Human Pathology Chapter 2. Inflammation and Tissue Repair
Overview of Inflammation
Definition: A protective response of the body to tissue injury, chemical agents, physical agents, or pathogenic microorganisms.
Process: Initiated by various agents, leading to the release of mediators of inflammation.
Effects:
Capillary Dilatation: Leads to increased blood flow, resulting in: Heat, Redness, Tenderness.
Increased Capillary Permeability: Causes extravasation of fluid, leading to: Swelling, Pain.
Attraction of Leukocytes: Results in the migration of white cells to the site of injury.
Systemic Response: Manifests as: Fever, Leukocytosis
Inflammation can lead to various observable signs, as illustrated by medical imaging where signs of inflammation are evident.
Tissue Repair: Initiation of healing processes, including regeneration and fibrosis, which are crucial for restoring function and structural integrity.
Cells Involved in Inflammation
White Blood Cells (Leukocytes):
Polymorphonuclear neutrophil (PMN)
Basophil
Monocyte
Lymphocyte
Eosinophil
Mast cell
Other Relevant Cells/Components:
Platelets
Macrophage
Plasma cell
Phagocytosis
Definition: The process by which certain cells engulf solid particles, such as bacteria.
Steps of Phagocytosis of Bacteria:
A. Invagination of Cell Membrane: The bacterium comes into contact with the cell membrane, which then invaginates around it.
B. Phagocytic Vacuole Formation: The bacterium is enclosed within a phagocytic vacuole (phagosome) inside the cell.
C. Degranulation: Granules within the phagocyte (e.g., neutrophils) release enzymes and reactive oxygen species (like O2 and H2O_2) into the phagocytic vacuole to destroy the bacterium. The nucleus remains intact.
Key Receptors: FC (Fc receptor) and C_3 (Complement component 3 receptor) are involved, indicating the role of opsonization in enhancing phagocytosis.
Circulatory Changes Due to Inflammation
Initial Response: Transient constriction of arteriolar smooth muscles, immediately followed by prolonged vasodilatation.
Active Hyperemia: Increased blood flow to the affected area, leading to increased temperature and redness.
Edema Formation:
Increased hydrostatic pressure within capillaries leads to fluid accumulation in the extravascular compartment and interstitial tissues.
Edema: Accumulation of fluid within these tissues.
Red Blood Cell (RBC) Rouleaux Formation: RBCs stack together like coins (stacked dime formation) due to changes in blood viscosity and adherence.
Leukocyte Dynamics:
Margination: Neutrophils move from the central axial bloodstream towards the endothelial lining of blood vessels.
Adhesion: Leukocytes adhere to the endothelial cells via leukocyte adhesion molecules and platelets/fibrin.
Pavementing: Leukocytes (primarily neutrophils) flatten and spread along the endothelial surface.
Diapedesis/Emigration: Involves the reorganization of white blood cells (WBC) and the insertion of pseudopods into the gaps between endothelial cells, allowing them to migrate out of the vessel into the injured tissue.
Edema: Characteristics and Types
Mechanism: Increased permeability of vessel walls and increased hydrostatic pressure drive fluid out of circulation.
Types of Edema Fluid:
Transudate: Edema fluid with a low protein content (specific gravity < 1.020 – though transcript states < 1.0).
Exudate: Edema fluid with a high protein concentration (specific gravity > 1.020 – though transcript states > 1.0), frequently containing inflammatory cells. Often indicates a more severe inflammatory process.
a) Serous Exudate: Has a yellow, straw-like color and is characterized by the absence of a prominent cellular response.
b) Fibrinous Exudate: Contains large amounts of fibrin due to the activation of the coagulation system.
c) Purulent Exudate (Pus): Contains abundant cellular components, predominantly polymorphonuclear neutrophils (PMN). It is associated with pyogenic (pus-forming) bacterial infections.
Key points **
Fibrin= glue for coagulation and helps stabilize the clot.
Transudate fluid indicates inflammation is not aggressive, follows the laws of physics and gravity
Exudate fluid has a higher protein concentration and indicates a more severe injury has occurred
Purulent fluid is a type of exudate that contains pus, typically resulting from infection, and indicates a significant inflammatory response as the body attempts to fight off pathogens.
Mediators of Inflammation
Major Groups:
Plasma-Derived: Circulate in an inactive form and are activated (e.g., complement system).
Cell-Derived: Stored within leukocytes and platelets, released upon activation.
Biochemical Diversity: Include biogenic amines, peptides, and arachidonic acid derivatives.
Multifunctional: Act on a wide range of cells and tissues to propagate the inflammatory response.
Specific Mediators: Histamine & Bradykinin
Histamine:
A biogenic amine containing an NH_2 group.
Commonly released from platelets and mast cells.
Increases inflammatory responses, primarily by causing vasodilation and increased vascular permeability.
