that becomes impaired in cancer cells.
p53 is a tetramer, even one mutant subunit makes the whole thing inactive. Unique of recessive mutations seen in other tumour suppressor genes. Dominant negative missense mutation.
Mutations in the p53 pathway enable cancer cells to survive and proliferation despite stress and DNA damage.
Li-Fraumeni syndrome: high risk for many types of cancer. Only have 1 TP53 gene.
Hereditary cancers: inherited genetic variants have been linked to 5-10% of cancers in the U.S.
Sporadic cancers: caused by the accumulation of mutations that were not inherited from an individual’s parents.
Increasing mutation rate is sufficient enough to cause cancers
Carcinogen: an agent that causes cancer (first direct demonstration of chemical causing cancer in rabbit with coal tar)
Mutagen: an agent that causes genetic mutation.
Strong correlation between ability of chemical to cause cancer in lab rodents and its mutagenicity.
Many factors increase cancer risk by affecting the tissue microenvironment: tobacco, biochemically, and physically (hormones, diet, physical activity).