3.4.2 mechanisms for addition reactions of alkenes
electrophilic addition of hydrogen halides to alkenes
overall equation:
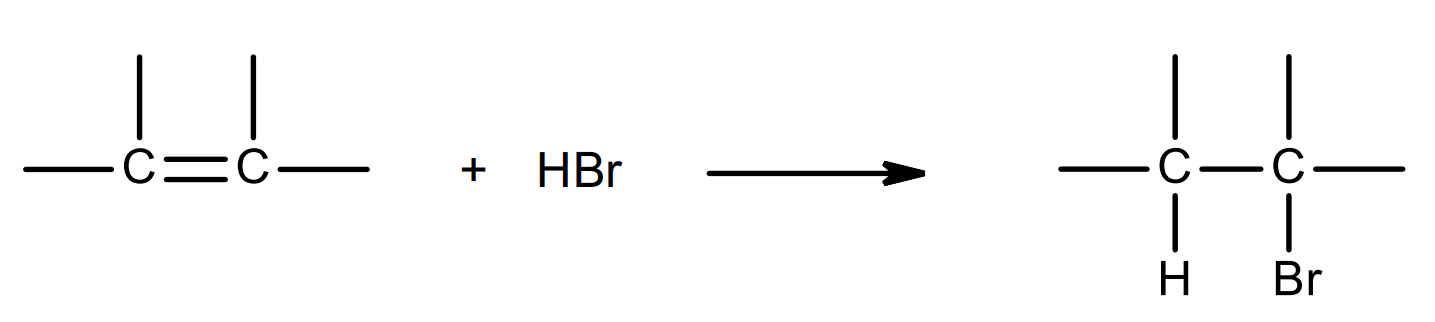
mechanism:
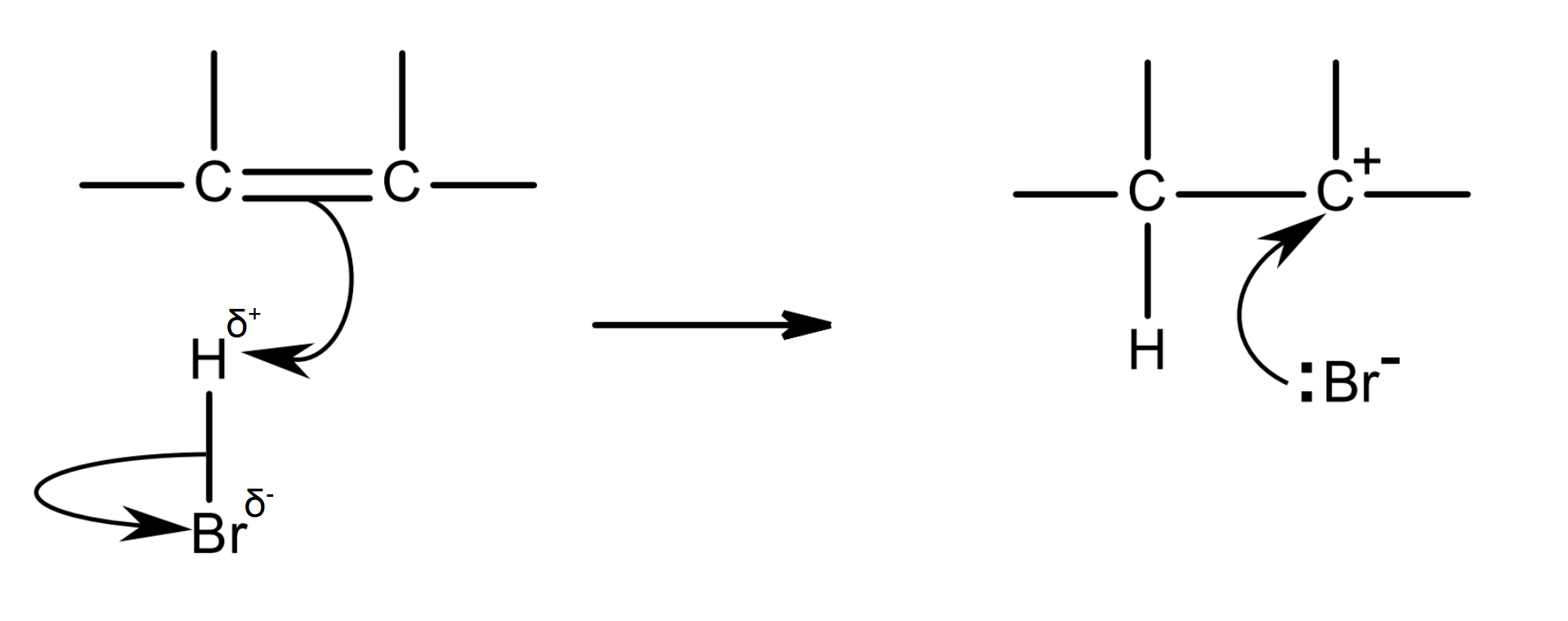
halogen atoms are more electronegative than hydrogen atoms, so hydrogen halide molecules have a permanent dipole.
