Chapter 2: Dissecting Atoms: Atomic Structure and Bonding
Organic Chemistry for Dummies 2nd Edition by Arthur Winter, PhD
Chapter 2: Dissecting Atoms: Atomic Structure and Bonding
==Electron House Arrest: Shells and Orbitals==
- Protons: number of protons cannot be changed without changing the identity of the atom itself; if the number of protons change, it becomes a different element.
- The number of protons of an element can be found by looking at its atomic number on the periodic table.
- If the atom is electrically neutral, it will have the same number of protons and electrons.
- Ion: an atom that has more or fewer electrons than the amount of protons. The atom is also electrically charged.
- Anion: an atom with more electrons than the number of protons in its nucleus.
- Cation: an atom with fewer electrons than the number of protons in its nucleus.
- Electrons: they are located in the shells surrounding the nucleus, not in the nucleus itself.
- First Shell: closest to the nucleus of the atom, has the lowest energy, and can hold up to 2 electrons.
- Second Shell: higher in energy, farther away from the nucleus, and can hold up to eight electrons.
- Third Shell: higher in energy than the first and second shells, even farther away from the nucleus, and can hold up to 18 electrons.
- %%Note: there are other higher shells, but they are not dealt with in organic chemistry.%%
@@Electron apartments: Orbitals@@
Orbitals: electron shells are further subdivided into these; they are the actual location in which an electron can be found.
Difference between Shells and Orbitals
- @@Shell:@@ indicates the energy level of a certain electron; a full shell will be spherical in shape.
- @@Orbital:@@ status the actual location in space of where the electron resides.
Analogy to Better Understand Electrons:
- @@Electron Shell:@@ Floor in the apartment complex (energy level).
- @@Orbital:@@ Apartment where the electron resides.
- Electrons in atoms are under house arrest. They are restricted to their orbital apartments, but you cannot know for sure where an electron is at any given moment.
Heisenberg uncertainty principle: the uncertainty in knowing the locations of electrons at a given moment.
Orbitals (Used in Organic Chemistry)
- Each orbital can hold up to 2 electrons, but if there are 2 in one orbital, they have opposite spins.
- s orbital: spherical in shape.
- p orbital: shaped like a dumbbell.
- p orbitals hold six electrons in total. This is because there are 3 individual p orbitals in a p level, in which each holds 2 electrons.
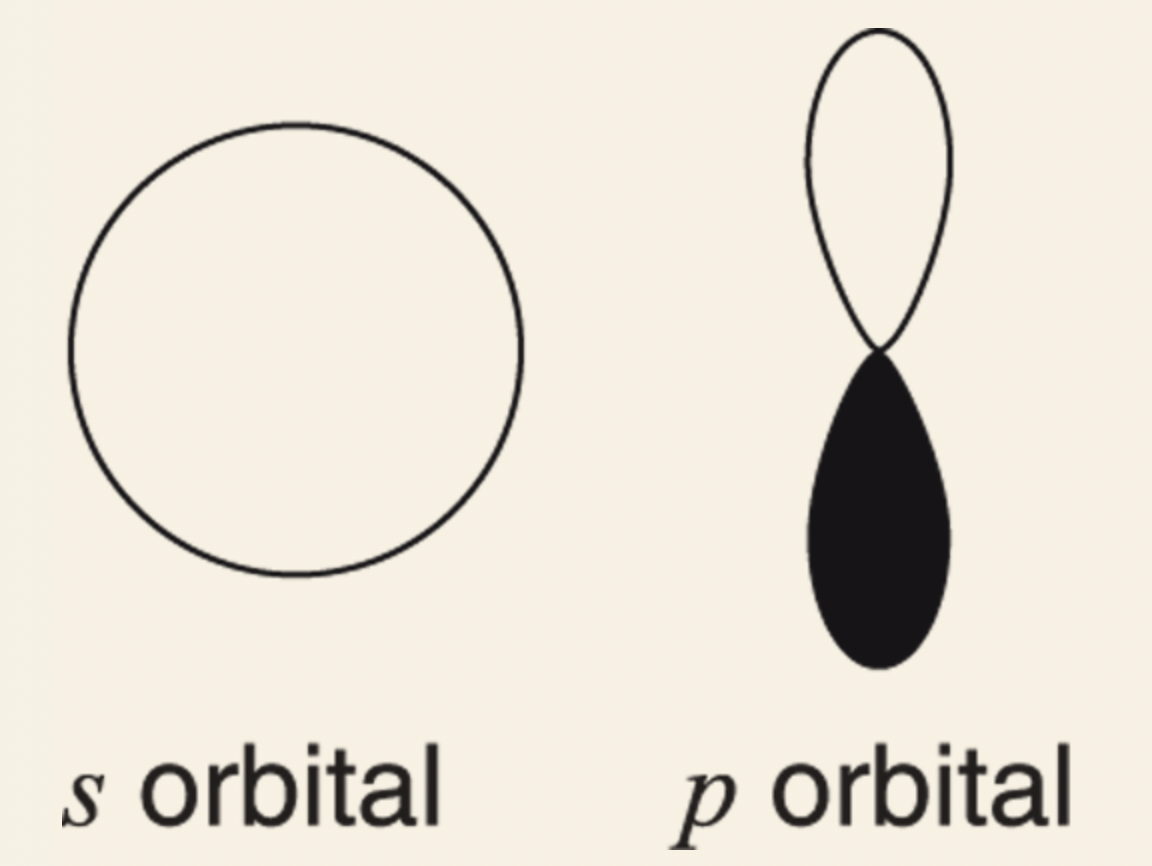
1s orbital: spherically symmetric, holds 2 electrons, only orbital in the first shell.
@@Second Shell:@@ contains both s and p orbitals, holds up to 8 electrons.
- 2s orbital: spherical shape like 1s orbital, but larger and higher in energy.
