Organic Lecture 3/5
Physical Properties and Reactivity of Phenols
Influence of Substituents:
The nitro group (NO2) has a significant effect on acidity and reactivity compared to other groups.
Groups like carboxylic acids may require removal of water for reactions to proceed efficiently.
Electrophilic Aromatic Substitution (EAS) Reactions
OH group is a powerful activating group in EAS and ortho/para director.
Mechanism Overview:
Reactions are acid-catalyzed (e.g., sulfuric acid, H2SO4).
Water removal might be necessary to shift equilibrium to favor product formation.
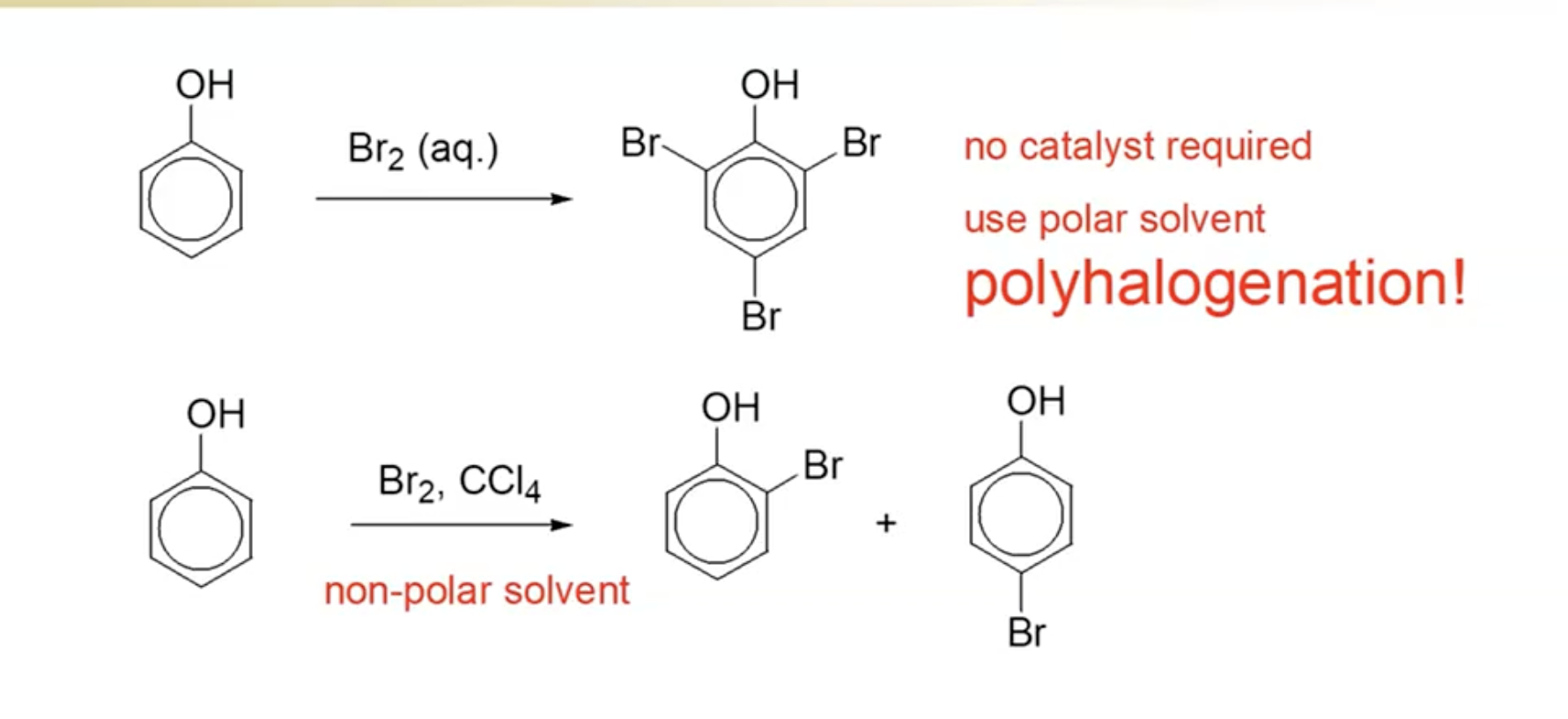
Halogenation with Bromine Water:
Phenols easily react with bromine in water, demonstrating high reactivity as an activated benzene ring.
Reaction must be done in a polar solvent (like water) to stabilize the bromide ion (Br-).
Multiple additions can occur, confirming the presence of excess phenol.