H is δ+ and Br is δ-.
the H δ+ acts as an electrophile: it is attracted to the high electron density of the C=C bond, and accepts the electron pair in the pi bond.
as this C-H bond forms, the bonding pair between H and Br moves completely onto Br; this is heterolytic fission.
a Br- ion and a carbocation intermediate are formed.
the C atom in the carbocation is positive as it has lost its share of the electron pair that were in the pi bond (it has lost an electron).
the lone pair of the Br- ion is attracted to the C+ in the carbocation, forming a C-Br bond.
Markovnikov’s rule:
when a hydrogen halide reacts with an unsymmetrical alkene, the hydrogen is more likely to bond to the carbon which is already attached to the greater number of hydrogen atoms.
this will form a more stable carbocation so will form the major product.
tertiary carbocations are the most stable.
electrophilic addition of halogen molecules to alkenes
overall equation:
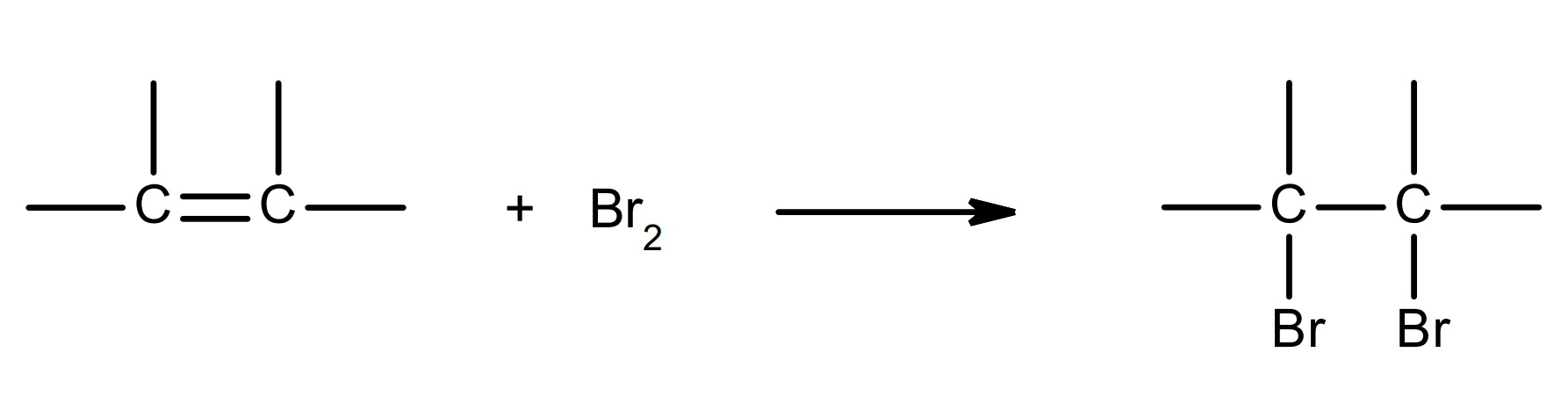
mechanism:
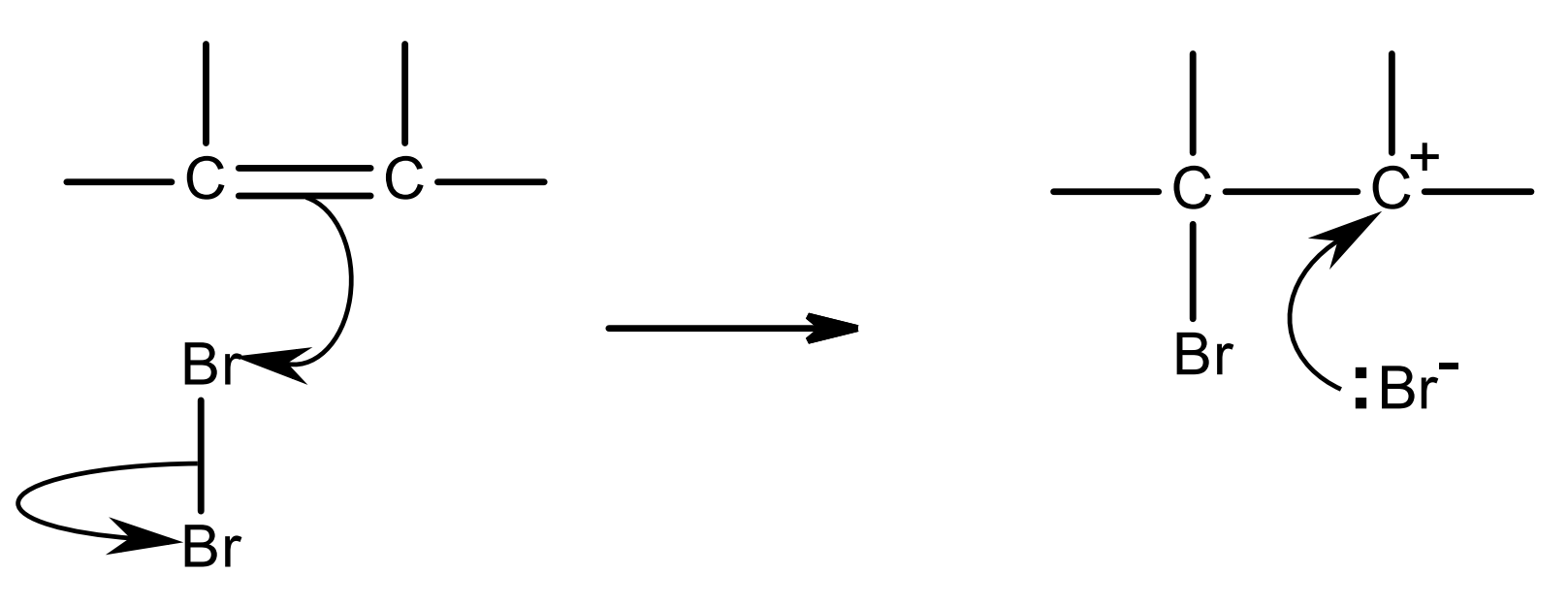
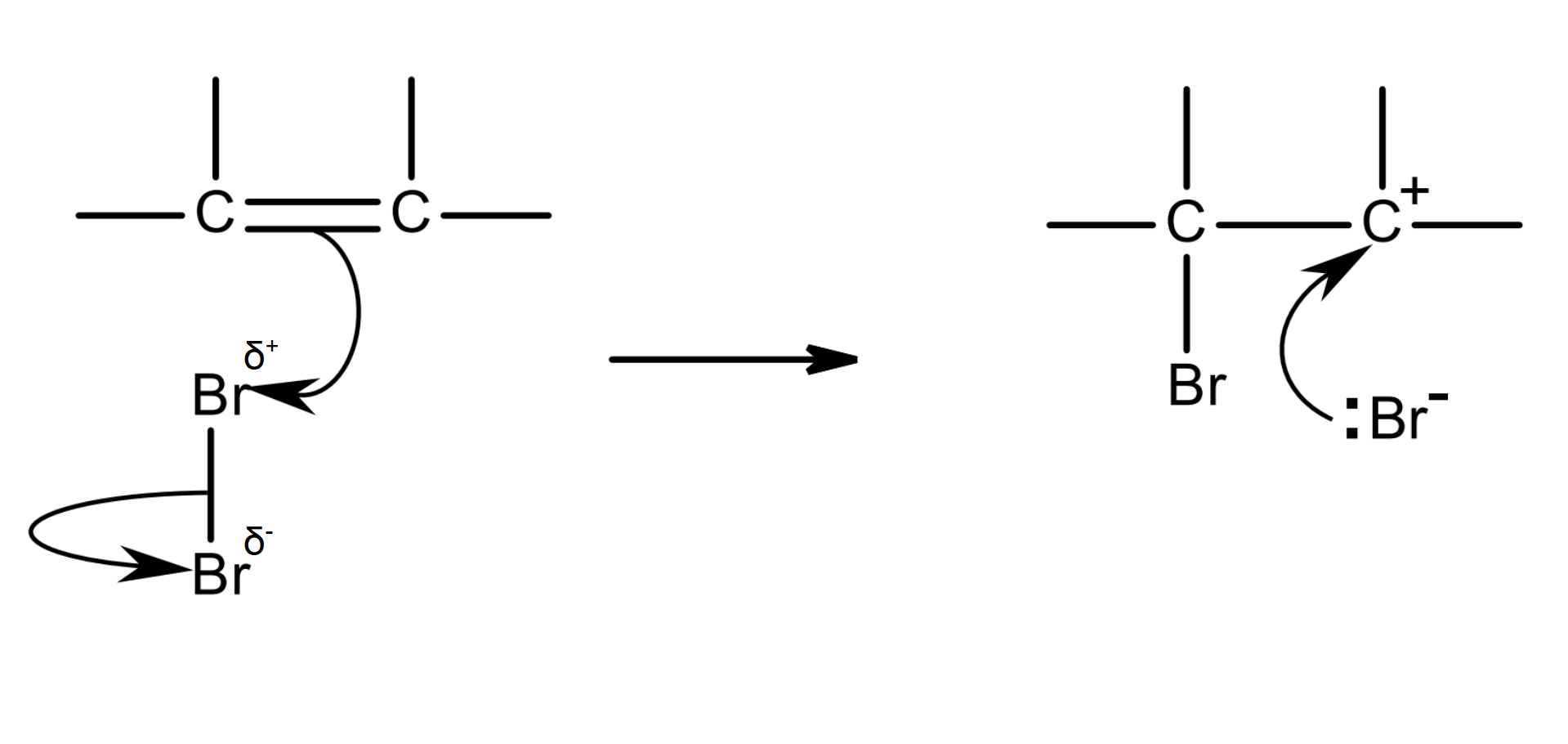
Br2 is non-polar.
the high electron density of the C=C bond attracts the Br2 molecule and repels the electron pair of the Br-Br bond.
Br-Br becomes polarised and now has an induced dipole.
Brδ+ acts as an electrophile and is attracted to C=C, and it accepts the electron pair in the pi bond.
as this C-Br bond forms, the bonding pair in the bromine molecule moves completely onto the other Br atom: this is heterolytic fission.
a Br- ion and a carbocation intermediate are formed.
the C atom in the carbocation is positive as it has lost its share of the electron pair that were in the pi bond (it has lost an electron).
the lone pair of the Br- ion is attracted to the C+ in the carbocation, forming a C-Br bond.