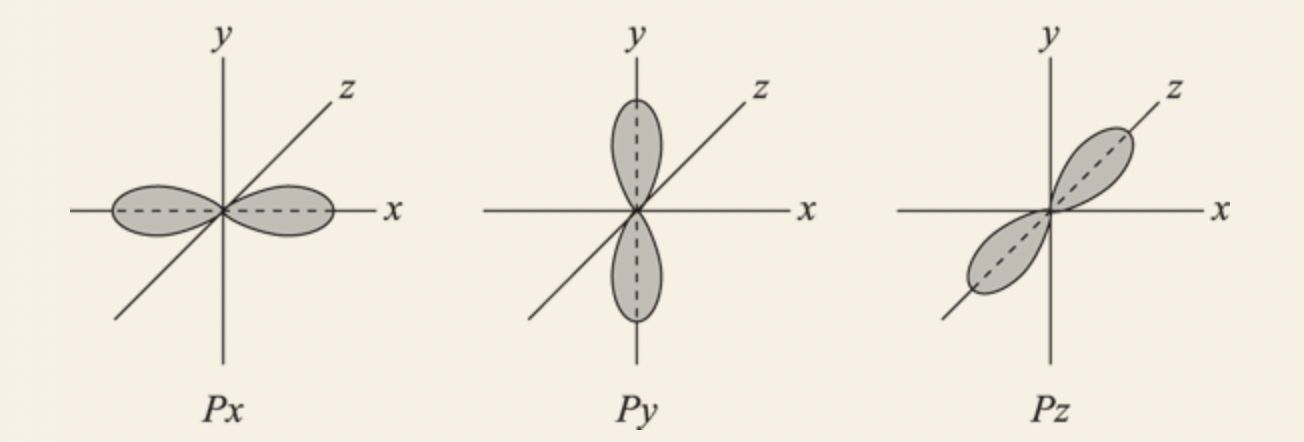
- 2p level: consists of 3 individual p orbitals:
- Px: an orbital that points in the x direction.
- Py: an orbital that points in the y direction.
- Pz: an orbital that points in the z direction.
@@Electron instruction manual: Electron configuration@@
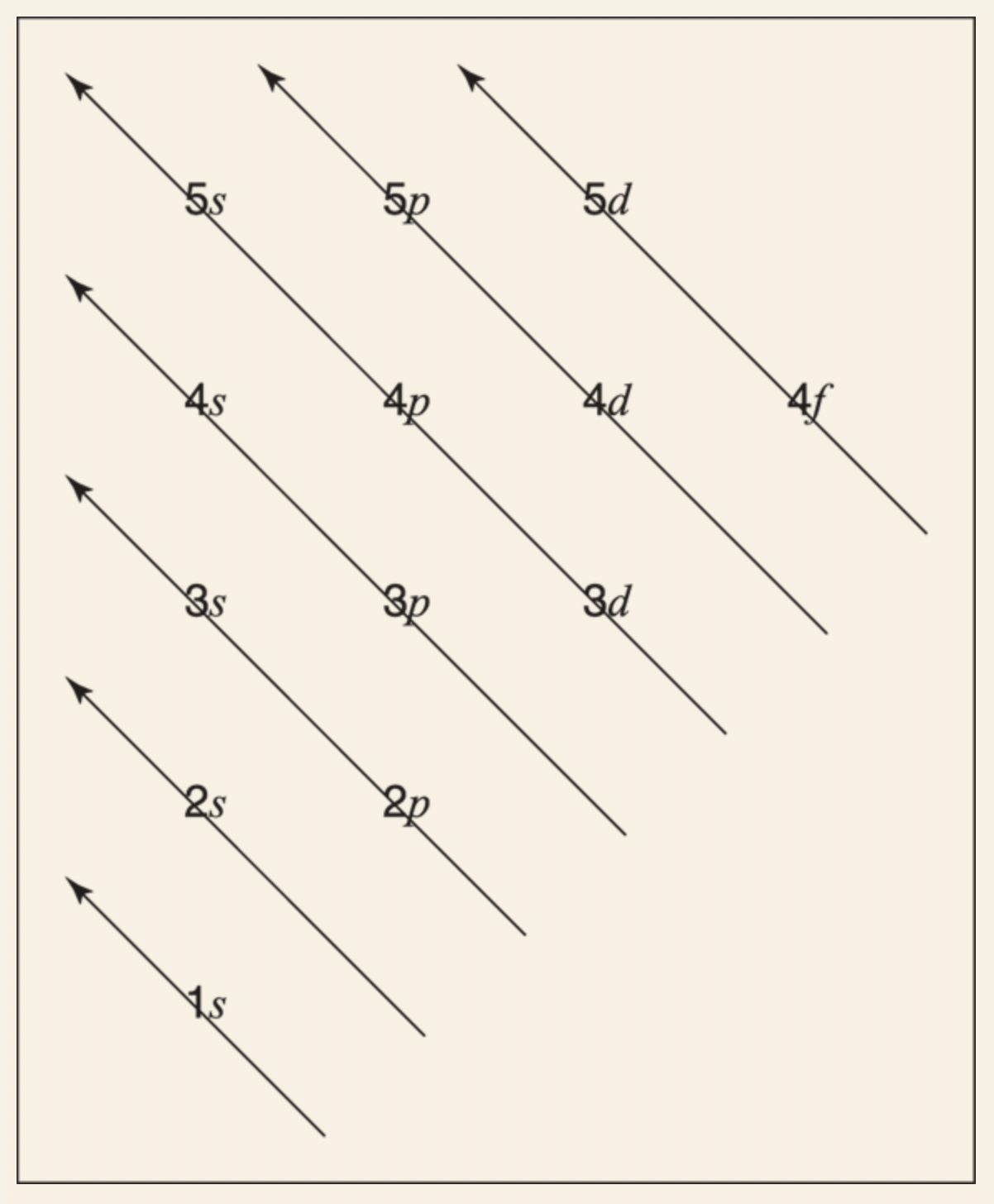
Ground-state electron configuration: list of orbitals occupied by electrons in a particular atom.
You start by placing electrons into lower energy orbitals and build up from there.
The lowest-energy orbital is 1s, followed by 2s, 2p, 3s, 3p, 4s, and so on.
The Aufbau chart is helpful for remembering which orbitals to fill first.
Filling Orbitals
- Fill two electrons per orbital, starting with lowest-energy orbitals and work up until you run out of electrons.
- However, the last electrons in an orbital must be placed according to the Hund’s rule.
- Hund’s rule: the electrons should go into different orbitals with the same spin, instead of pairing up into a single orbital with the opposite spin.
- Electrons repel each other and want to be as far away from each other as possible.
