Structure of atoms
Models of atoms
• Thomson's model of the atom:
J.J Thomson performed experiments with cathode ray tubes and concluded that it consisted of identical particles independent of the element used for the cathode or filling gas, so the particles must be present in atoms of every element.
Thomson said an atom is a homogeneous, positively charged liquid-like substance completely filled with tiny, negatively charged electrons in it(Plum-pudding model).
• Rutherfords model:
They bombarded a piece of thin metal foil with alpha particles(helium atoms stripped of electrons). Most of the particles suffered a deflection when passing through the foil but some of them bounced back, so they concluded that most of its mass is stored in a small nucleus and the other space is empty.
Particles went straight through
Some scattered at low angles
Some scattered back.
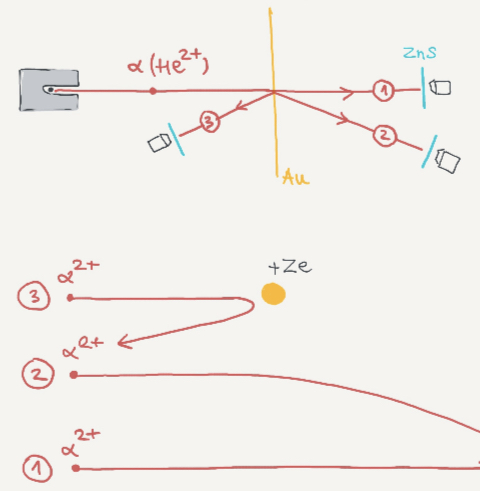
Rutherford said an atom is like a solar system with electrons orbiting around the nucleus.
Problems of this model
An atom with constantly accelerating electrons will not be stable cause they will radiate and lose energy then collapse on the nucleus.
• Bohrs postulate:
Electrons can be found on certain orbits and don’t radiate there. L = mvr = n(h/2 pi).
L is angular momentum, n is integer(1, 2, 3)
Electrons can jump between shells to emit radiation.
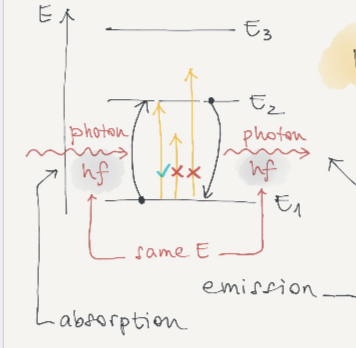
Bohr said electrons in an atom only occupy certain distinct orbits. Atoms radiate only when electrons jump from one level to another.
Frequency of light emitted : hf= E2 - E1 E1 = -13.6ev
h is Planck constant, f is frequency, E is energy levels.
Balmer formula: hv= hc/wavelength
v = c/wavelength
c is speed of light, v is frequency
Rydbergs equation: 1/wavelength=R(1/n2 - 1/k2)
n is lower energy level, k is higher energy level.
Line spectra of atoms
When atoms undergo excitation they emit light of different wavelengths which corresponds to different colours.
For hydrogen it is an emission spectrum which forms lines of colour against the dark spaces.
Franck Hertz experiment
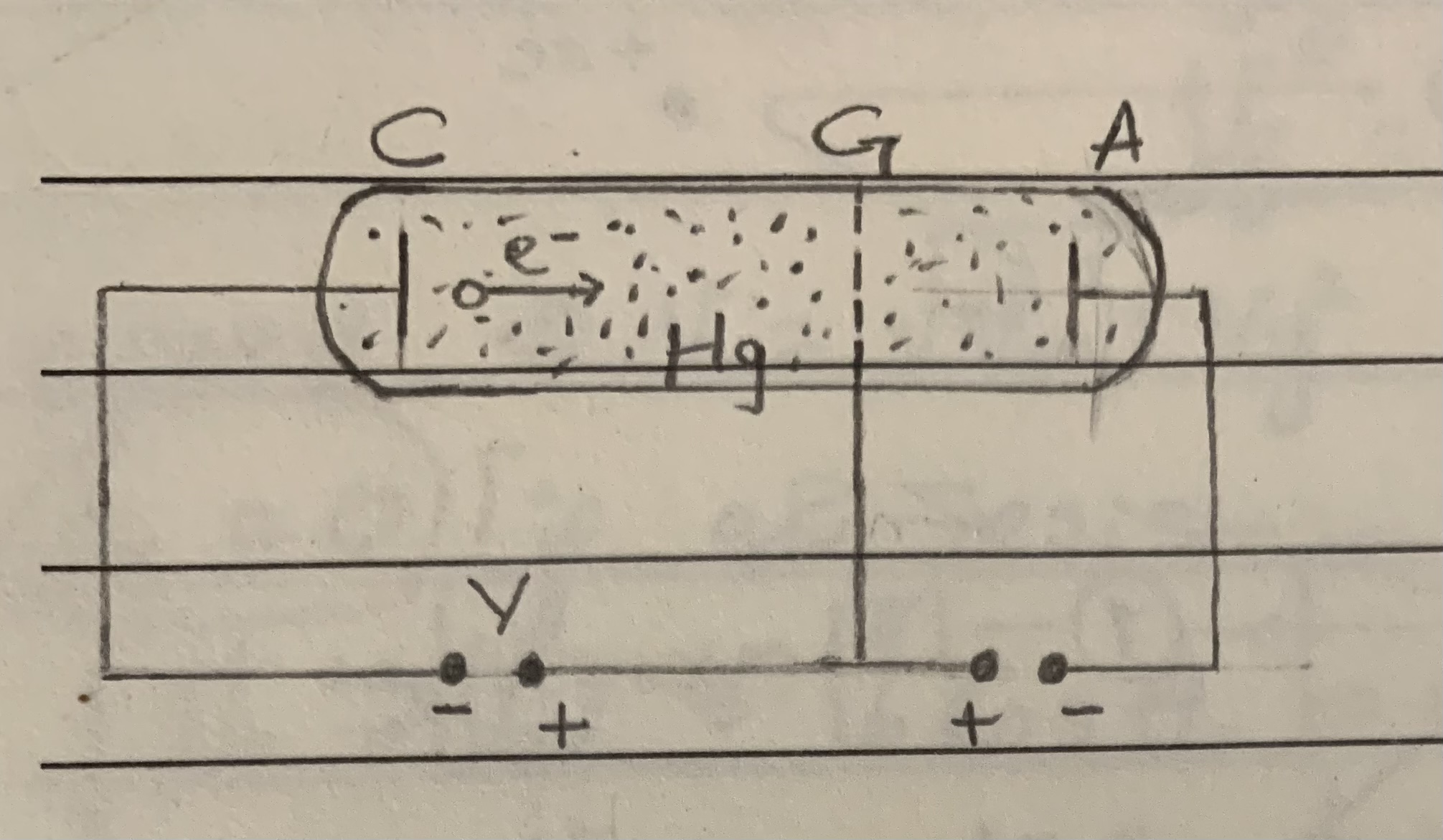
Electron collision with Hg atoms, electrons are accelerated by the electric field between the cathode (C) and the grid (G) in a tube filled with Hg vapour. When they pass through a field of opposite voltage they slow down and are not able to reach the anode(A) as they don’t have enough K.E . The electrons reaching the anode form a measurable current (I).
The experiment was to prove evidence of energy quanta.