Intro To Solutions
Types of Solutions
Solution
- A homogenous mixture (only one phase visible)
- Uniform composition throughout.
- The simplest solutions contain 2 substances, but there can be multiple substances involved.
- Solutions are composed of at least one solute and one solvent
- Eg) saltwater, chocolate milk, apple juice
Solvent
- The substance in which a solute is dissolved in
- A substance that is present in the largest amount in the solution
- Often liquid
- Eg) universal solvent = water
- Always more than the solute
Solute
- A substance that is dissolved in a solvent (to form a solution)
- Solutions are of variable composition which means that different ratios of solvent to solute are possible. (Recall that pure substances are of fixed composition.)
- When a solute dissolves in a solvent, no chemical reaction occurs. Solutions can be separated via physical means (boiling point, melting point, etc.).
- A solution can be a gas, liquid, or solid. Various combinations of solute and solvent states are possible.
- Eg) Salt, sugar, chocolate syrup
Aqueous Solution
- A solution in which the solvent is water
Alloys
- A solution of metals.
Miscible
- Liquids that dissolve readily in each other in any proportion.
Immiscible
- Liquids that do not dissolve readily in each other.
Solubility & Saturation
The ability of a solvent to dissolve a solute depends on the forces of attraction between the
particles. There is always some attraction between solvent and solute particles, so some
solute always dissolves.
Solubility
- The amount of solute that dissolves in a given quantity of solvent at a certain temperature.
Soluble
- Solubility is greater than 1 g per 100 mL of solvent.
Insoluble
- Solubility is less than 0.1 g per 100 mL of solvent.
Saturated Solution
- When no more solute will dissolve in a solution and excess solute is present.
Unsaturated Solution
- A solution which is not yet saturated; is able to dissolve more solute.
Precipitate
- A solid formed from a reaction
- * Review the solubility table
High Solubility
- The maximum solubility is greater than or equal to 0.1 mol/L (at SATP)
Low solubility
- The maximum solubility is less than 0.1 mol/L (at SATP)
Factors That Affect Rate of Dissolving and Solubility
Rate of Dissolving
- How quickly a solute dissolves in a solvent.
Factors that affect the Rate of Dissolving
Temperature
- For most solid solutes, the rate of dissolving is greater at higher temperatures.
- At higher temperatures, the solvent particles have greater kinetic energy. Thus, they collide with the undissolved solute more frequently and with more force.
Agitation
- Stirring or shaking a mixture in a container increases the rate of dissolving.
- Agitation brings fresh solvent into contact with undissolved solute.
Particle Size
- Smaller particle size increases the rate of dissolving.
- When a large mass of solute is broken into many smaller masses, the surface area of the solute that is in contact with solvent particles is increased.
Solubility & Particle Attractions
Solubility is related to the forces of attraction between particles. Attractions which need to be
considered are solvent-solvent, solute-solute, and solute-solvent.
The Process of Dissolving at the Molecular Level
- Forces between solute particles must be broken → requires energy.
- In an ionic solid, the forces holding the ions together must be broken, in a molecular solid, the forces holding the molecules together must be broken.
- Some forces between solvent particles must be broken → requires energy.
- There is an attraction between the particles of the solute and the solvent → releases energy.
If the energy change in step 3 is greater than the sum of the energy changes in steps 1 & 2, then the solute is likely to dissolve in the solvent.
Polar & Non-Polar Substances
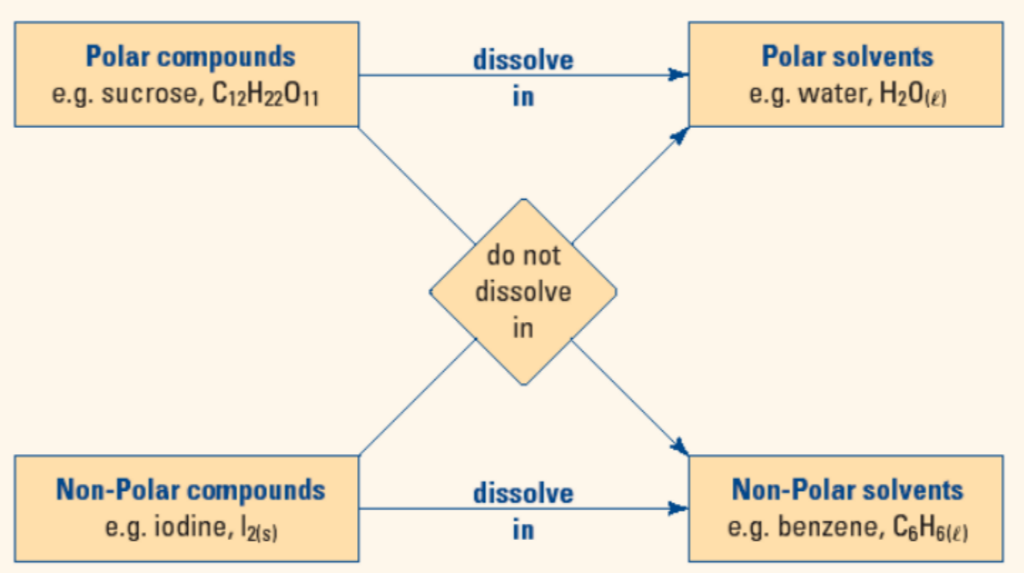
- Polar substances ONLY dissolve other polar substances
- Non-polar substances ONLY dissolve other non-polar substances.
- Like dissolves like
Solubility of Ionic and Polar Covalent Compounds
Ion-Dipole Attractions
The attraction between an ion and a polar molecule.
Generally, an ionic compound will dissolve in a polar solvent because the negative poles of the polar solvent are attracted to the cations and the positive poles of the polar solvent are attracted to the anions of the ionic solid. Ions in aqueous solutions are hydrated (i.e. surrounded by water molecules).
Hydrated ions can conduct electricity and solutions that conduct electricity are called electrolytes. There are some ionic solids which are insoluble, due to the fact that the attractive forces within the compound are too strong to break.
Dipole-Dipole Attractions
The attraction between the opposite charges on two different polar molecules.
Intermolecular attractions are only about 1% as strong as ionic or covalent bonds. Hydrogen bonding is a special dipole-dipole attraction that is stronger than an ordinary dipole-dipole attraction, however, it is still significantly weaker than any intramolecular force. Some covalent compounds contain polar bonds and are therefore soluble in polar solvents (such as water). Covalent compounds which dissolve remain neutral molecules and do not conduct electricity. They are non-electrolytes.
Solubility of Non-Polar Covalent Compounds
Many covalent compounds do not have negative and positive charges (no dipoles) and therefore are not soluble in water. These compounds are more likely to dissolve in a non-polar solvent (such as hexane).
Factors That Affect Solubility
Molecule Size
Small molecules are often more soluble than larger molecules. Consider the relative solubilities of alcohols as the carbon chain increases in length.
Temperature Size
- The solubility of most solids increases with temperature.
- The solubility of most liquids is not greatly affected by temperature.
- The solubility of gases decreases with higher temperatures.
Pressure
- the solubility of gases is directly proportional to the pressure of the gas above the liquid.