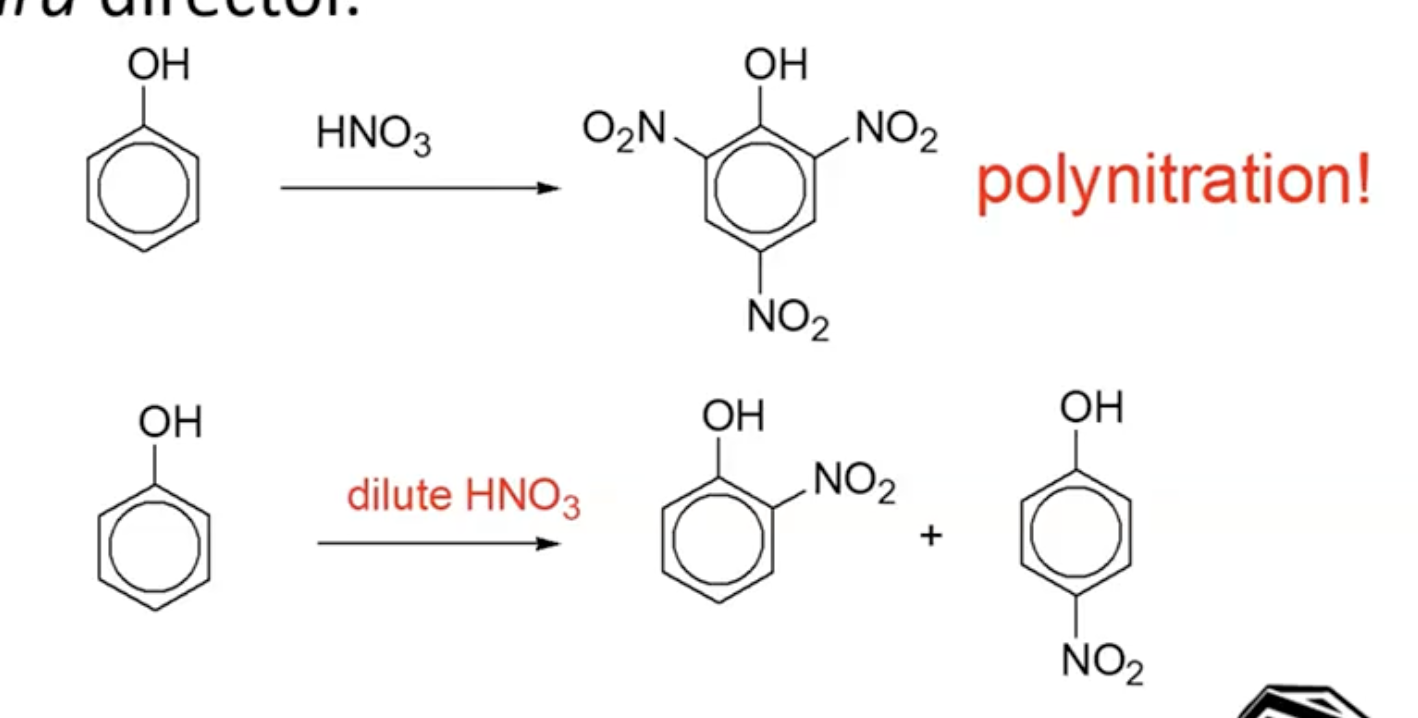
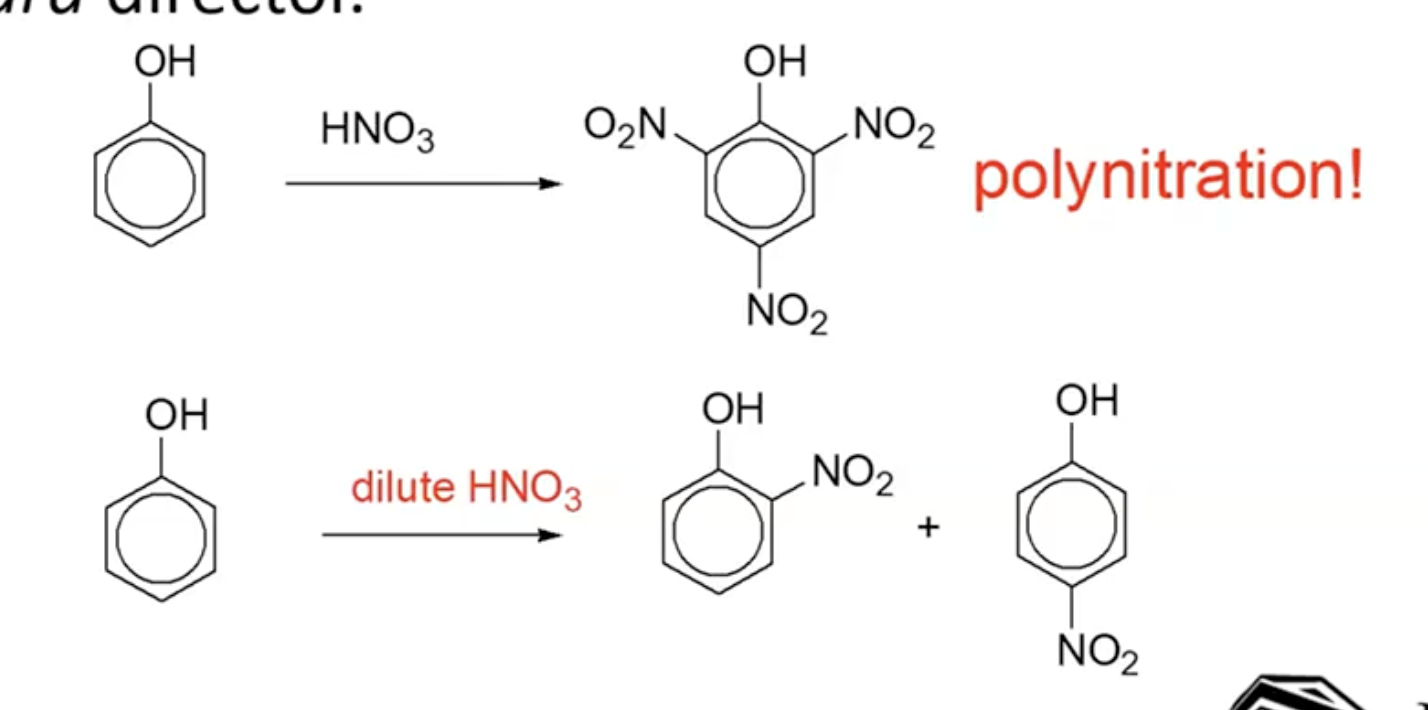
Nitration:
Using concentrated nitric acid will lead to multiple substitutions; dilute nitric acid can limit this to one substitution.
The hydroxyl (-OH) group acts as a strong electron-donor, enhancing reactivity.