- %%Example:%% Carbon’s electron configuration is 1s^2 2s^2 2px^1 2py^1 2pz^0 (not 1s^2 2s^2 2px^2 2py^0 2pz^0, which violates Hund’s rule).
==Atom Marriage: Bonding==
- Atoms strive to be like noble gases, so they bond with other atoms. It is a major driving force of chemical reactions.
- Noble gases: the only atoms that have their outermost shells filled with electrons; unreactive.
- Filled shell of electrons is the most stable possible electron configuration.
- Octet rule: the desire of atoms to have filled electron shells.
- This refers to the desire of atoms in the second row of the periodic table to fill their outer shells with 8 electrons.
- Valence electrons: electrons in the outermost shell of an atom; most important for bonding.
- Core electrons: electrons in the inner shells; don’t participate in bonding.
==To Share or Not to Share: Ionic and Covalent Bonding==
- Ionic Bonding: two electrons in a bond are not shared between the bonding atoms.
- Covalent Bonding: two electrons in a bond are shared between the two bonding atoms.
@@Mine! They’re all mine! Ionic Bonding@@
Example of Ionic Bonding reaction:
Sodium (Na) and Chlorine (Cl) to make (NaCl), also known as table salt.
Sodium is an atom in the first column of the periodic table and has 1 valence electron.
Chlorine is an atom in the second-to-last column of the periodic table and has 7 valence electrons.
In order to achieve valence octet, sodium could either gain 7 electrons or lose 1; chlorine could either lose 7 electrons or gain 1.
However, atoms generally do not gain or give up more than 3 electrons, so sodium gives up 1 of its valence electron to chlorine. Chlorine, then, has a full octet.
Sodium becomes a cation as it lost 1 electron, and chlorine becomes an anion because it gained 1 electron. Hence, the positive and negative signs. The dots symbolize the valence electrons.
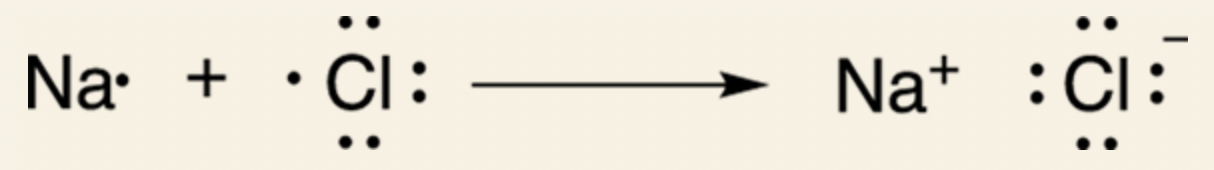
When giving up an electron, sodium imitates the electron configuration of noble gas neon (Ne), which has a full electron configuration.
When gaining 7 electrons, chlorine imitates the noble gas argon (Ar).
Attraction between sodium cation and chlorine anion in sodium chlorine is an ionic bond.
Electrons are NOT shared. They are taken away from one atom by the other atom.
@@The name’s Bond, Covalent Bond@@
- Example of covalent bonding: hydrogen gas (H2)
A hydrogen atom has 1 electron, so it needs another 1 to fill its shell.
Because both hydrogen atoms need 1 electron to fill its shell, they share their electrons equally (instead of grabbing an electron from each other).
Now, they both achieved the electron configuration of noble gas helium (He).

@@Electron piggishness and electronegativity@@
How to know whether a bond is ionic or covalent?
- Look at the difference in electronegativities between the two atoms.
- Electronegativity: tendency of an atom to attract electrons towards itself.
- @@Ionic bond:@@ when the electronegativity difference is very large; greater than 2.
- This is because the atom with the larger electronegativity will attract all the electrons.
- Polar covalent bond: if the electronegativity difference is smaller; between 0 and 2.
- Electrons are shared, but not equally between the atoms.
- Purely covalent bond: if the electronegativity difference is 0.
- Electrons are equally shared between the 2 atoms.
Ionic bonds are usually found in inorganic compounds
- Inorganic compounds: non-carbon-containing compounds.
- Atoms on the left side and atoms on the right side of the periodic table usually make up inorganic compounds. Examples include:
LiF, NaCl, KBr, and MgBr2.
Covalent bonds are found in organic compounds.
- Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) are the most prominent elements in organic compounds.
- Also, phosphorous (P), sulfur (S), and most of the halogens (elements such as fluorine [F], chlorine [Cl], bromine [Br], and iodine [I] that are found in the second-to-last column).
==Separating Charge: Dipole Moments==
Dipole moment: separation of charge in the bond because the more electronegative atom “bullies” most of the bonding electrons away from the less electronegative atom.
For example: in hydrochloric acid (HCl), chlorine is the more electronegative atom of the two, so the electrons between hydrogen and chlorine are “hogged” mostly by chlorine.
Because electrons spend most of the time around chlorine, chlorine gets a partially negative charge.
Because electrons spend less time around hydrogen, hydrogen gets a partially positive charge.
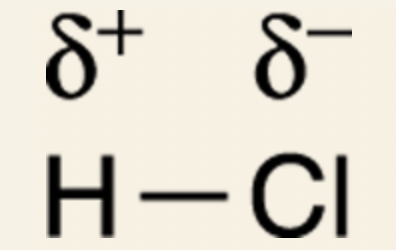
Dipole vector: a distinct arrow that is used to show the direction of the dipole moment, or separation of charge.
Head of the arrow points in the direction of the partial negative charge, and the tail (which looks like a plus sign) points in the direction of the partial positive charge.
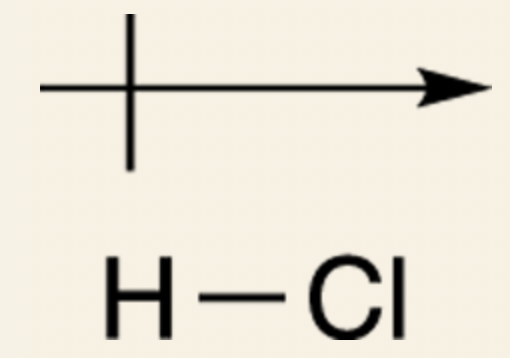
@@Problem solving: Predicting bond dipole moments@@
- How to draw the dipole vector for a bond?
- The atom with the higher electronegativity becomes partially negative, because this atom is the greater electron hog.
