Expression Systems Lecture Review
Upstream Processing: Expression Systems
Biopharmaceuticals and Recombinant Expression
Biopharmaceuticals: These are therapeutic proteins produced through genetic engineering. Their creation relies on various recombinant expression systems.
Recombinant Proteins: Defined as proteins expressed by an organism that does not naturally possess the genetic material (gene) to produce that specific protein.
Process Overview: The fundamental process involves inserting a gene (coding for a desired protein) into a target 'expression system.' This system is then engineered to transcribe and translate the inserted gene, thereby producing the desired protein.
Criteria for Selecting an Expression System
The choice of an optimal expression system is critical and depends on several key factors:
Protein Complexity and Post-Translational Modifications (PTMs):
The inherent complexity of the protein (e.g., presence of disulfide bonds, multimeric structure) and the absolute requirement for specific PTMs (e.g., glycosylation, phosphorylation, cleavage) significantly influence the system choice.
PTMs: These are crucial for the protein's proper folding, stability, function, and immunogenicity in humans. Systems vary widely in their ability to perform human-like PTMs.
Yield and Scalability:
Different expression systems offer varying levels of protein yield, referring to the amount of protein produced per unit volume or cell mass.
Scalability: This refers to the ease and feasibility of expanding production to large industrial volumes while maintaining quality and efficiency.
Cost and Infrastructure:
Cost factors: These are paramount in manufacturing, including the expense of culture media, reagents, labor, and energy.
Infrastructure: The requirements for specialized equipment, facilities, and environmental controls (e.g., sterile environments, bioreactors) are important considerations.
Regulatory and Speed Considerations:
Regulatory requirements: The pathway for approval by bodies like the FDA significantly impacts system selection, especially concerning product safety, purity, and consistency.
Production speed: The time required from gene insertion to protein harvest can be a crucial factor for time-sensitive applications or market entry.
General Trends in Expression System Selection
Based on criteria, systems generally trend as follows:
Speed (Low \rightarrow High): Mammalian \rightarrow BEVS/Insect \rightarrow Yeast \rightarrow Bacteria
Cost (Low \rightarrow High): Bacteria \rightarrow Yeast \rightarrow BEVS/Insect \rightarrow Mammalian
Typical Yield (Low \rightarrow High): Mammalian \rightarrow BEVS/Insect \rightarrow Bacteria \rightarrow Yeast
Post-Translational Modifiability (Low \rightarrow High): Bacteria \rightarrow Yeast \rightarrow BEVS/Insect \rightarrow Mammalian
FDA Approval (Complexity/Scrutiny for Products) (Low \rightarrow High): Bacteria \rightarrow BEVS/Insect \rightarrow Yeast \rightarrow Mammalian (reflecting the higher complexity and need for human-like PTMs in products often sourced from mammalian systems, leading to stringent regulatory scrutiny for these complex biopharmaceuticals).
Choosing an Expression System Based on Protein Characteristics
For Simple Proteins (e.g., lack complex PTMs, small, soluble):
Preferred Systems: Bacteria (e.g., E. coli) and Yeast.
Advantages: Relatively simpler fermentation processes, high scalability, and lower cost of goods.
Limitations: PTMs are generally not human-like, which can be an issue for therapeutic proteins requiring specific human glycosylation patterns.
For Complex Proteins (e.g., requiring human-like PTMs, large, multi-domain, intricate folding):
Preferred Systems: Mammalian cells (often human cell lines).
Advantages: Capability to perform human-like PTMs, crucial for protein quality and suitability for human therapeutic use.
Limitations: Involve more complex culture conditions, are generally less scalable than microbial systems, and incur higher costs of goods.
Overall Principle: The ultimate decision hinges on protein quality and suitability for its intended therapeutic application.