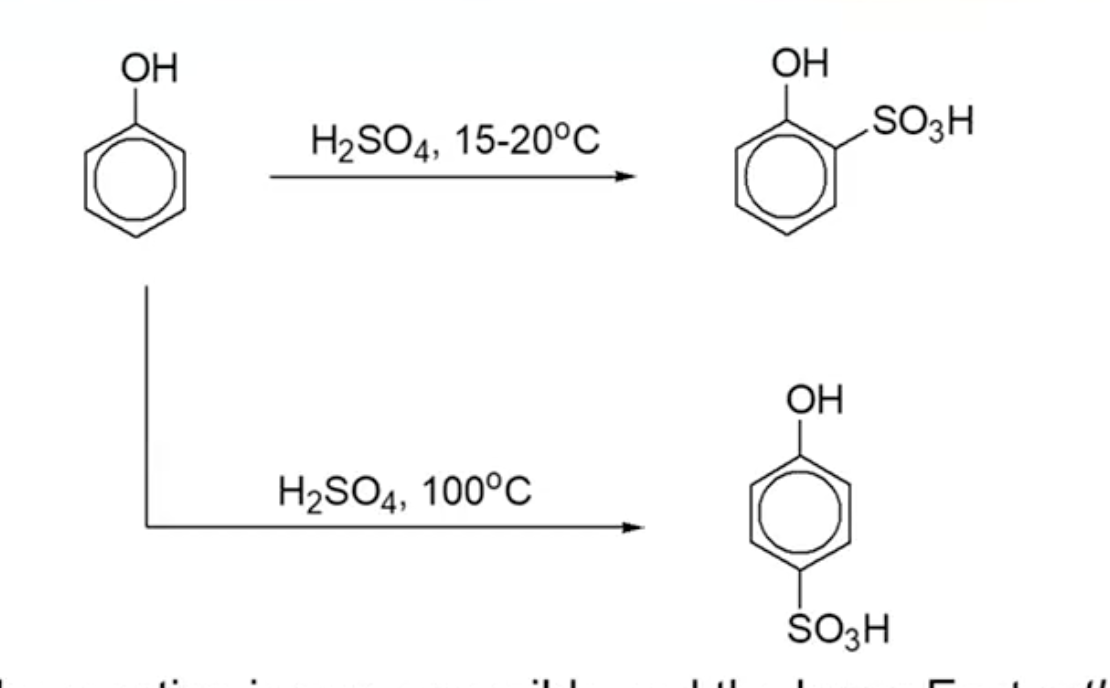
Sulfonation
Position Dependence on Temperature:
At low temperatures, sulfonyl group (-SO3H) prefers ortho substitution (rate control)
At high temperatures, para substitution is favored (kinetic control)
Sulfonyl group acts as a meta director and deactivator due to positive charge on sulfur.
Regeneration and Removal:
Sulfonyl group can be removed using diluted sulfuric acid, demonstrating phenol's high reactivity.
Functional Group Tests
Testing for Presence of Phenols:
Bromine water is a specific test for phenols, indicating their reactive nature in electrophilic substitution.
The reaction proceeds only in water, confirming phenol presence based on consistent reaction outcomes.
Acylation Reaction of Phenols: Alkylation is super simple
Mechanism of O-Acylation:
Acid halides or anhydrides react with phenols to form esters via O-acylation (similar to Fischer esterification).
Lewis acids can facilitate acylation, changing reaction mechanism to involve EAS type reaction for electrophilic substitutions.
Normal catalytic conditions (AlCl3), EAS occurs
Absence of aluminum chloride, O-acylation occurs
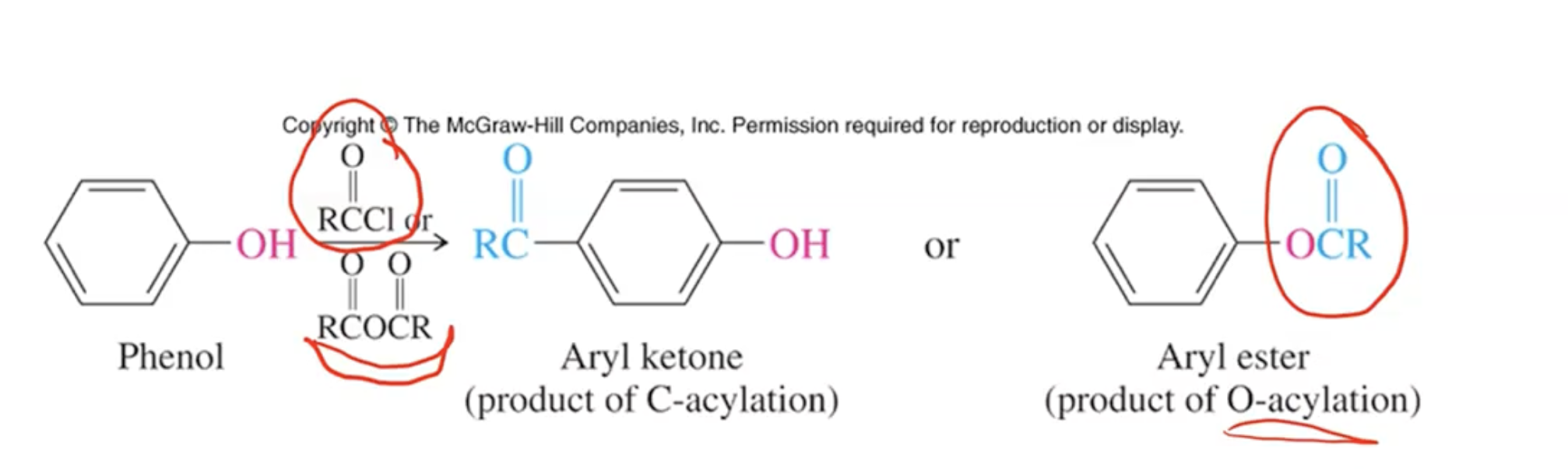
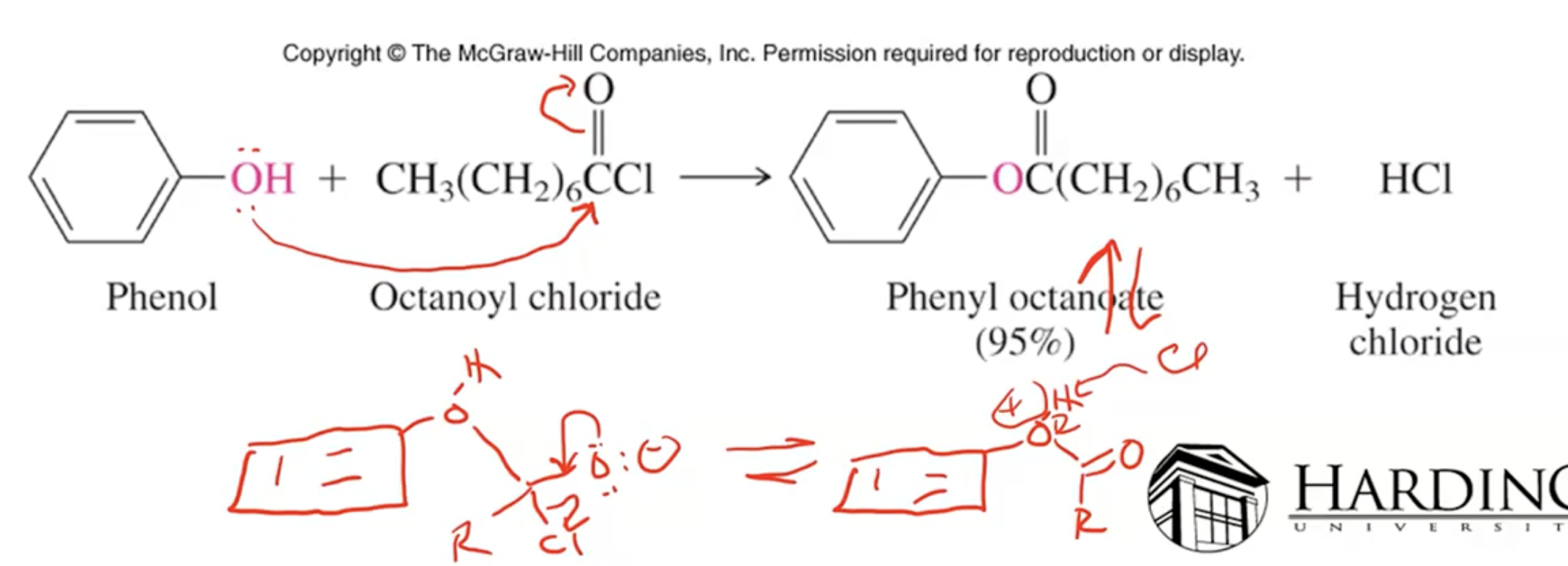
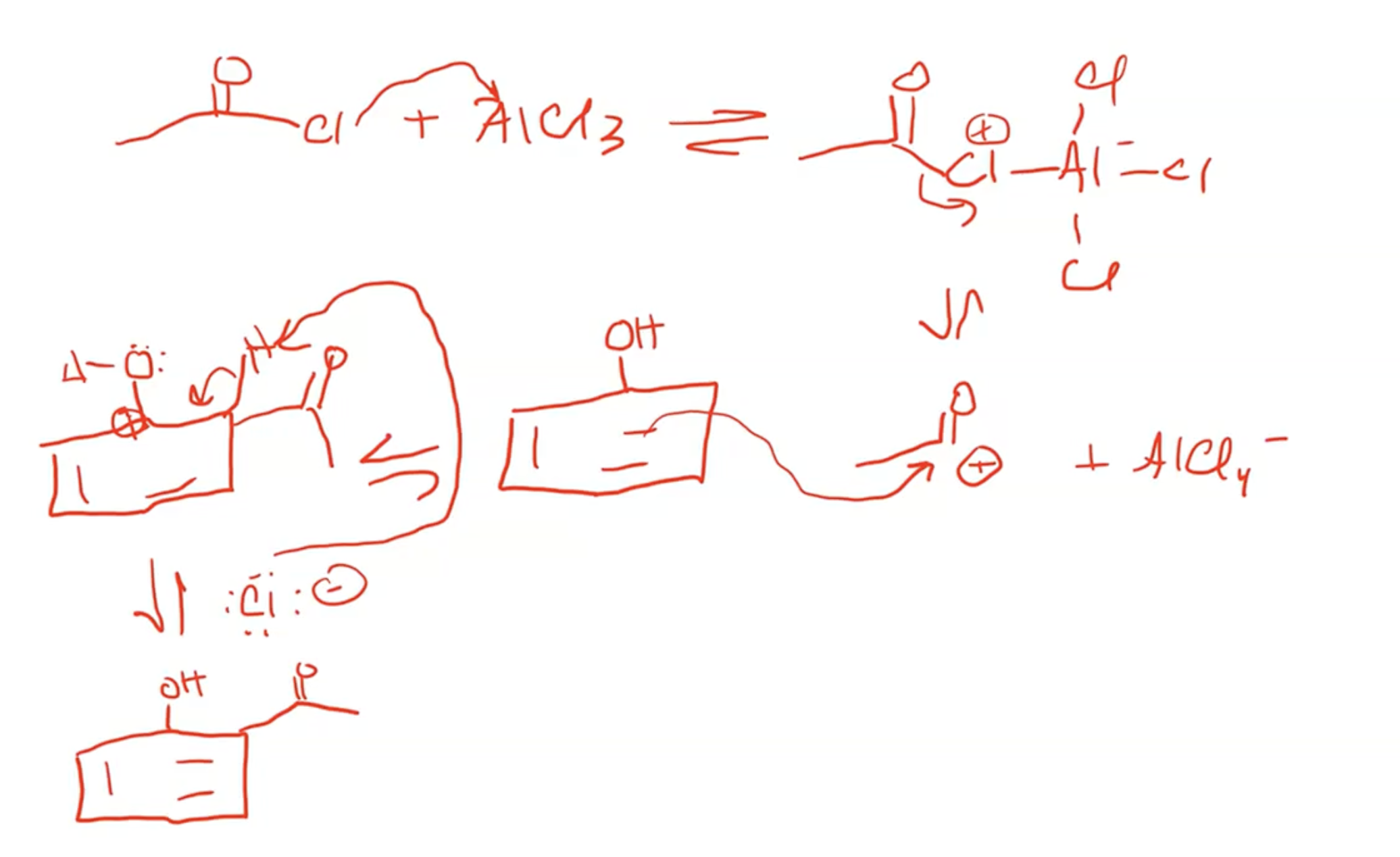
anything larger than acyl group will prefer going para over ortho
Nitrosation
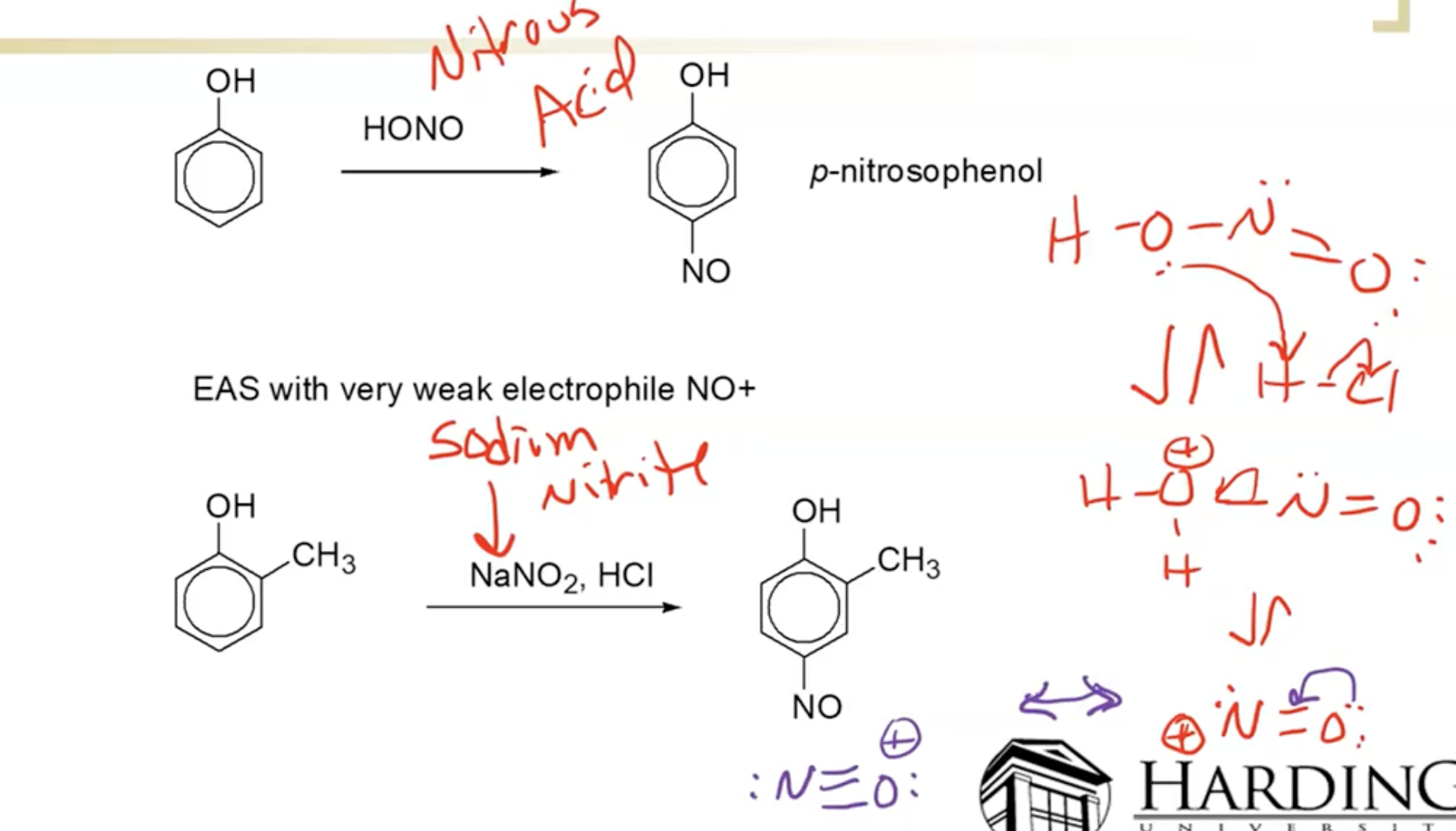
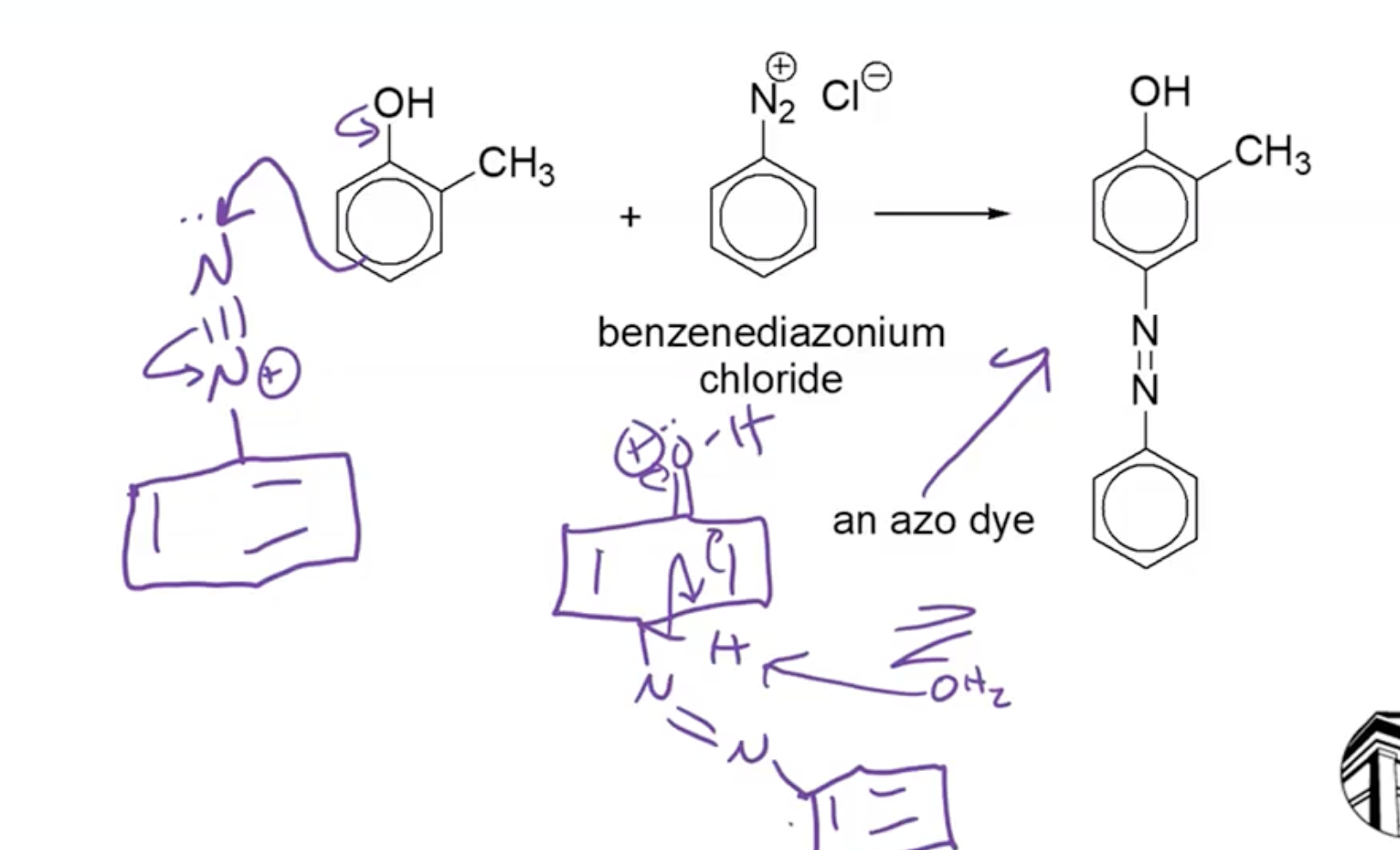
Diazotization and Azo Dye Formation
Formation of Azo Compounds:
A diazonium ion reacts with phenol to form azo compounds, useful as dyes.