- The atom with the lower electronegativity becomes partially positive.
- Draw the dipole vector with the head pointing toward the more electronegative atom and the tail pointing toward the partially positive atom.
- The size of the vector depends on the difference in electronegativity.
- Draw a long vector for large differences in electronegativity, and a short vector for smaller differences.
@@Problem Solving: Predicting molecule dipole moments@@
How to predict dipole moment for a molecule?
Find the dipole vectors of each of the individual bonds.
Add up each of the individual bond vectors.
Line up the vectors from head to tail (the order doesn't matter).
Example: Chloroform: The dipole moment points to the right.
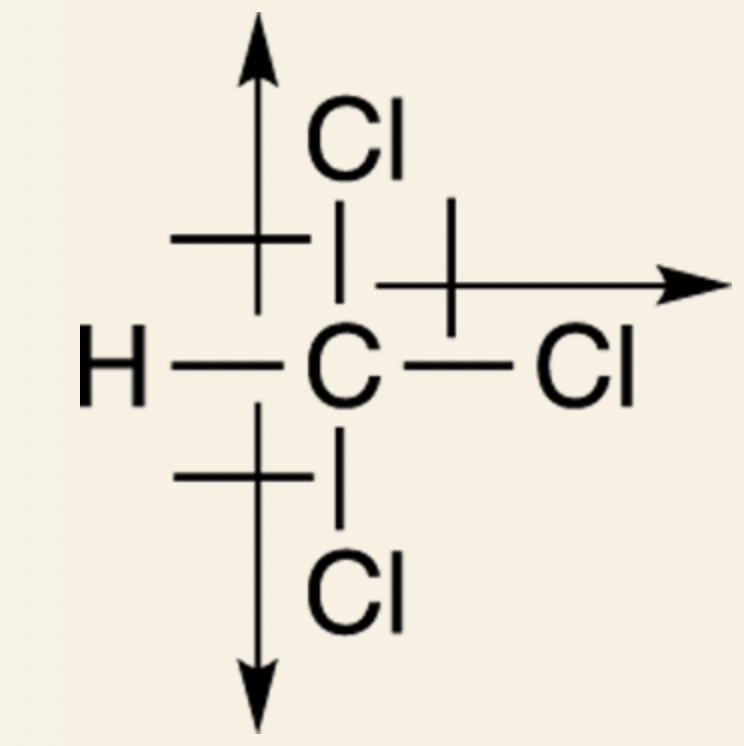
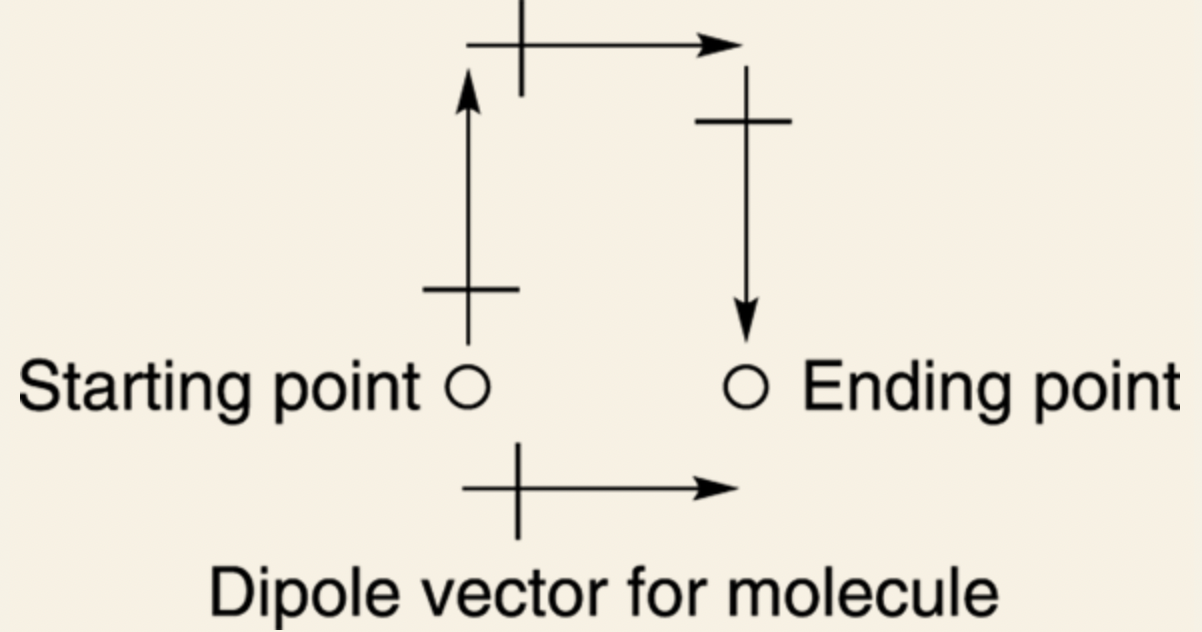
However because individual bonds have dipole moments, it doesn't mean that those molecules have dipole moments.
Example: Carbon Dioxide: Oxygen is more electronegative than carbon, so two dipole vectors point out.
The net dipole moment is zero because the oxygens are pulling in equal and opposite directions; therefore, they cancel each other out.
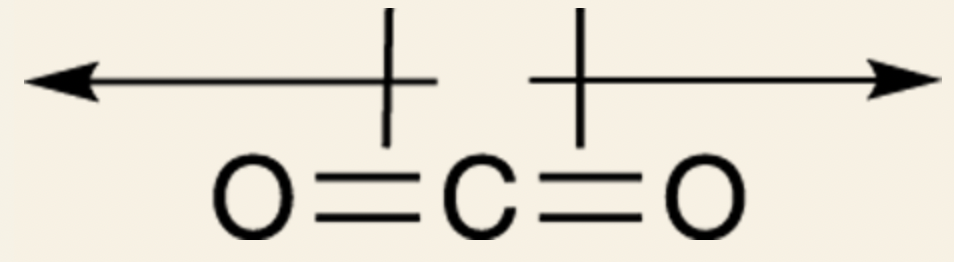
==Seeing Molecular Geometries==
VSEPR theory: stands for valence shell electron pair repulsion; predicts the approximate geometry of bonds around an atom.