Examples of Expression Systems for Biopharmaceutical Products
Biopharmaceutical Product | Source |
|---|---|
Tissue Plasminogen Activator | E. coli, Mammalian (CHO) |
Insulin | E. coli, Yeast |
Interferon \alpha | E. coli |
Interferon \gamma | E. coli |
Interleukin-2 (IL-2) | E. coli |
Human Growth Hormone | E. coli |
Follicle Stimulating Hormone | Mammalian CHO (Chinese Hamster Ovary) |
Interferon \beta | Mammalian CHO (Chinese Hamster Ovary) |
Erythropoietin | Mammalian CHO (Chinese Hamster Ovary) |
Factor VIIa | Mammalian BHK (Baby Hamster Kidney) |
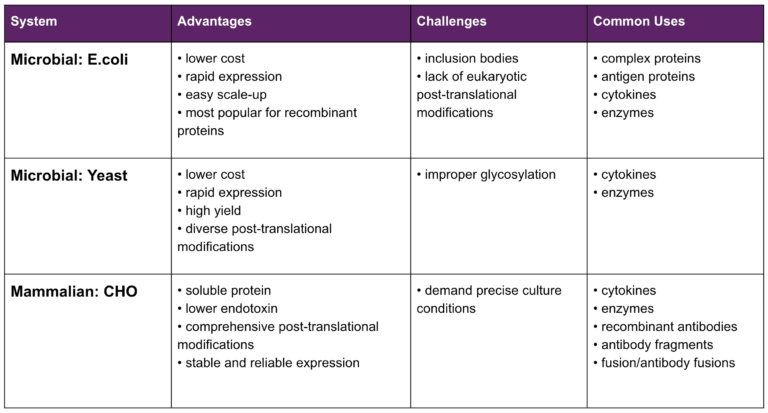
Prokaryotic Cells: E. coli
Characteristics: E. coli is a Gram-negative, rod-shaped, facultative anaerobic bacterium, widely used as a workhorse in biotechnology.
Historical Significance: The first recombinant protein produced was human insulin, achieved by expressing its gene in E. coli cells.
Advantages of E. coli Expression System
Ease of Culture & Genome Modifications: Relatively simple to grow in defined media and genetically engineer.
Rapid Expression: Short generation times allow for rapid protein production.
High Yields: Can produce large quantities of protein per unit time.
Ease of Scale-up: Microbial fermentation processes are well-established for large-scale industrial production.
Inexpensive: Culture media and operational costs are generally low.
Disadvantages of E. coli Expression System
Endotoxin Production: As a Gram-negative bacterium, E. coli produces lipopolysaccharides (LPS), also known as endotoxins, which are pyrogenic and must be rigorously removed from therapeutic products.
Acetate Formation: Can produce acetate as a byproduct, leading to cell toxicity and reduced yield at high cell densities.
Intracellular Proteins: Proteins are typically produced intracellularly, requiring cell lysis and subsequent extraction, which adds to downstream processing complexity.
Inclusion Bodies: Recombinant proteins often misfold and aggregate into inactive inclusion bodies, necessitating a refolding step which can be inefficient and costly.
Lack of Eukaryotic PTMs: Cannot perform complex eukaryotic PTMs (e.g., glycosylation), limiting its use for proteins requiring such modifications.
Endotoxins
Definition: Endotoxins are pyrogenic lipopolysaccharides (LPS), which are a major constituent of the outer leaflet of the outer membrane of virtually all Gram-negative bacteria.
Significance: Since E. coli is Gram-negative, any biopharmaceutical produced in it must undergo stringent endotoxin removal and detection.
Endotoxin Removal Methods
Common methods to remove endotoxins from E. coli-produced biopharmaceuticals include:
Ultrafiltration: A membrane separation technique.
Anion-exchange chromatography: Utilizes positively charged (+ ext{ve}) resins to bind negatively charged LPS.
Histamine-immobilised affinity resins: Specific affinity-based removal.
Endotoxin Detection: Limulus Amebocyte Lysate (LAL) Assay
Development: Developed in the 1960s.
Nature: An in-vitro assay.
Source: The LAL is an aqueous extract of blood cells (amoebocytes) from the horseshoe crab (Limulus polyphemus).
Mechanism: The assay is based on the clotting reaction of horseshoe crab blood in the presence of endotoxin.
Advantages: It is faster, more economical, and significantly more sensitive than the older rabbit pyrogen test, making it the most sensitive, reliable, and specific detection method for pyrogen-producing Gram-negative bacterial endotoxins.