Modification of the phenolic structure allows tailoring of dye colors.
Azodyes are prevalent in commercial applications, though not as commonly used in textiles due to fading issues.
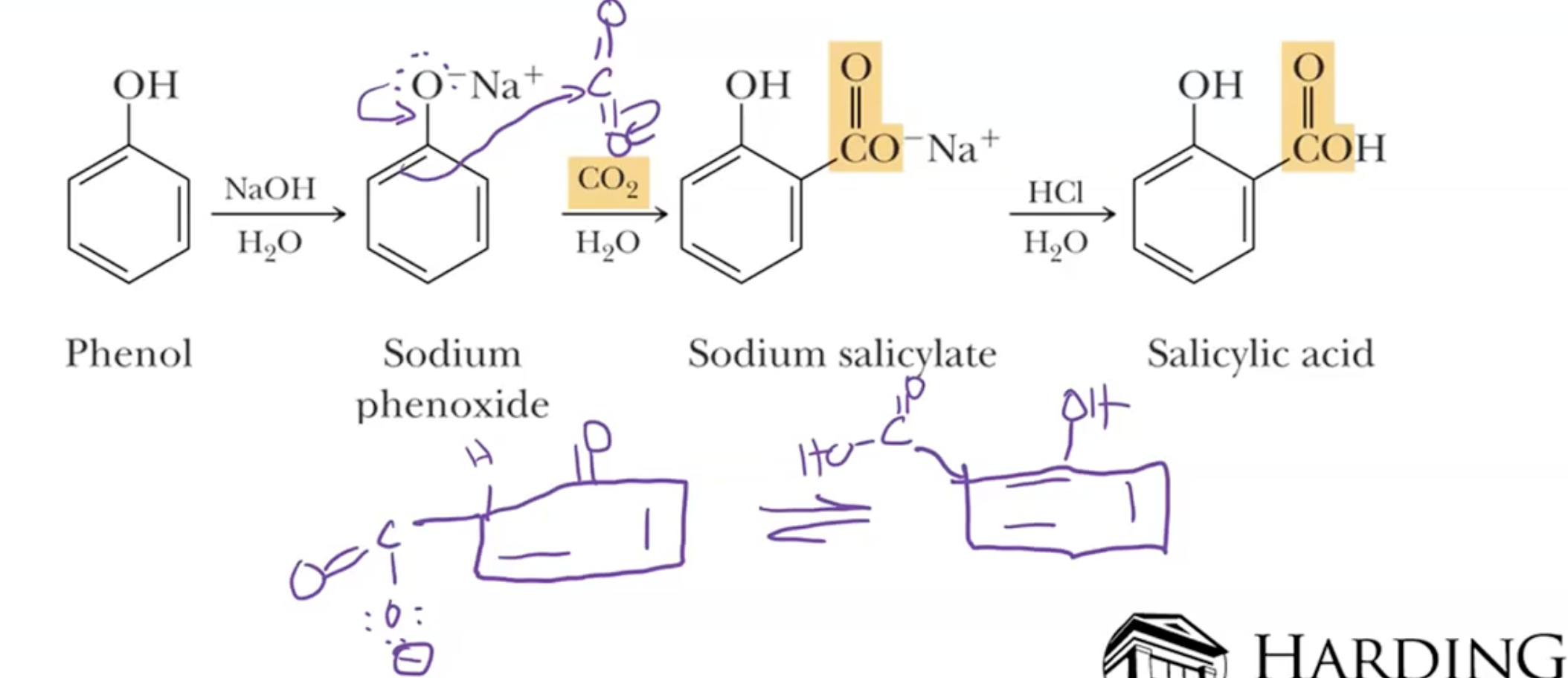
Kolby Carboxylation
Phenoxide Ion Formation and CO2 Reaction:
Phenol is converted to phenoxide ion using NaOH and H2O/ can also used solid sodium.
Phenoxide ion then reacts with CO2 gas and H2O under pressure to form carboxylic acids.