- Because electrons repel each other, bonds and lone pairs (non-bonding electron pairs) around an atom want to be as far away from each other as possible.
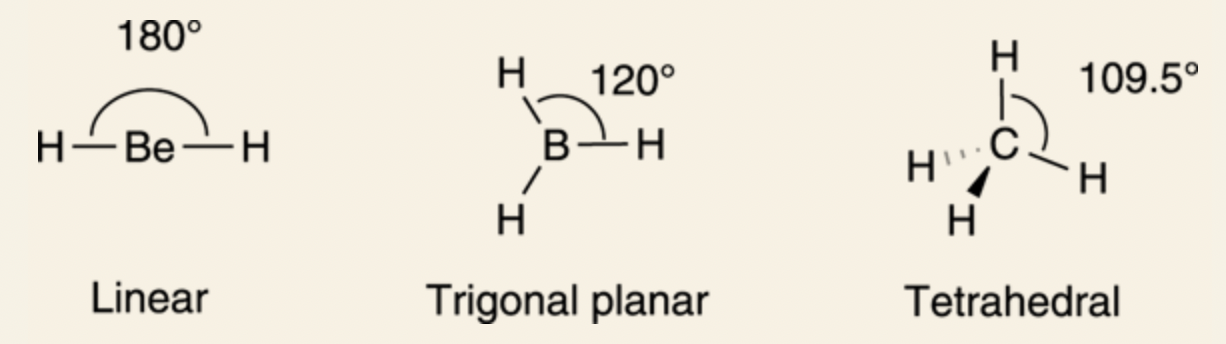
Main Geometries in Organic Chemistry
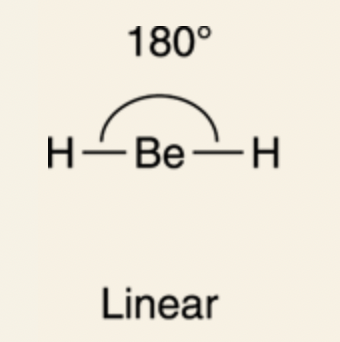
Linear: 180°
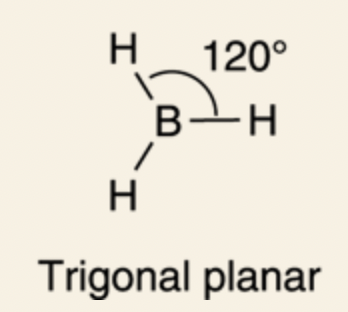
Trigonal Planar: 120°
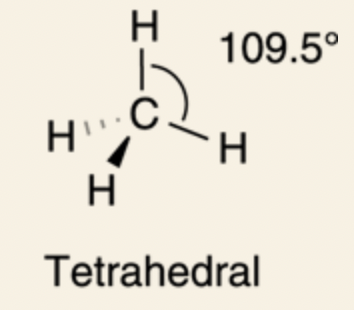
Tetrahedron: 109.5°
@@Mixing things up: Hybrid orbitals@@
Carbon has 4 valence electrons, therefore it makes 4 bonds to fill its octet (and mimic Ne)
In carbon’s electron configuration: 1s and 2s orbitals are completely filled, and there are 2 electrons in the p orbitals to be shared in a covalent bond.
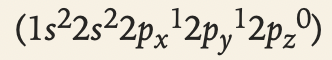
Carbon also wants to make 4 bonds that are oriented in a tetrahedral shape with bond angles of 109.5°.
- However the p orbitals are oriented at 90° angles, perpendicular to each other.
What is the solution, then?
Carbon atom promotes an electron from the filled 2s orbital into the last empty p orbital.
- This leaves the atom with four orbitals, each of which contains one electron, perfect for making four covalent bonds.
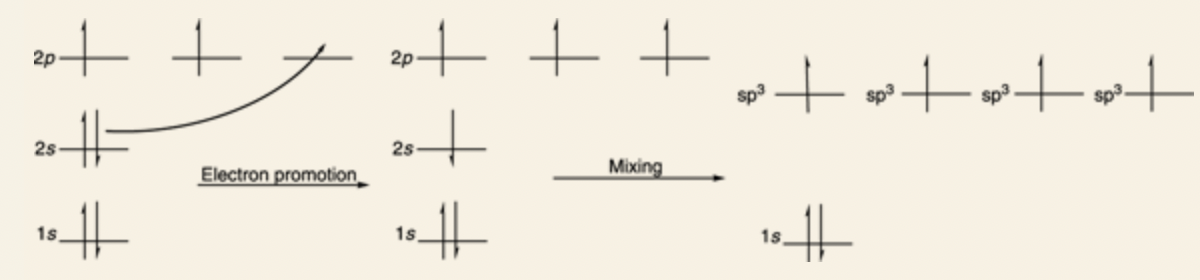
But why would carbon promote the electron?