Benadryl is a common histamine blocker
Has a short action (termed "immediate transient reaction") because it is rapidly inactivated by histaminase.
Pharmacological Relevance: Benadryl (Diphenhydramine) is an antagonist of the histamine H_1 receptor, effectively blocking histamine activity and reducing allergic/inflammatory symptoms.
Bradykinin:
Has actions similar to histamine, particularly in increasing vascular permeability and vasodilation.
A significant mediator that induces pain, contributing to one of the cardinal signs of inflammation.
Normally occurs when an injection is given
Complement System
The complement system is a complex network of proteins that enhances the body's immune response by opsonizing pathogens, promoting inflammation, and recruiting immune cells to sites of injury. Additionally, it plays a crucial role in tissue repair by facilitating the clearance of debris and promoting regeneration, ultimately aiding in the resolution of inflammation.
Composition: A group of plasma proteins primarily produced by the liver, circulating in an inactive form.
Activation Pathways: Can be activated through a classical pathway (e.g., antigen-antibody complexes) or an alternative pathway (e.g., microbial surfaces).
Outcome of Activation: Leads to the formation of biologically active fragments, intermediate complexes, and ultimately, the terminal Membrane Attack Complex (MAC).
Activated Mechanisms:
Opsonization: Marking pathogens for phagocytosis, thereby enhancing the uptake of bacteria by phagocytes.
Anaphylaxis: Triggers histamine release, leading to increased vessel wall permeability and smooth muscle contraction.
Chemotaxis: Attracts and guides the migration of leukocytes (e.g., neutrophils and macrophages) to the site of injury.
