Unit 5 The Periodic Table >
5.1 Parts of the Periodic Table
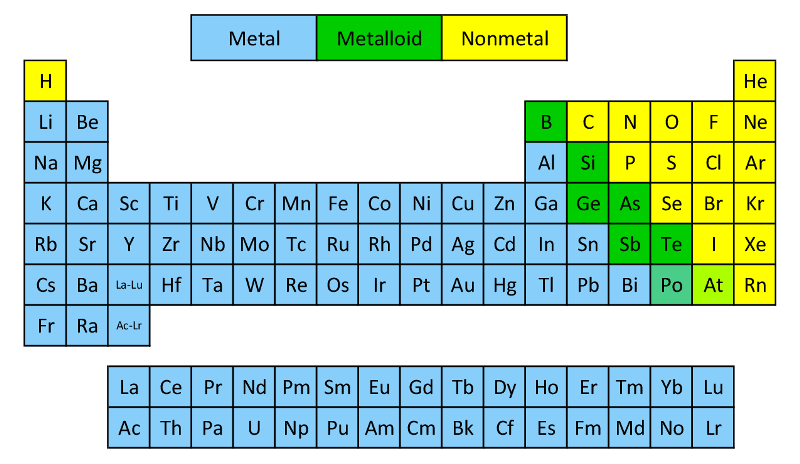 Metals:
Metals:
Nonmetals:
Metalloids:
Transition Metals:
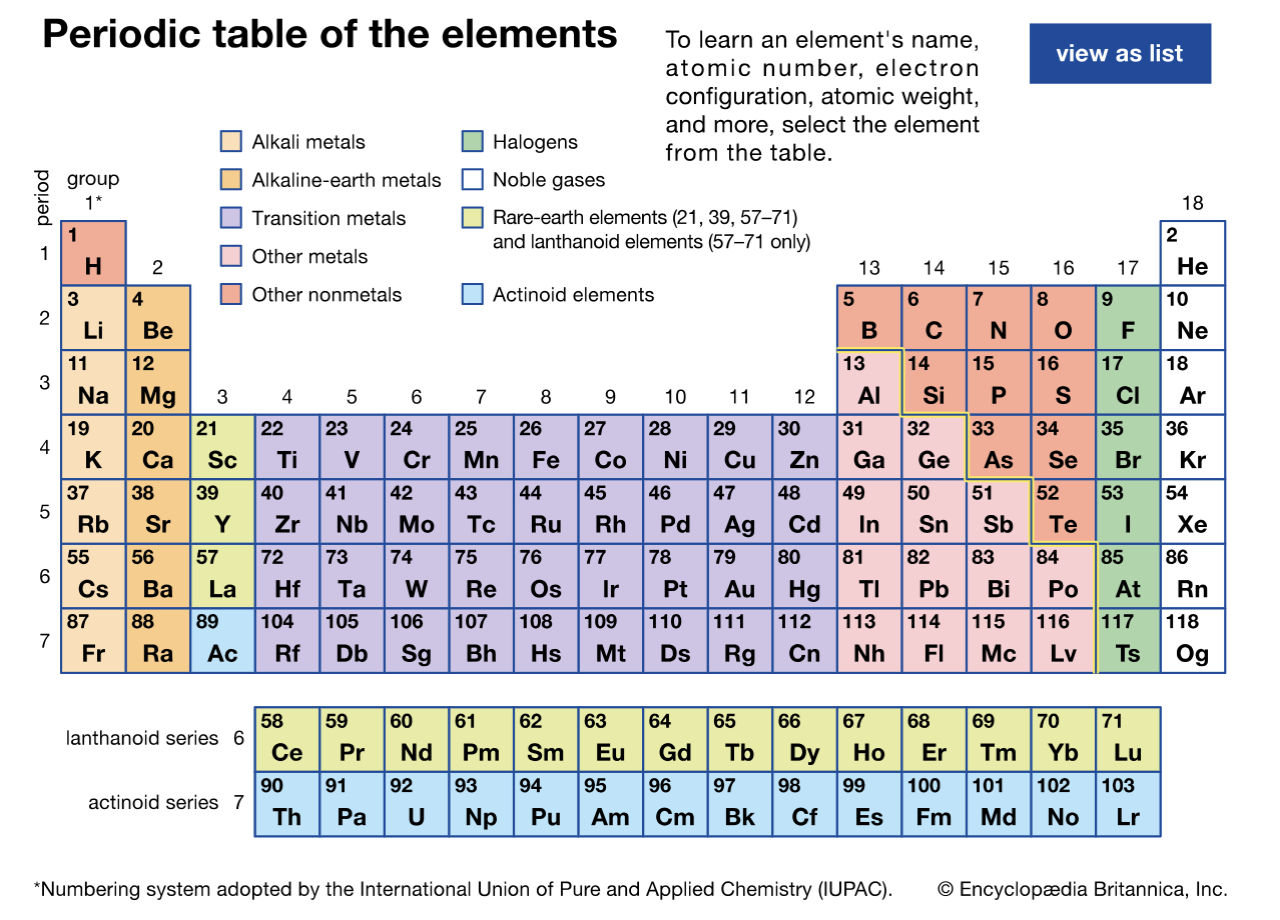
Periods vs. Groups
there are 7 periods (rows)
there are 18 groups (columns)
Group 1 elements (hydrogen omitted): Alkali metals
✩They are soft metals that quickly become coated with a black coating when exposed to air
✩These elements are kept in oil to prevent corrosion/oxidation with air
✩All of these metals react in water aggressively and oxidize to air
✩When going down the line, these metals seem to corrode more quickly and explode more aggressively in water
✩Soft, silver metals
Group 2 elements: Alkaline earth metals
✩Each has two valence electrons and shares different properties
✩These metals, except for magnesium, are stored in oil
✩Melting and boiling points decrease down the line, and the metals get softer
✩Magnesium will only react with steam, not cold water
✩The reaction of each metal with water becomes more vigorous as it goes down the line
✩Reactive but not as reactive as Alkali metals
✩Soft, silver metals
Group 18 elements: Noble Gases (Lazy gases)
✩Most stable or inactive non-metals
Group 17: Halogens (Only the first four compared)
✩Most reactive nonmetals
✩Halogen elements consist of diatomic molecules
✩Very soluble in water
✩React with aluminum