only one possible product
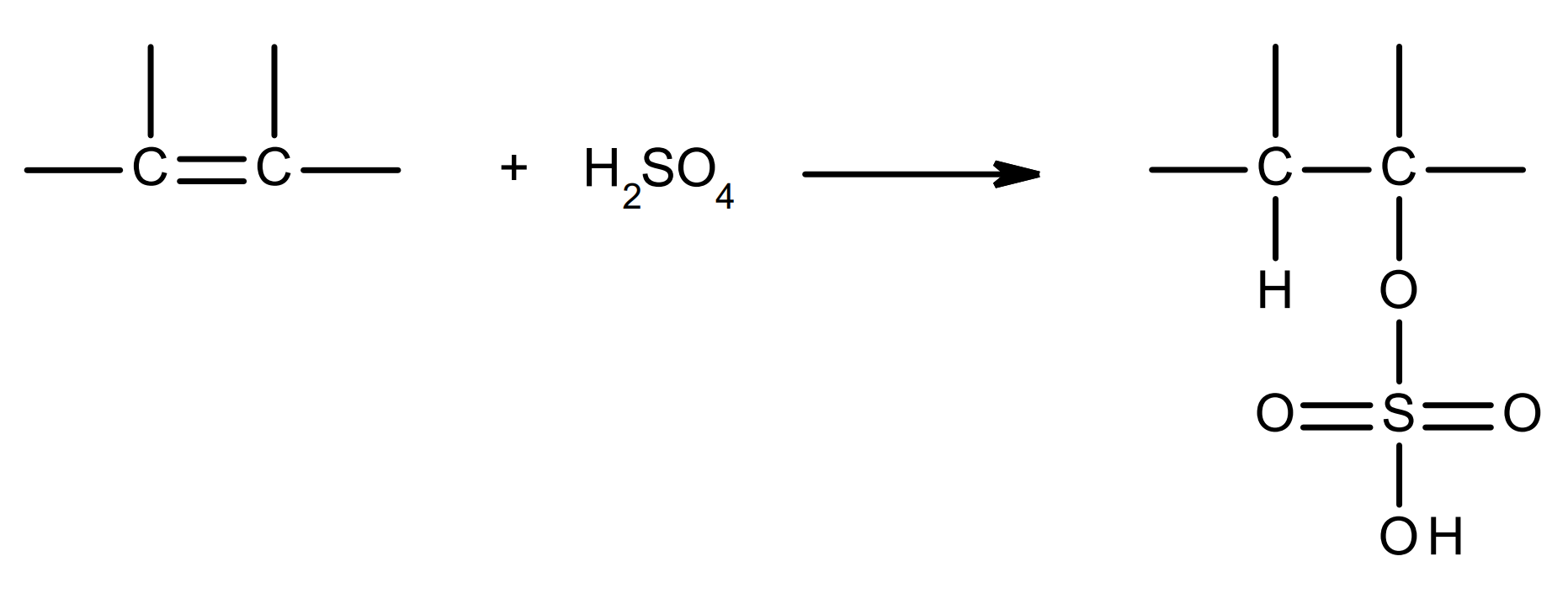
electrophilic addition of sulfuric acid to alkenes
overall equation:
mechanism:
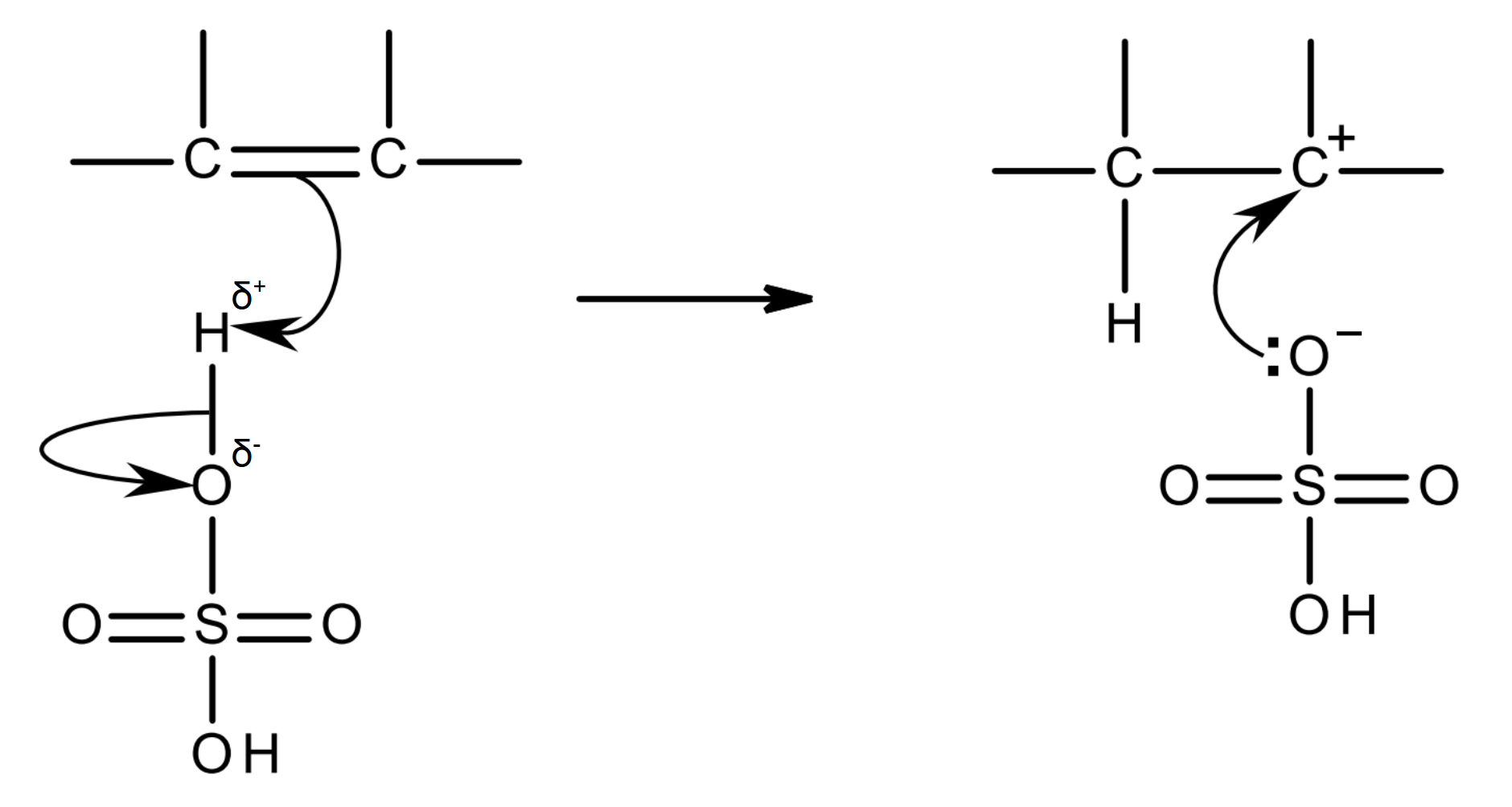
in the sulfuric acid, one of the H δ+ acts as an electrophile: it is attracted to the high electron density of the C=C bond, and accepts the electron pair in the pi bond.
as this C-H bond forms, the bonding pair between H and O moves completely onto O; this is heterolytic fission.
a HO-O2S-O- ion (O-SO2OH, hydrogen sulfate ion) and a carbocation intermediate are formed.
the C atom in the carbocation is positive as it has lost its share of the electron pair that were in the pi bond (it has lost an electron).
the lone pair of the O- in the hydrogen sulfate ion is attracted to the C+ in the carbocation, forming a C-OSO2OH bond.