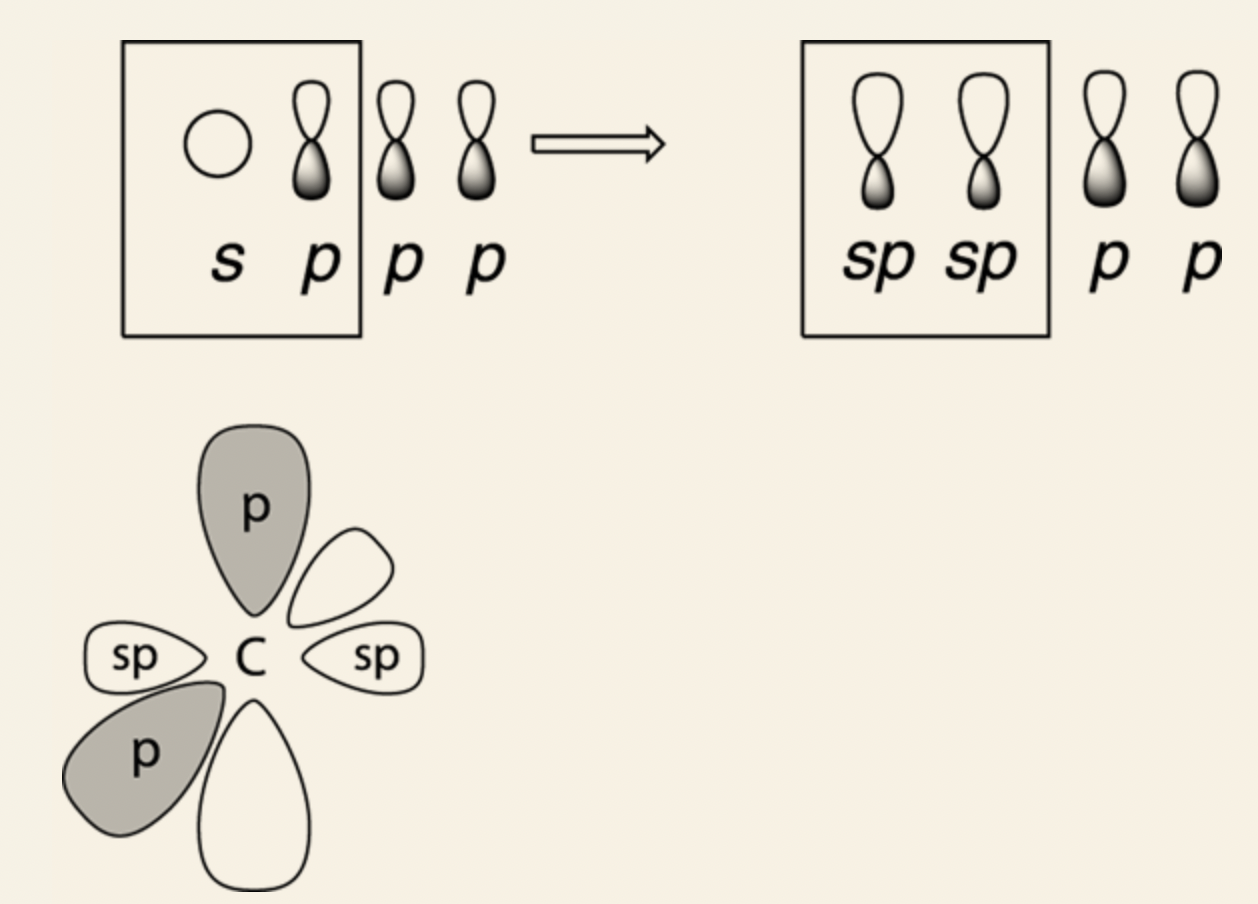
- It allows carbon to mix the four orbitals together — the three 2p orbitals and the 2s orbital — and makes four new orbitals, called sp3 hybridized orbitals.
- Each identical, point at 109.5-degree bond angles away from each other.
- sp3 orbitals are called hybridized orbitals because they’re hybrids of the original orbitals.
Naming Hybridized Orbitals
- The superscript indicates the number of orbitals of each type that mix to form the hybrid.
- But if only one s or p orbital is involved in the hybridized orbital, the superscript is omitted.
- A hybridized orbital made from one s and three p orbitals is written as sp3.
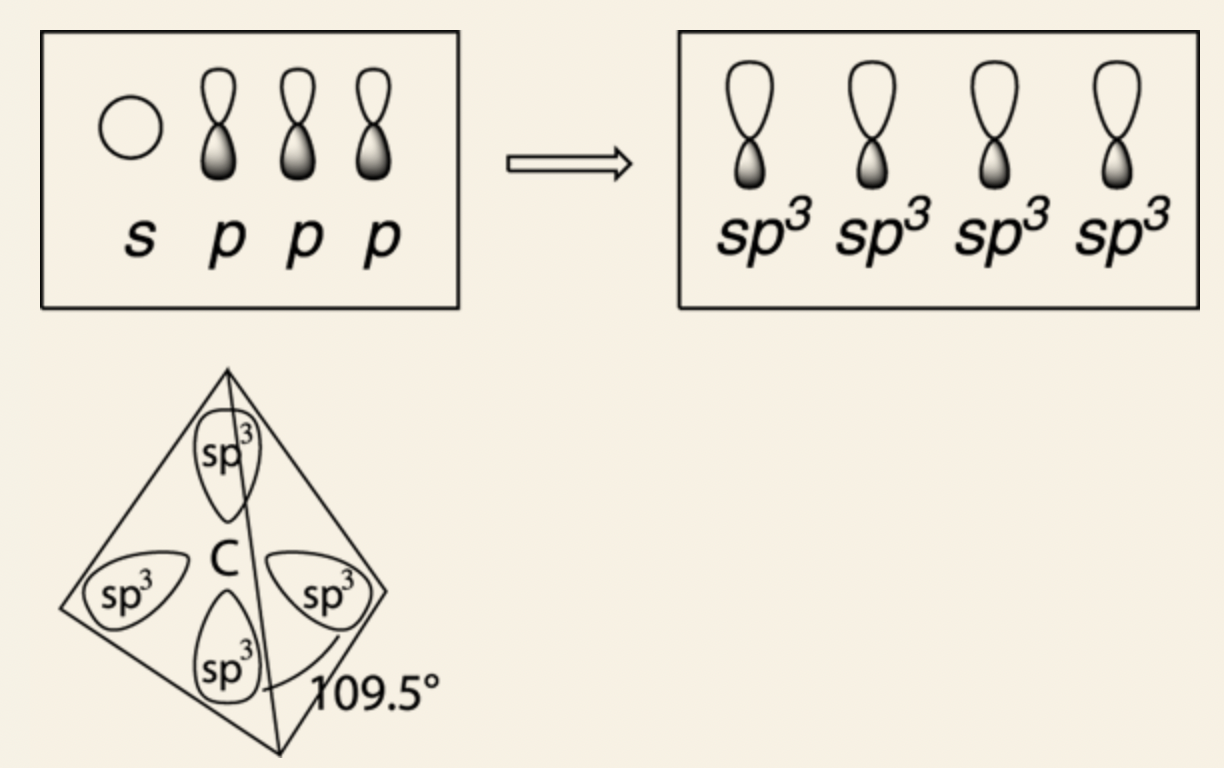
sp3 orbital
- Mixing three p orbitals and one s orbital makes the four output sp3 hybridized orbitals three-quarters p in character and one-quarter s in characteristics.
%%Note:%% The numbers of orbitals that are mixed must equal the number of hybridized orbitals that come out at the end. If four atomic orbitals are mixed, four hybridized orbitals must come out.
sp3 orbital: four sp3 hybridized orbitals (three 2p orbitals and 2s orbital), bond angles are at 109.5°, tetrahedron formation.
