Chapter 19 - Organic Chemistry
19.2 Alkanes
hydrocarbons: compounds composed only of carbon and hydrogen atoms
alkanes: hydrocarbons whose molecules contain only single bonds
- saturated hydrocarbons: maximum ratio of hydrogen to carbon atoms
- unsaturated hydrocarbons: lower hydrogen-carbon ratios
kekule strucutres: structures of organic molecules that show all bonds with lines but omit any lone pairs
- carbon-skeleton structure: no letters used for carbon and hydrogen, each line segment represents one carbon-carbon bond with 109.5°. Each end of the zigzag line is a -CH3 and every intersection of two line segments is a -CH2- group. Assumed each carbon has a steric number of 4.
Alkane properties
- low reactivity (paraffins)
- widely used as fuels and lubricants; highly exothermic with a significant activation energy barrier
- homologous series: series of compounds win which members can be described by a general formula and similar chemical properties
- linear straight-chain hydrocarbons: general formula of CnH2n+2
- methylene group: -CH2- unit
- methyl group: -CH3 unit
- branch: side chain attached to the longest chain
- structural (constitutional) isomers: compounds with same molecular formula but atoms connected in different bonding patterns
Naming alkanes
| prefix | condensed alkane structure | name |
|---|---|---|
| meth- | CH4 | methane |
| eth- | CH3CH3 | ethane |
| prop- | CH3CH2CH3 | propane |
| but- | CH3(CH2)2CH3 | butane |
| pent- | CH3(CH2)3CH3 | pentane |
| hex- | CH3(CH2)4CH3 | hexane |
| hept- | CH3(CH2)5CH3 | heptane |
| oct- | CH3(CH2)6CH3 | octane |
| non- | CH3(CH2)7CH3 | nonane |
| dec- | CH3(CH2)8CH3 | decane |
- select longest chain of carbon atoms and use prefix given in table to name this as the parent chain ex. pentane
- identify each branch and name it with prefix that matches the number of carbon atoms in the branch, append with suffix -yl; name of branch comes before the name of the parent chain ex. methylpentane
- number carbon atoms in parent chain, place branches on lowest possible number starting on the left ex. 2-methylpentane
- if the same group is attached more than once to the parent chain, we use prefixes di-, tri- and tetra- to indivate number of groups present. ex. 2,4-dimethylpentane
- if different groups are attached to a parent chain, they are named in alphabetical order. ex. 4-ethyl-3-methylheptane (not considering prefixes)
Calcoalkanes: alkanes formed in a ring structure, general formula of CnH2n.
- Has one more carbon-carbon bond and two fewer hydrogen atoms per molecule than an alkane with same number of carbon atoms, bond angle of 109.5
- chair form: two possible configurations of cyclohexane with greatest structural stability; cyclohexane spends 99% of its time in one chair form or the other
- boat conformation: higher-energy transition-state configurations (repulsion between the two hydrogen atoms across the ring from each other adds to internal energy and reduces stability) required to flip between chair conformations
- axial position: hydrogen atoms above and below the carbon ring
- equatorial position: hydrogen atoms more parallel to the ring
19.3 Alkenes and Alkynes
alkenes: hydrocarbons with one or more carbon-carbon double bonds
- one c-c double bond has one degree of unsaturation
- capacity to be reduced allows alkenes and alkynes to be more reactive than alkanes; among the most versatile functional groups in organic chemistry