apply Markovnikov’s rule
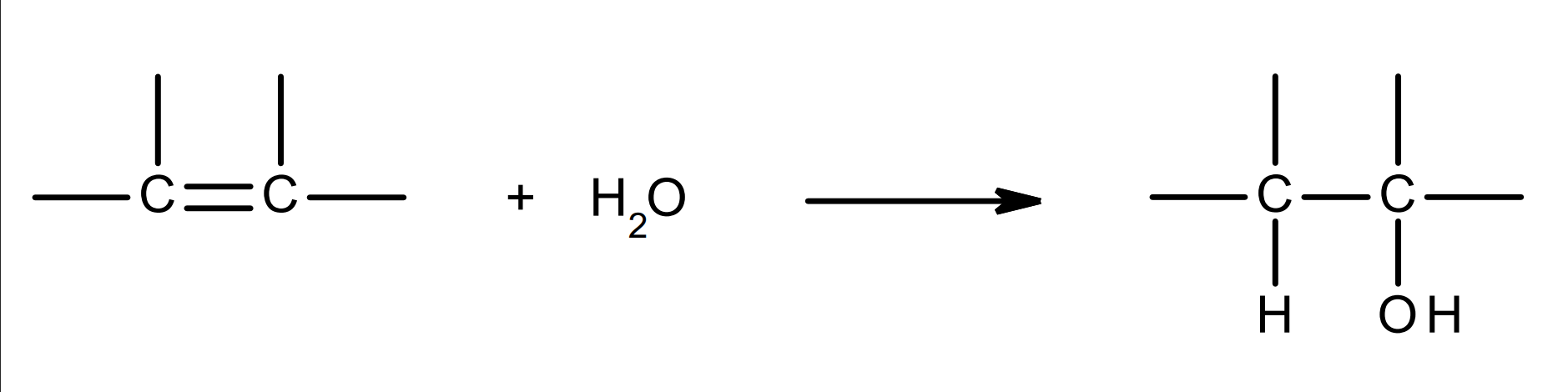
hydration of alkenes - electrophilic addition of water to alkenes
overall equation:
mechanism:
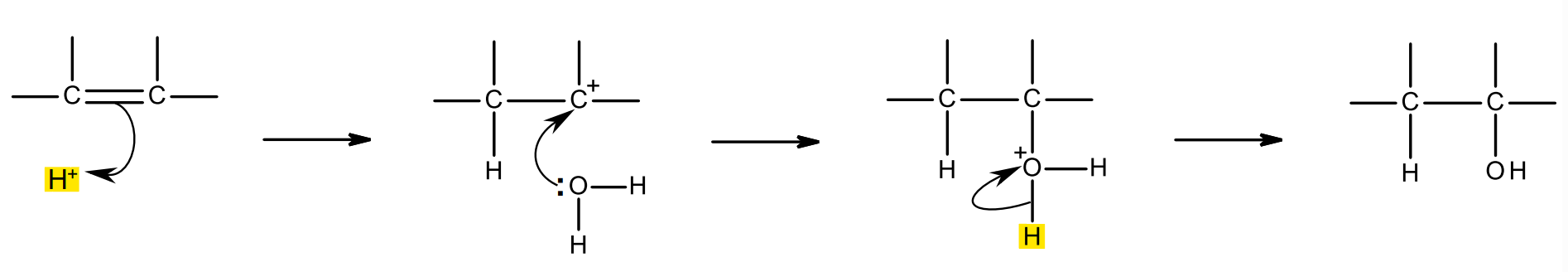
H+ from phosphoric/sulfric acid catalyst acts as an electrophile, it is attracted to the high electron density of the C=C bond, and accepts the electron pair in the pi bond.
C-H bond forms, so a carbocation intermediate and a dihydrogen phosphate ion is formed.
a lone pair on a water molecule joins to C+ of the carbocation
an intermediate molecule with a O+ is formed; the O is + as water is neutral, but now O has lost an electron/is sharing it with C.
H+ is lost from the intermediate and bonds to dihydrogen phosphate ion, so the acid catalyst is regenerated.
as H+ leaves the intermediate, the bonding pair in the O-H bond moves completely to O: heterolytic fission.
apply Markovnikov’s rule