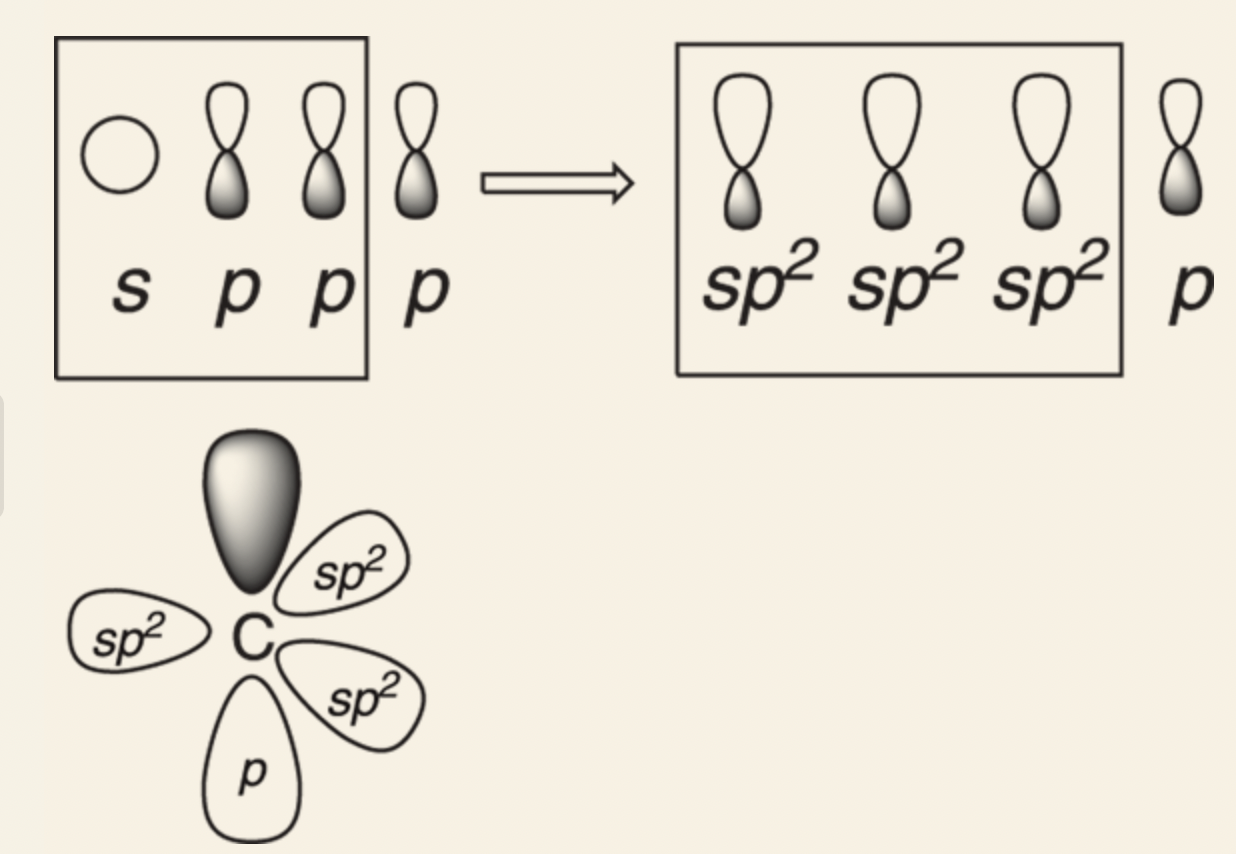
sp2 orbital: three sp2 hybridized orbitals (two 2p orbitals and 2s orbital); since one of the p orbitals are not mixed, it remains in its original unhybridized form, bond angles are at 120°, trigonal planar formation.
This formation is preferable for atoms with 3 bonds and need 120° bond angles for maximum separation of charges from the bonds.
sp orbital: two sp hybridized orbitals (one 2p orbital and 2s orbital); since two of the p orbitals are not mixed, they remain in their original unhybridized form, bond angles are at 180°, linear formation.
This formation is preferable for atoms with 2 bonds and need 180° bond angles for maximum separation of charges from the bonds.
@@Predicting hybridization for atoms@@
Count the number of substituents (or number of atoms bonded to that particular atom) and lone pairs of electrons around that atom.
For BeH2, the beryllium (Be) has two substituents (two identical H atoms), so it’s sp hybridized.
For BH3 (also refer to Figure 2-15), the boron (B) has three substituents (three H atoms), so it’s sp2 hybridized.
In methane, CH4, which has four substituents, the carbon is sp3 hybridized.
==It’s All Greek to Me: Sigma and Pi Bonding==
- Covalent bonds occur when the orbitals of bonding atoms overlap each other.
- Two kinds of covalent bonds can be formed in organic molecules:
- Sigma bonds: bonds in which orbital overlap occurs between the two bonding nuclei.
- Examples:
- Two s orbitals could overlap to make a sigma bond (such as in the bond between the two hydrogens in H2).
- A hybridized orbital and an s orbital could overlap (such as in a C-H bond)
- Two hybridized orbitals could overlap (such as in a C-C bond).
- Pi bonds: bonds where orbital overlap occurs above and below the nuclei, and not directly between them.
- Example:
- Side-by-side overlap between p orbitals.
- With the side-by-side overlap of p orbitals, there is no overlap directly between the bonding nuclei, because the p orbitals have nodes in this region.
- There is, however, overlap above and below the nuclei.
- Node: a region of zero electron density
- Pi bonds are less common than sigma bonds because they’re found only in double and triple bonds, not in single bonds.
Draw the Orbital Diagram of a Molecule
- Determine the hybridization of each of the atoms.
- Draw all the valence orbitals for each atom.
- Determine which orbitals will overlap to make the bonds.
- Sigma bonds: all single bond.
- Double bonds: one sigma and one pi bond.
- Triple bonds: one sigma bond and two pi bonds.