Mammalian Cell Lines
General Characteristics: Mammalian cell systems are known for their ability to produce complex proteins with human-like PTMs, which are often essential for the biological activity and safety of therapeutic proteins.
Limitations: They are generally expensive culture systems and are susceptible to infections, particularly by viruses.
Protein Output: Despite the costs, they can achieve high titers (concentrations) of recombinant proteins.
Examples:
Chinese Hamster Ovary (CHO) cells: Widely used for monoclonal antibody production.
Baby Hamster Kidney (BHK) fibroblast cells: Another common commercial cell line.
Advantages of Mammalian Cell Lines (e.g., CHO cells)
Complex Human-like PTMs: CHO cells can secrete proteins with complex post-translational modifications that are very similar to those produced by human cells. This is crucial for efficacy and reduced immunogenicity of therapeutic proteins.
Extracellular Secretion: Proteins are often secreted extracellularly, simplifying initial recovery steps compared to intracellular expression.
Environmental Tolerance: Extremely tolerant to changes in oxygen concentrations, pH, temperature, and pressure within bioreactors, aiding process stability.
Disadvantages of Mammalian Cell Lines
High Production Cost: Involves very high production costs due to expensive media, reagents, and stringent sterile culture conditions.
Low Protein Yield: Compared to microbial systems, mammalian cells generally have lower protein yields per unit volume or time, although the quality is superior for complex proteins.
Fragility: Cells are more fragile and susceptible to shear stress in bioreactors.
Susceptibility to Viral Infections: Requires rigorous biosecurity measures to prevent contamination that can lead to batch loss.
Insect Cell Lines
Type: Eukaryotic cells, offering a middle ground between microbial and mammalian systems for complexity.
Expression Capability: Efficiently express complex recombinant proteins, with PTMs similar to, though not identical to, mammalian cell lines.
Availability: Over 100 insect cell lines are currently available for research and production.
Examples: Spodoptera frugiperda (Sf9 & Sf21) are commonly used cell lines derived from armyworm ovaries.
Advantages of Insect Cell Lines
Post-Translational Modifications: Capable of many eukaryotic PTMs, including glycosylation, allowing for more complex proteins than yeast. They can produce large and intricate proteins.
High Protein Yield: Can achieve high protein yields.
Scalable Production: Suitable for large-scale production, often using baculovirus expression vector systems (BEVS).
Stable Integration: Allow for stable integration of the gene of interest (GoI) into the host genome.
Disadvantages of Insect Cell Lines
Lysis Prone: Cells are prone to lysis, which can halt protein production prematurely and release proteases.
Limited Glycosylation: Lack some specific glycosylation pathways necessary for precisely human-like proteins, potentially impacting efficacy or immunogenicity for certain biopharmaceuticals.
Higher Cost: More expensive than bacterial or yeast cell lines, though generally less expensive than mammalian systems.
Less Extensive Regulatory Records: Regulatory pathways and records for products derived from insect cells are not as extensive as for well-established mammalian or bacterial systems.
Protease Degradation: Naturally occurring proteases from the host cells can lead to the degradation of the expressed recombinant protein, requiring careful process optimization.
Yeast Expression Systems
Type: Monocellular eukaryotic fungi.
Examples:
Saccharomyces cerevisiae (baker's yeast) is the most common and well-studied.
Pichia pastoris is another popular methylotrophic yeast, known for high-level protein expression.
Clinical Example: Eptinezumab (Vyepti), an antibody approved in 2020 for migraine prevention, is the sole antibody produced in P. pastoris approved for clinical use, highlighting its growing potential.
Advantages of Saccharomyces cerevisiae (Yeast)
Rapid Growth & Scalability: Exhibits rapid growth rates and can achieve very high cell mass densities, making it highly scalable.
Easy to Manipulate: Well-understood genetics and molecular tools make it easy to genetically manipulate.
Inexpensive Culture: Simple and inexpensive media requirements and culture conditions.
Post-Translational Modifications: Capable of performing some eukaryotic PTMs, an advantage over bacteria.
