chemistry
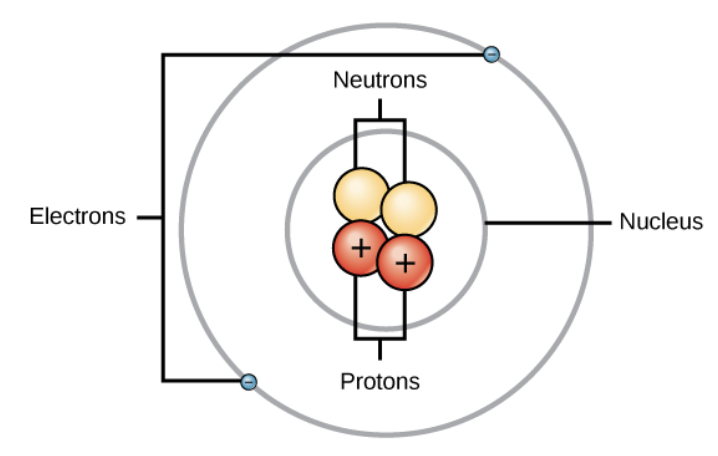
atoms are the smallest units of matter and consist of three main subatomic particles: protons, neutrons, and electrons
protons are positively charged particles found in the nucleus. the number of protons determines an element’s atomic number (EX: carbon has 6 protons and its atomic number is 6)
neutrons are neutral particles that are located in the nucleus. they contribute to the mass of the atom but do not affect its charge. different isotopes of an element have the same number of protons, but different numbers of neutrons.
electrons are negatively charged particles that orbit the nucleus in various energy levels or “shells”. the number of electrons in a nucleus atom equals the number of protons, balancing the overall charge.
Reading the periodic table of elements
Each tile on the periodic table of elements corresponds to a unique element. The number of protons determines an element’s atomic number
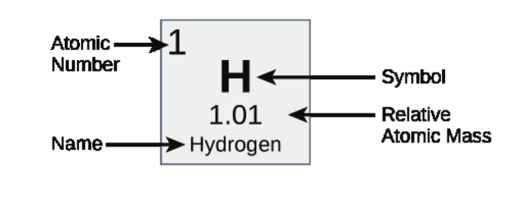
The number of protons and neutrons determine an element’s mass number. The atomic mass is a weighted average of all isotopes for a given element.
The weight of electrons is negligible, so it isn’t considered in the atomic mass.
-Changing the number of electrons an atom has will affect its charge.
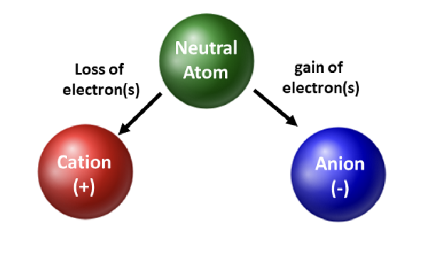
No charge → # of electrons equals # of protons
Positive charge → # of electrons is less than # of protons
Negative charge → # of electrons is greater than # of protons
Charged atoms are called ions
The Bohr Model: depicts atoms as a central nucleus with electrons in “shells” or different energy levels at specific distances from the nucleus.
an help us find the # of valence e-’s
- Innermost first (1n) - 2 electrons max
- The next two (2n and 3n) - 8 electrons max
The outermost shell of electrons (valence shell) determines the chemical properties of an atom.
Atoms with a full outer shell tend to be inert, while atoms without a full shell will be more likely to react in a way to gain, lose or share electrons
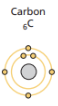
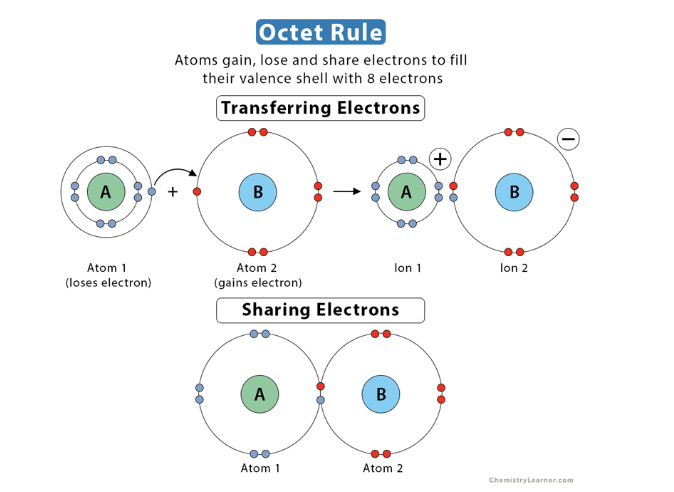
octet rule - atoms want a full valence shell
The “octet rule” - atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell.