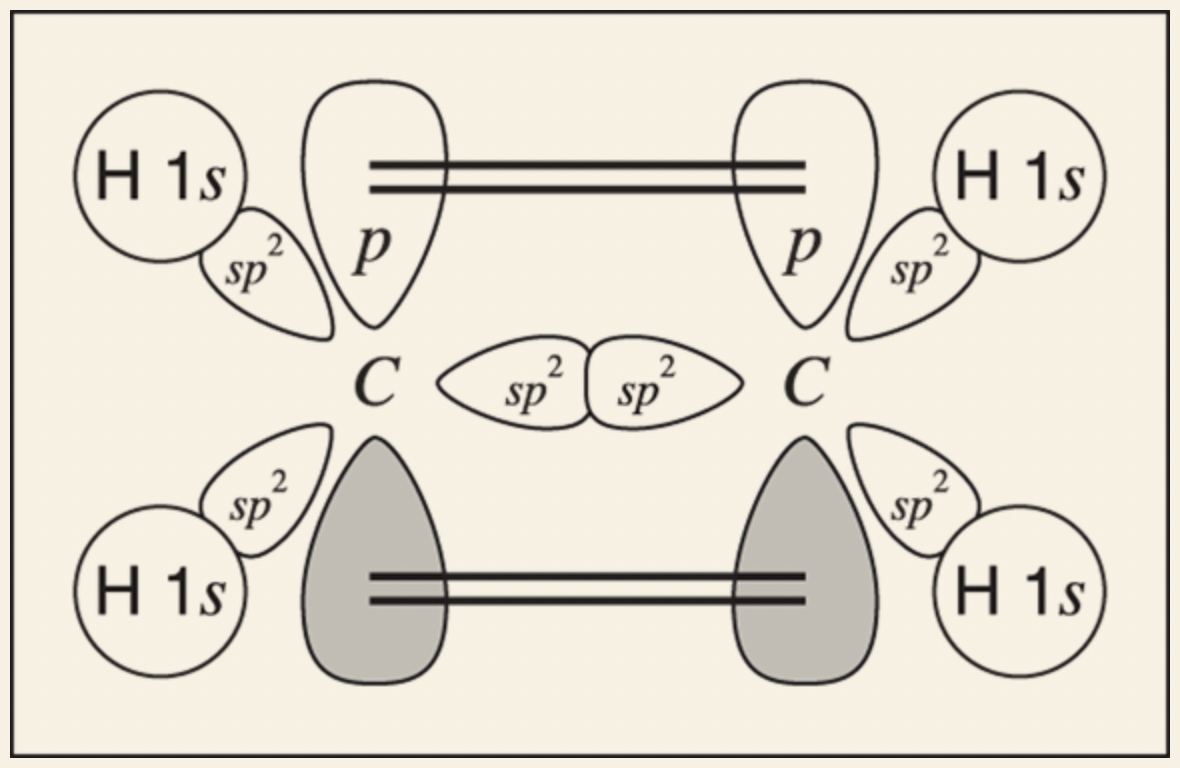
Example of drawing orbital picture for ethylene:
Determine the hybridization of each of the atoms
- Both carbons have substituents (each attached to three other atoms). They are both sp2 hybridized, so carbon has three sp2 orbitals plus one p orbital available for bonding.
- Hydrogen is not hybridized and, thus, has just the 1s orbital available for bonding.
Draw each of the atoms with its valence electrons.
Determine which orbitals overlap to make the bonds.
For each of the C-H bonds (sigma bond), the bond will result from the overlap between an sp2 orbital on the carbon and the 1s orbital on the hydrogen
For the double bond (between the C-C bond), one of the bonds comes from the two sp2 hybridized orbitals overlapping between the carbon nuclei to make a sigma bond, while the other bond comes from the two p orbitals overlapping sideways to make a pi bond above and below the carbon nuclei.
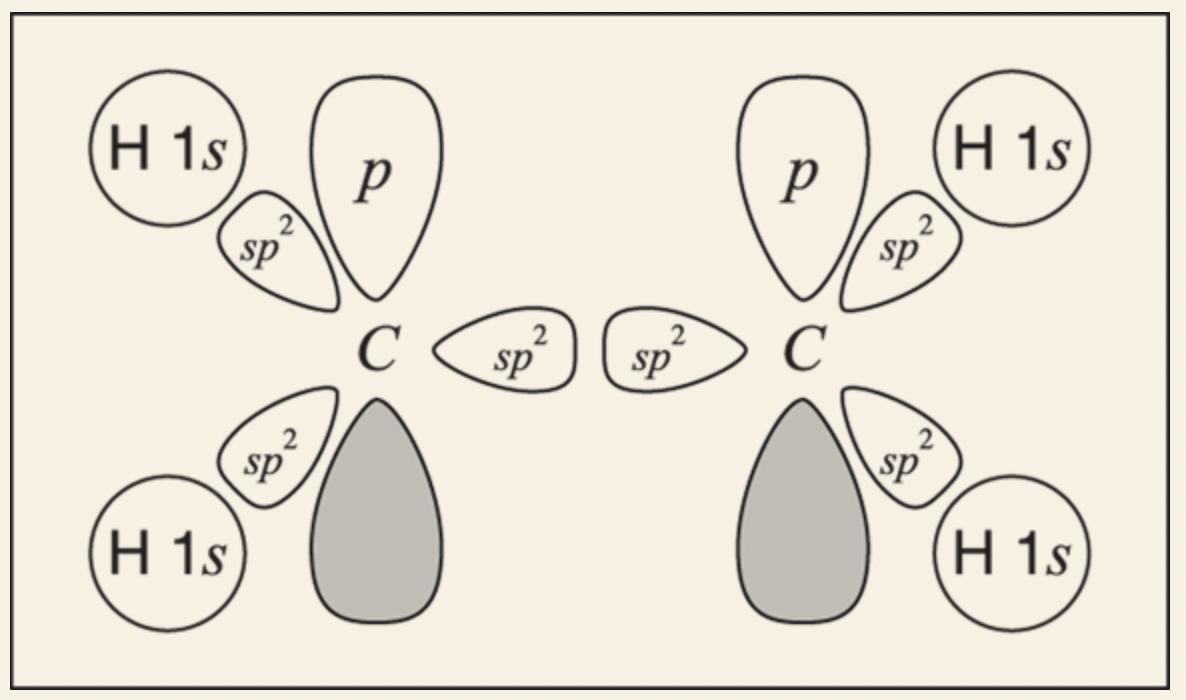
Final Step: orbitals that overlap to make each bond in the molecule are accounted for.