ORTHO SELECTIVE
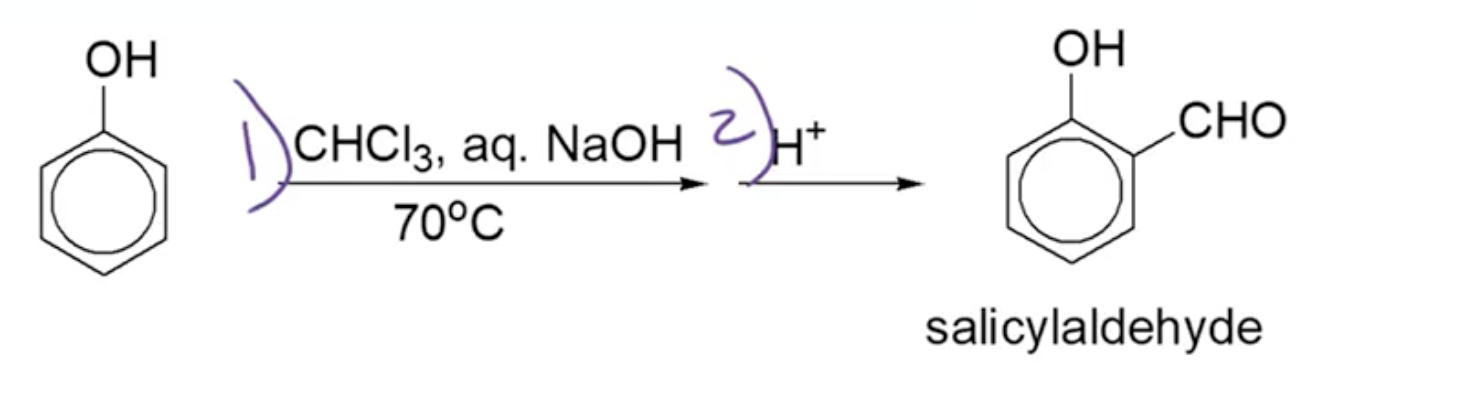
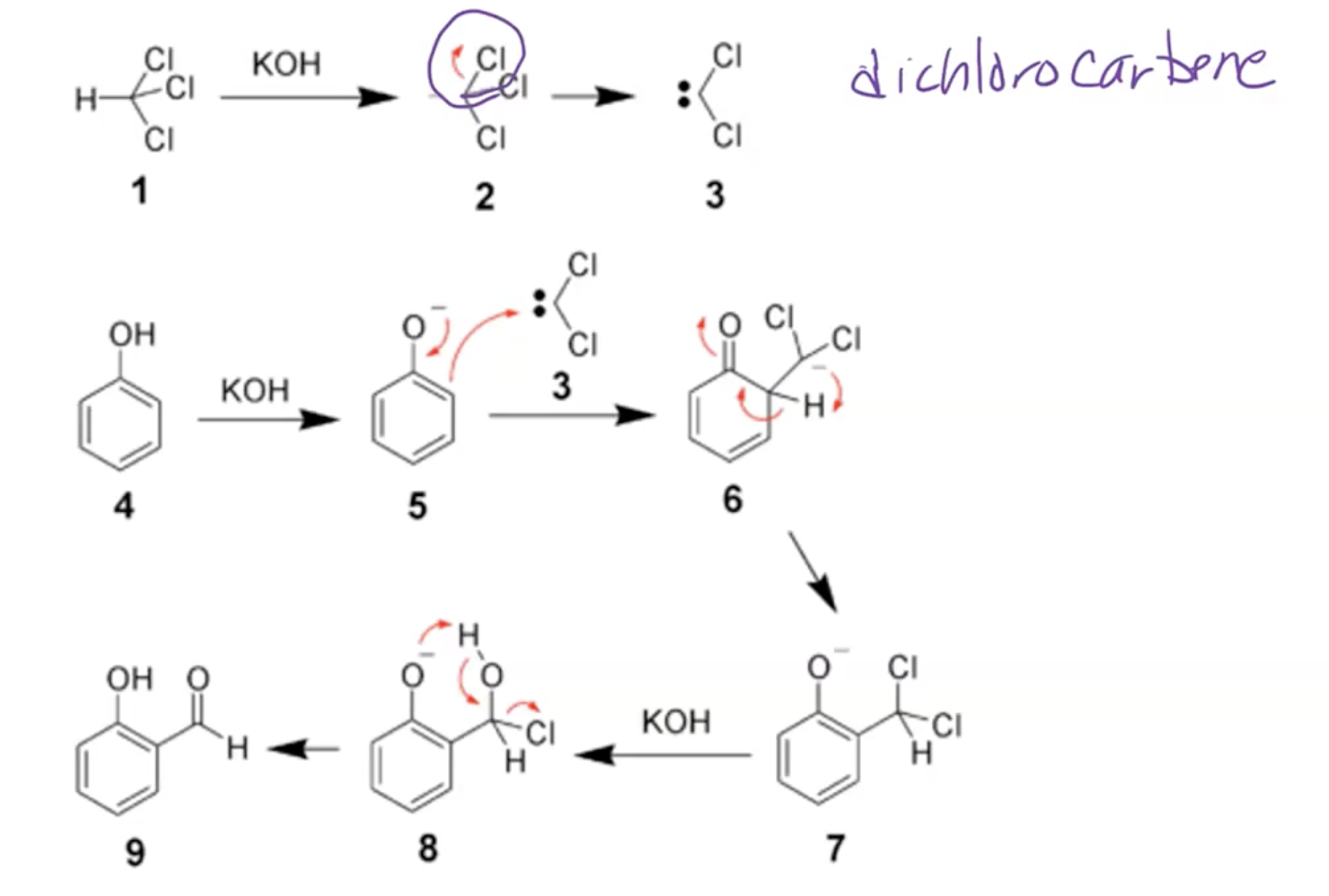
Reimer-Tiemann Reaction
Mechanism Overview:
Reagent: chloroform (CHCl3) and NaOH at 70 Celsius then acidify to form ortho-substituted phenols upon heating.
The EAS type mechanism yields carbonyl products that restore aromaticity.