- Atoms will gain, lose, or share electrons to fill their valence shell with 8 electrons, usually resulting in the formation of chemical bonds

covalent bonds: electron pairs are shared equally between two atoms. Two or more atoms held together by covalent bonds are called molecules.
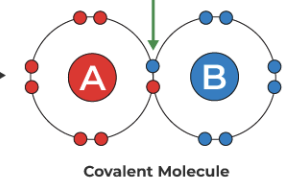
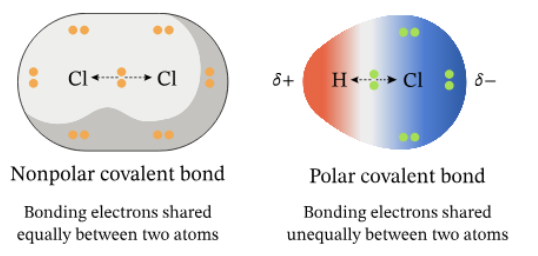
electronegativity The attraction of a particular atom for the electrons of a covalent bond
Atoms with similar electronegativities share e- equally in nonpolar covalent bonds.
Atoms with different electronegativities share e- unequally in polar covalent bonds.
- Electrons are pulled closer to the more electronegative atom.
Ionic bonds (this is stealing/ filling each others electrons!)
In some cases, two atoms are so unequal in their attraction for valence electrons that one can steal an electron from the other completely.
An ion forms when an atom or molecule gains or loses an electron and becomes charged. An ionic bond is the attraction between two oppositely charged ions.
hydrogen bonds
The non-covalent attraction between a hydrogen and an electronegative atom (e.g. oxygen) is called a hydrogen bond.
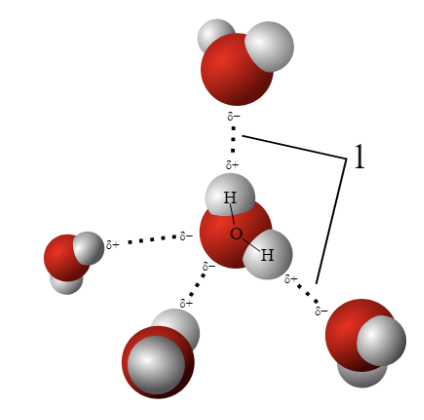
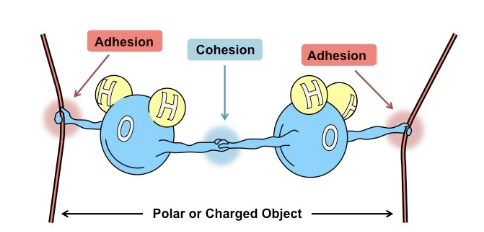
The ability of water to form hydrogen bonds contributes to:
1. Cohesion - the ability of water to stick to itself
2. Adhesion - the ability of water to stick to other things
3. Solubility - the ability water to dissolve a solvent into solution
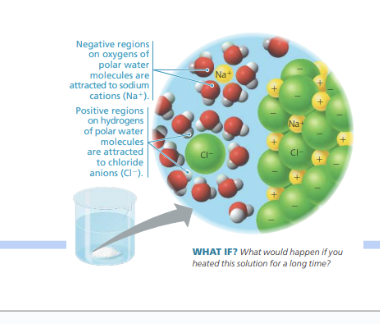
This image is showing how water dissolves salt,
-The negative part of water molecules (oxygen) is attracted to the positively charged sodium ions (Na⁺)
-The positive part of water molecules (hydrogen) is attracted to the negatively charged chloride ions (Cl⁻)
also, heating the water makes it evaporate, causing the salt to reform.
“Van der Waals” interactions
Electrons don't always spread out evenly around the nucleus of an atom, which creates brief moments where one part of the atom has a slight positive or negative charge. These temporary charges allow atoms to attract each other through weak forces called van der Waals interactions.
Molecular shape- determines how molecules recognize and respond to one another with specificity. Biological molecules often bind temporarily to each other through many weak interactions, but only if their shapes “fit”.
Chemical reactions:
Chemical reactions make and break chemical bonds.
Starting material = reactants
Resulting material = products
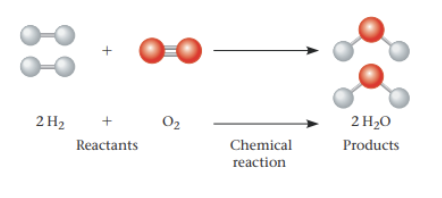
All chemical reactions can, in theory, go both ways—forward or backward. The point where the reactions balance each other out is called chemical equilibrium.
- At equilibrium, the amounts of reactants and products aren’t necessarily equal; instead, their levels stay steady at a specific ratio.
 Knowt
Knowt
