Practical Work
Soluble salt formation
Steps to prepare copper(II) sulfate crystals:
Add excess copper(II) oxide to dilute sulfuric acid and dissolve (heating in a water bath if needed).
Filter the solution to remove unreacted solid, leaving a blue copper(II) sulfate solution.
Evaporate half the water by heating, then allow the remaining solution to cool and evaporate slowly over several days to form blue crystals.
Slow evaporation = larger crystals; fast evaporation produces white anhydrous copper(II) sulfate.
Reaction equation:
H2SO4+CuO→CuSO4+H2O
Insoluble salt formation
Insoluble salts are made by precipitation reactions, where an insoluble solid forms when two solutions mix.
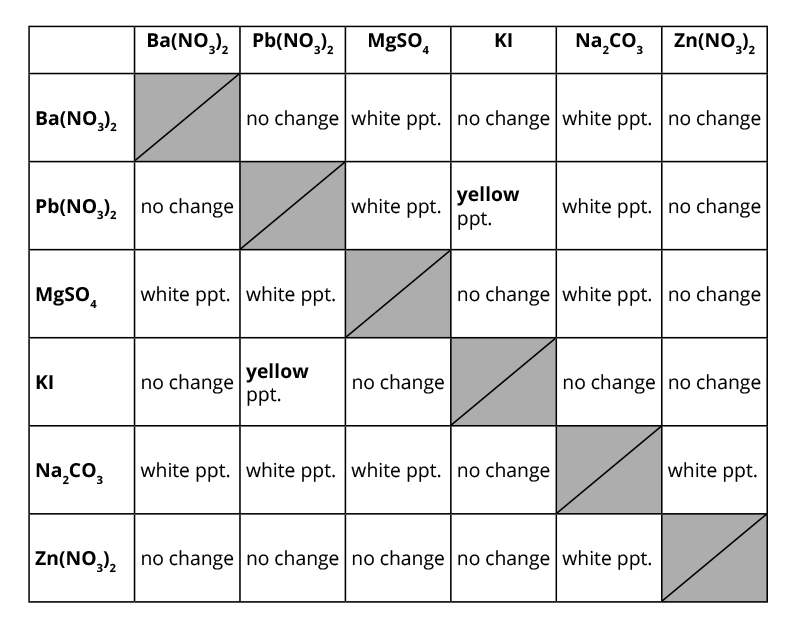
Example: Silver nitrate + halide solution forms a precipitate.
Lead nitrate + potassium iodide forms bright yellow lead(II) iodide, used to test for lead ions:
Reaction:
Pb(NO3)2(aq)+2KI(aq)→PbI2(s)+2KNO3(aq)
The precipitate is separated by filtration and dried in an oven or on a windowsill.
Gravimetric Analysis
This practical involves using gravimetric analysis to determine the molar mass and concentration of an unknown chloride. The key steps include:
Weighing around 0.3 g of the unknown chloride, and transfer it to a 250cm3 beaker.
Dissolving it in deionised water and adding nitric acid.
Adding silver nitrate (AgNO₃) to precipitate silver chloride (AgCl).
Heating and then cooling the solution.
Filtering, washing, and drying the precipitate.
Weighing the dried AgCl to determine its mass.
The calculations involve:
Determining moles of AgCl using mass / molar mass.
Since Ag⁺ and Cl⁻ react in a 1:1 ratio, the moles of AgCl equal the moles of chloride.
Calculating chloride concentration using moles / volume of solution.
Qualitative Analysis