Disadvantages of Yeast Systems
Cell Disruption Difficulty: Possesses thick and hard cell walls, making cell disruption (for intracellular proteins) more difficult and energy-intensive.
Hyperglycosylation: Can lead to hyperglycosylation of recombinant proteins, which means attaching too many or non-human-like sugar residues. This can negatively affect protein function, stability, pharmacokinetic profile, and sometimes trigger immunogenic responses in humans.
“Molecular Farming” (Plant-Based Expression Systems)
Concept: Refers to the use of genetically engineered plants (entire plants or plant cell cultures) as bioreactors to produce pharmaceuticals, industrial enzymes, or other valuable proteins.
Most Used Host: Nicotiana benthamiana (a fast-growing tobacco plant relative) is a favored host due to its high efficiency in transient expression.
Yield & Speed: Offers significant advantages in speed and potential yield. Protein yields can reach gram levels of product per kilogram of leaves within 5-7 days post-DNA delivery. This rapid turnaround is a key benefit over traditional transgenic procedures that require stable integration and often over mammalian cell-based systems.
Notable Example: ZMapp, a cocktail of three monoclonal antibodies targeting the Ebola virus, was famously produced using N. benthamiana plants.
Advantages of Molecular Farming
Rapid & Affordable: Generally a rapid process, especially with transient expression, and can be very affordable, primarily requiring water and an energy source (sunlight/light).
Optimized Growth Conditions: Plants can be grown in agricultural settings or controlled environments, allowing for optimized growth.
Safety: Products are free from human pathogens and bacterial toxin contamination, mitigating some safety concerns associated with mammalian or bacterial systems.
PTMs Possible: Plants are eukaryotic organisms and can perform PTMs, though their glycosylation patterns may differ from human-like ones.
Disadvantages of Molecular Farming
Limited Glycosylation Capacity: Plant glycosylation pathways are not entirely identical to human ones, which can be a limitation for certain complex human therapeutic proteins.
Variable Expression Levels: Expression levels can vary depending on plant species, growth conditions, and specific gene constructs.
Generalized Plant-based Expression Process
Cloning and Transformation:
The gene encoding the desired protein is cloned into a suitable plant expression vector.
Often, transformation is achieved using Agrobacterium tumefaciens, which naturally transfers T-DNA into plant cells.
Infiltration (Transient Expression):
For rapid, temporary production, A. tumefaciens carrying the expression vector can be infiltrated directly into plant leaves. The plant cells transiently express the protein for a short period.
Stable Transformation (Stable Expression):
Alternatively, plant cells can be stably transformed, and a full transgenic plant line can be regenerated. This allows for long-term, consistent production, often suitable for large-scale cultivation or for generating plant suspension cultures (plant cells grown in liquid media for protein production).
Downstream Processing:
Disruption and Extraction: Plant material (leaves, cells) is harvested, disrupted, and the protein is extracted.
Purification: The target protein is then purified from plant endogenous proteins and other components.
Analysis: The purified protein undergoes rigorous analysis for quality, quantity, and activity.
Case Studies for Biopharmaceutical Production
CHO Cells for Antibodies:
Application: CHO cells are the leading platform for the production of monoclonal antibodies.
Reason: Their ability to perform human-like glycosylation is crucial for the therapeutic efficacy, stability, and reduced immunogenicity of these complex protein drugs.
E. coli for Insulin:
Application: E. coli cells are efficiently used for the production of recombinant human insulin.
Reason: Insulin is a relatively simple protein that does not require complex eukaryotic PTMs, making E. coli an economical and efficient choice for its expression.
Yeast for Vaccines:
Application: Yeast systems are employed to manufacture vaccines, such as the Hepatitis B vaccine.
Reason: They are effective in producing vaccine antigens safely and reliably, leveraging yeast's high-yield capabilities and established fermentation technologies.
Overall Principle: The selection of the right expression system involves carefully balancing the inherent protein complexity, the associated production costs, scalability requirements, and specific regulatory demands to achieve optimal biopharmaceutical manufacturing.
Learning Outcome
Upon completion of this lecture, students should be able to appropriately choose suitable host/vector systems for the production of recombinant proteins, considering the various criteria and system characteristics discussed.