- electron density is greatest around the bonding axis, making pi electrons more accessible to reactions than the sigma bonds between carbon atoms, explaining why unsaturated hydrocarbons are more reactive
alkynes: hydrocarbons with one or more carbon-carbon triple bonds
- one c-c triple bond has two degrees of unsaturation
hydrogenation: alkenes and alkynes combine with H2 to form alkanes; each degree of unsaturation requires one H2 ex. 2 degrees requires 2H2
Z (cis) isomer: methyl group and chain after the double bond are on the same side of the structure
E (trans) isomer: methyl group and chain after the double bond are on opposite sides of the structure
- these isomers exist because there is no free rotation around the bond, both are stereoisomers
Naming Alkenes and Alkynes
- prefixes used to identify length of chain, suffix -ene for alkene and -yne for alkyne
- carbon atoms in chain are numbered so the first carbon in the double/tripple bond has the lowest number possible and it precedes the name with a hyphen
- stereoisomers are identified by writing cis- or trans- before the number ex. cis-2-pentene or trans-2-pentene
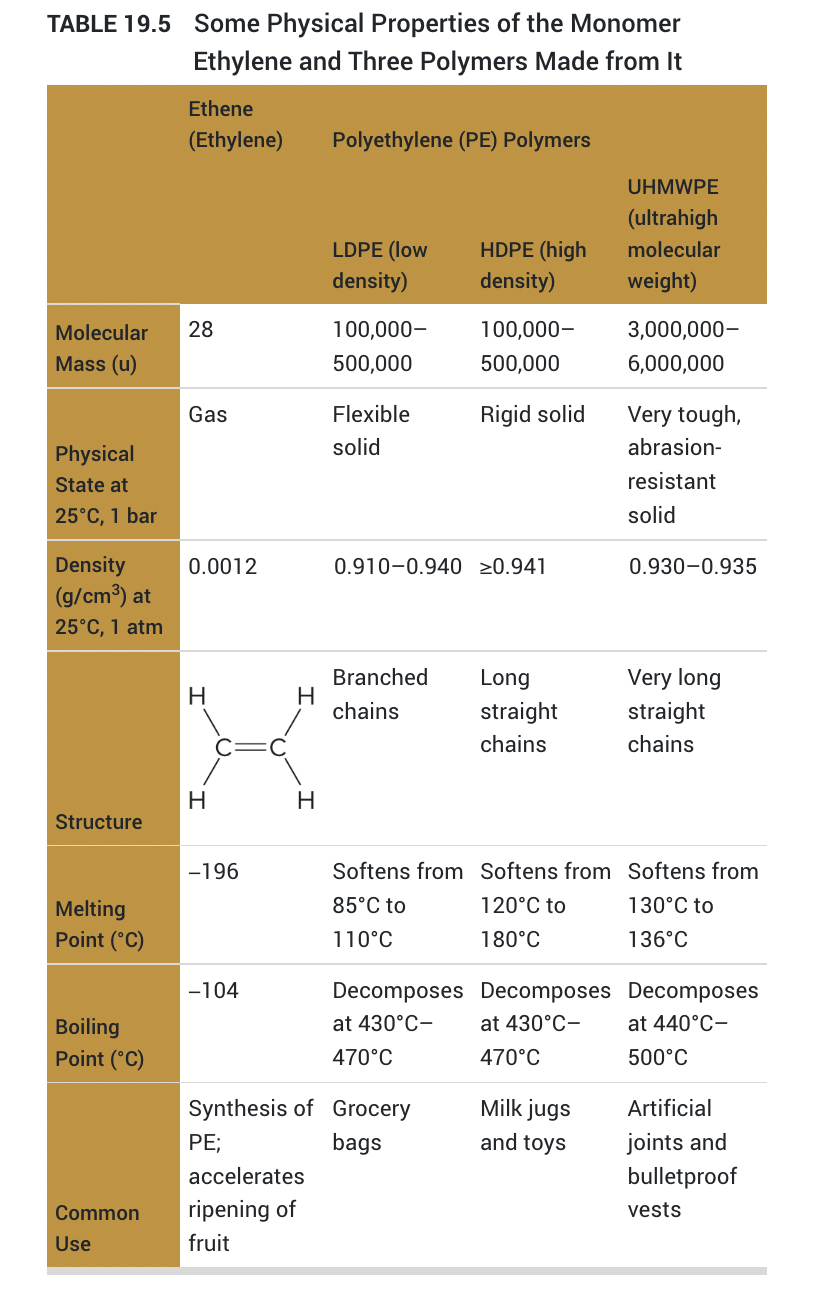
physical properties of monomer ethylene:
- Homopolymer: composed of one type of monomer
- Addition polymer: a polymer constructed by adding many molecules together to form the polymer chain
- vinyl polymer: CH2--CH-, where -- is a double bond
- Branched chains have lower densities and weaker intermolecular forces, causing them to be deormable and softer
- replacing hydrogens will change the chemical composition,
 19.5 Amines
19.5 Amines
Amines: nitrogen is the defining component of functional group, organic base
- Primary amine: one hydrogen in NH3 replaced with an R group, RNH2
- Secondary amine: two hydrogens in NH3 replaced with an R group, R2NH
- Tertiary amine: three hydrogens in NH3 replaced with an R group, R3N
Heteroatoms: atoms other than carbon, hydrogen or metals present in organic compounds
19.6 Alcohols, Ethers and Reformulated Gasoline
Alcohols: general formula of R-OH where R is any alkyl group
- small R group behaves like water and a large R group acts more like a hydrocarbon
- Methanol: widely used industrial organic chemical, starting material in preparation of organic compounds used to make polymers
- Ethanol: formed from fermentation of sugar from vegetable sources; added to gasoline to promote complete combustion and reduce air pollution
Ethers: general formula of R-O-R where R is any alkyl group or aromatic ring
- polar molecules with water solubilities comparable to alcohols of similar molar mass but lower boiling points - more similar to alkanes of similar molar mass
Polymers of Alcohols and Ethers
- used to make adhesives, emulsions and less porous, absorbent and smoother materials
- -OH chains make surface polar and resistant to organic solvents
- Copolymer: two different monomers
- Heteropolymer: three or more different monomers
- monomers forming these two types of molecules can form alternating, block and random co/heteropolymers
19.8 A Brief Survey of Isomers
Chain Isomers: molecules having different arrangements of their carbon skeletons
Positional isomers: same functional group (-OH group) bonded to different carbon atoms
Functional Isomers: different functional groups because of atom arrangement