Alpha elimination to form dichlorocarbene; dichlorocarbene acts as electrophile for EAS reaction, occurs at ortho position to give aldehyde
Oxidation and Reduction of Phenols
Oxidation Resistance:
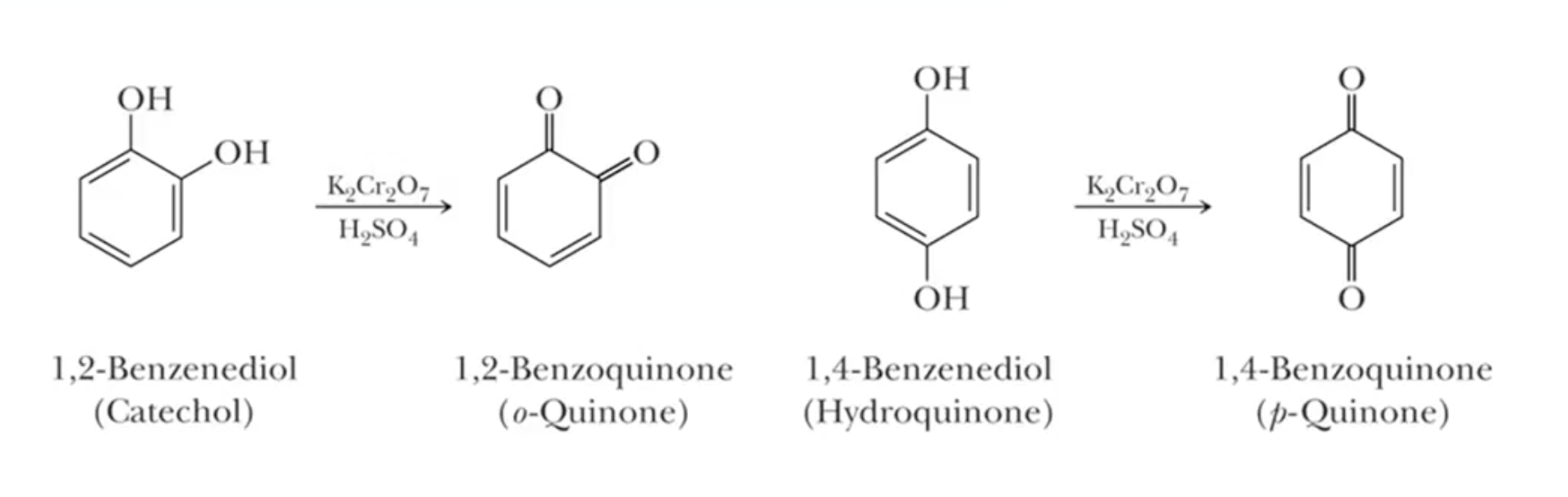
Phenols resist oxidation, but di-phenols can be oxidized to diketones under strong conditions.
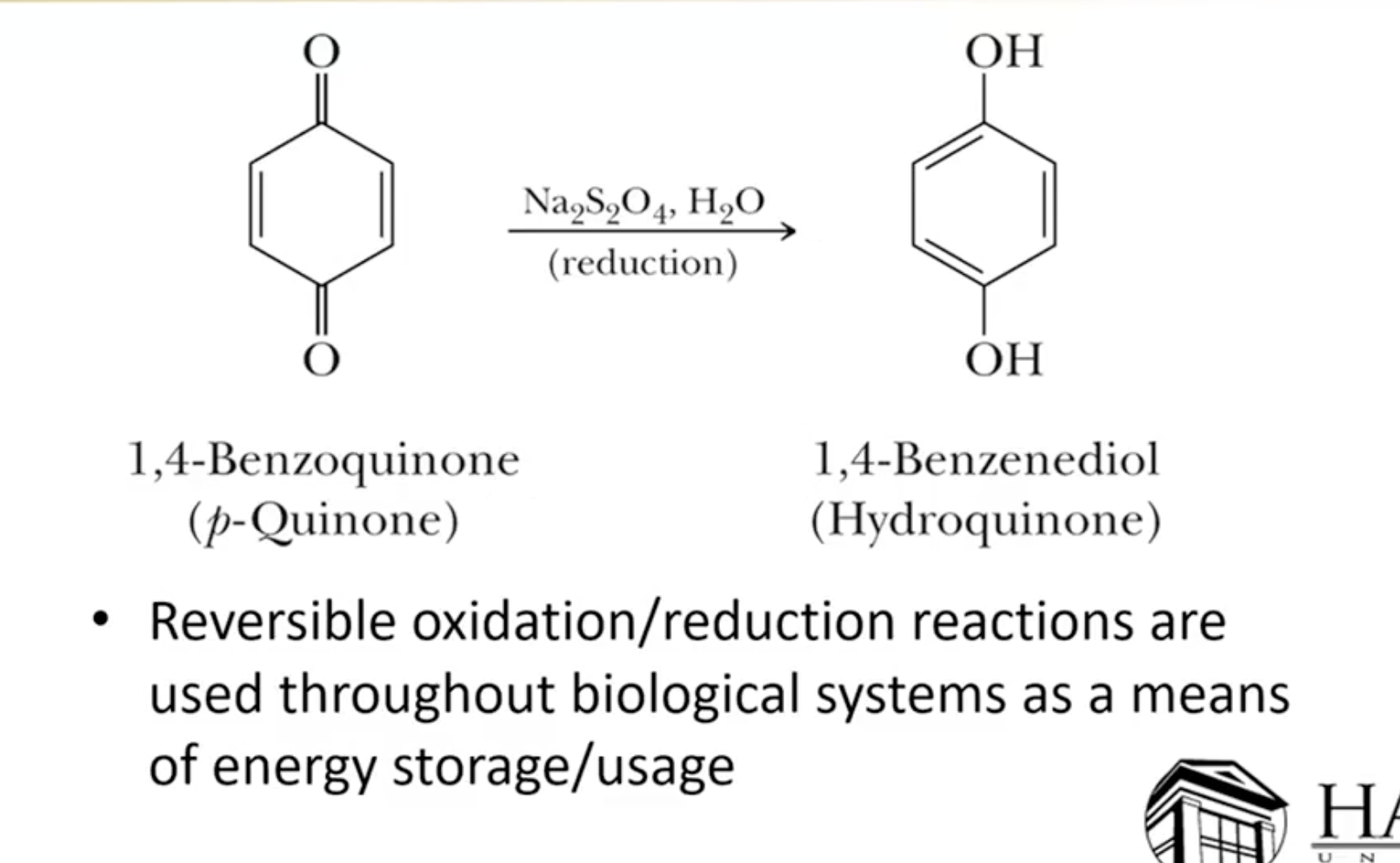
Mild Reduction Techniques:
Sodium thiosulfate (Na2S2O4) serves as a mild reducing agent for phenols, utilized in applications such as photography.
Spectroscopic Analysis of Phenols
Infrared (IR) Spectroscopy Peaks:
Key peaks around 3200 cm-1 indicate -OH stretch; distinguish from carboxylic acids (which show broader peaks).
Nuclear Magnetic Resonance (NMR) Peaks:
-OH protons show variable shifts due to hydrogen bonding; deuterium exchange can help confirm presence.
C-13 NMR indicates downfield shifts for carbons attached to oxygen due to deshielding effects.
 Knowt
Knowt