Cell Lysis: The MAC complex directly forms pores in the membranes of target cells (e.g., bacteria), leading to their lysis and destruction.
Arachidonic Acid Derivatives
Origin: Arachidonic acid is a fatty acid derived from membrane phospholipids through the action of phospholipases (specifically Phospholipase A_2 and Phospholipase C).
Mechanism: Corticosteroids inhibit phospholipase activity, thereby blocking the initial step in arachidonic acid metabolism and reducing inflammatory mediator production.
Metabolic Pathways: Arachidonic acid is further metabolized through two main pathways:
1. Lipoxygenase Pathway:
2. Cyclooxygenase Pathway🇦
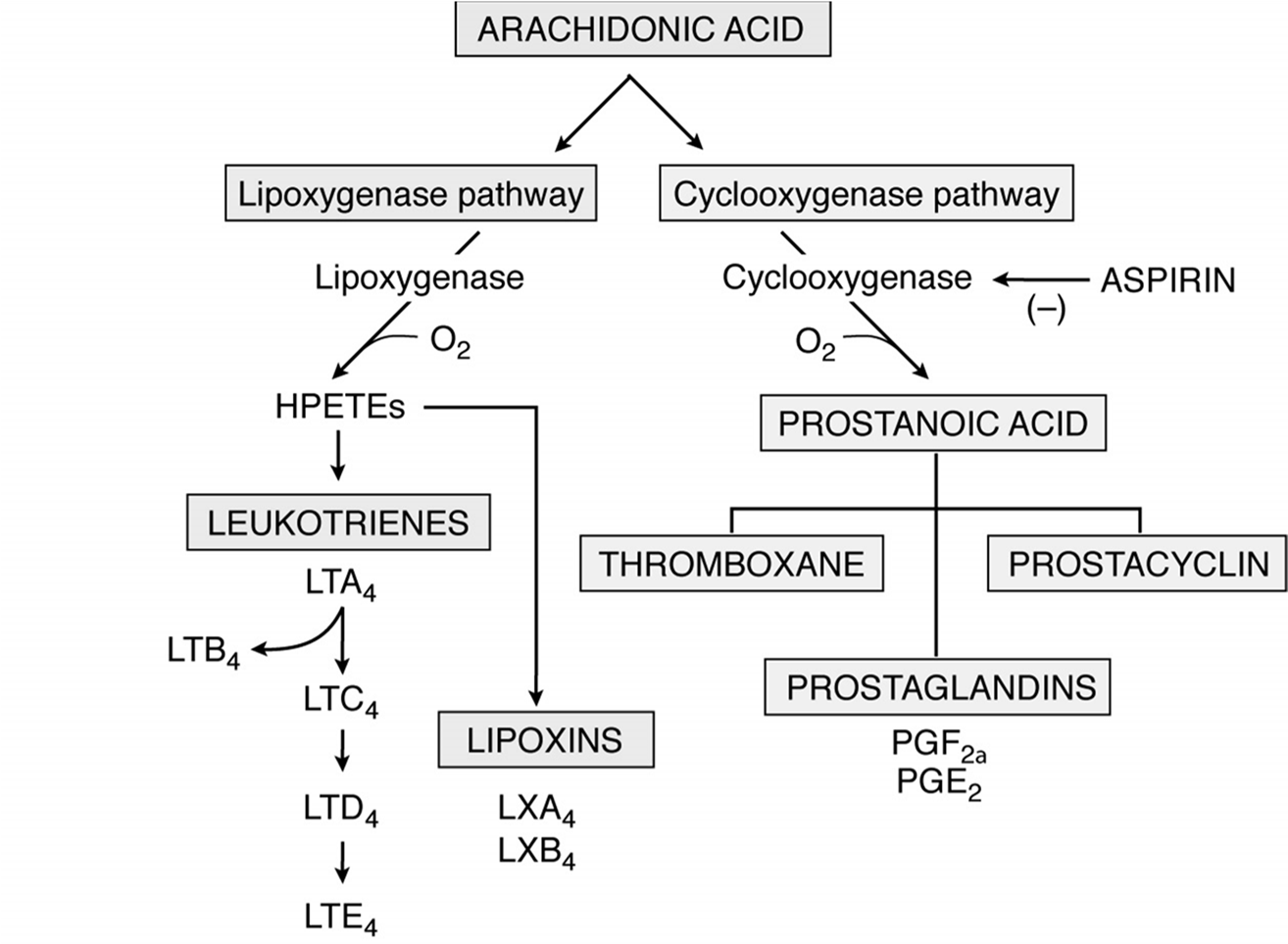
Other key points:
Prostaglandins in large sums gives pain. Decrease in prostaglandins= decrease in pain
During childbirth the uterine muscle contraction is dependent on prostaglandin (PGE2)
Anti-Inflammatory Drugs
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs):
Mechanism: Primarily inhibit the cyclooxygenase pathway, reducing the production of prostaglandins and thromboxane.
Examples: Aspirin, Diclofenac, Ibuprofen, Indomethacin, Ketoprofen, Ketorolac (parenteral), Naproxen, Oxaprozin, Piroxicam, Sulindac.
COX-2 Inhibitors (Selective NSAIDs):
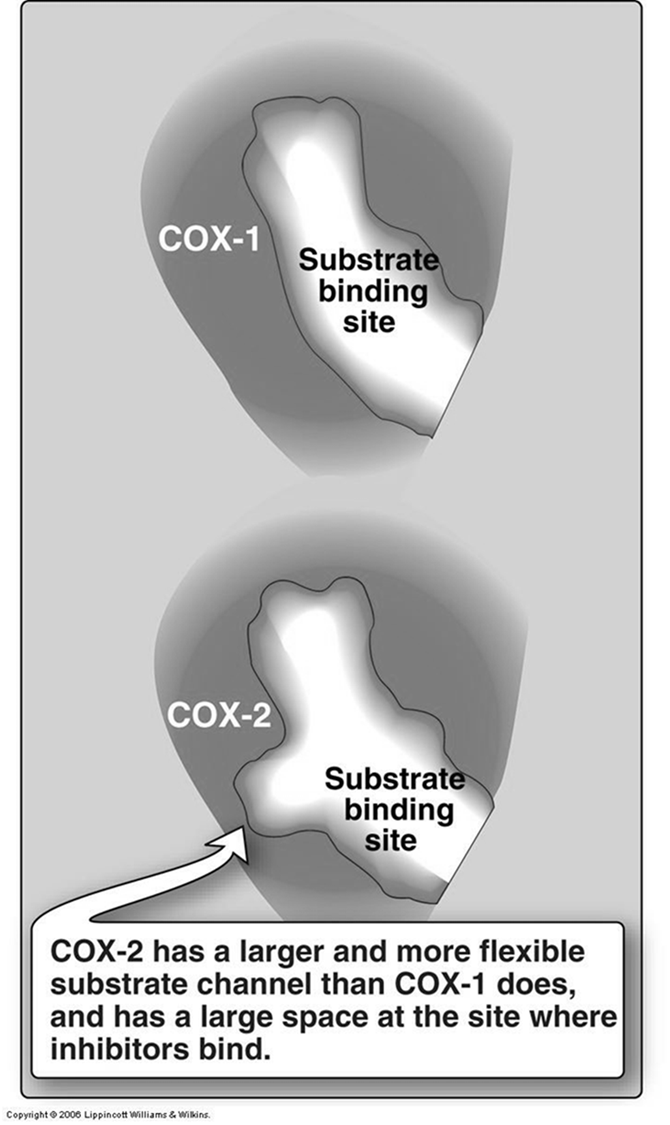
Mechanism: Selectively inhibit the COX-2 enzyme, which is primarily induced during inflammation, while sparing COX-1 (involved in protective functions like gastric mucosal maintenance).
Structural Difference: COX-2 has a larger and more flexible substrate channel and a larger space at the inhibitor binding site compared to COX-1. This structural difference allows for selective inhibition.
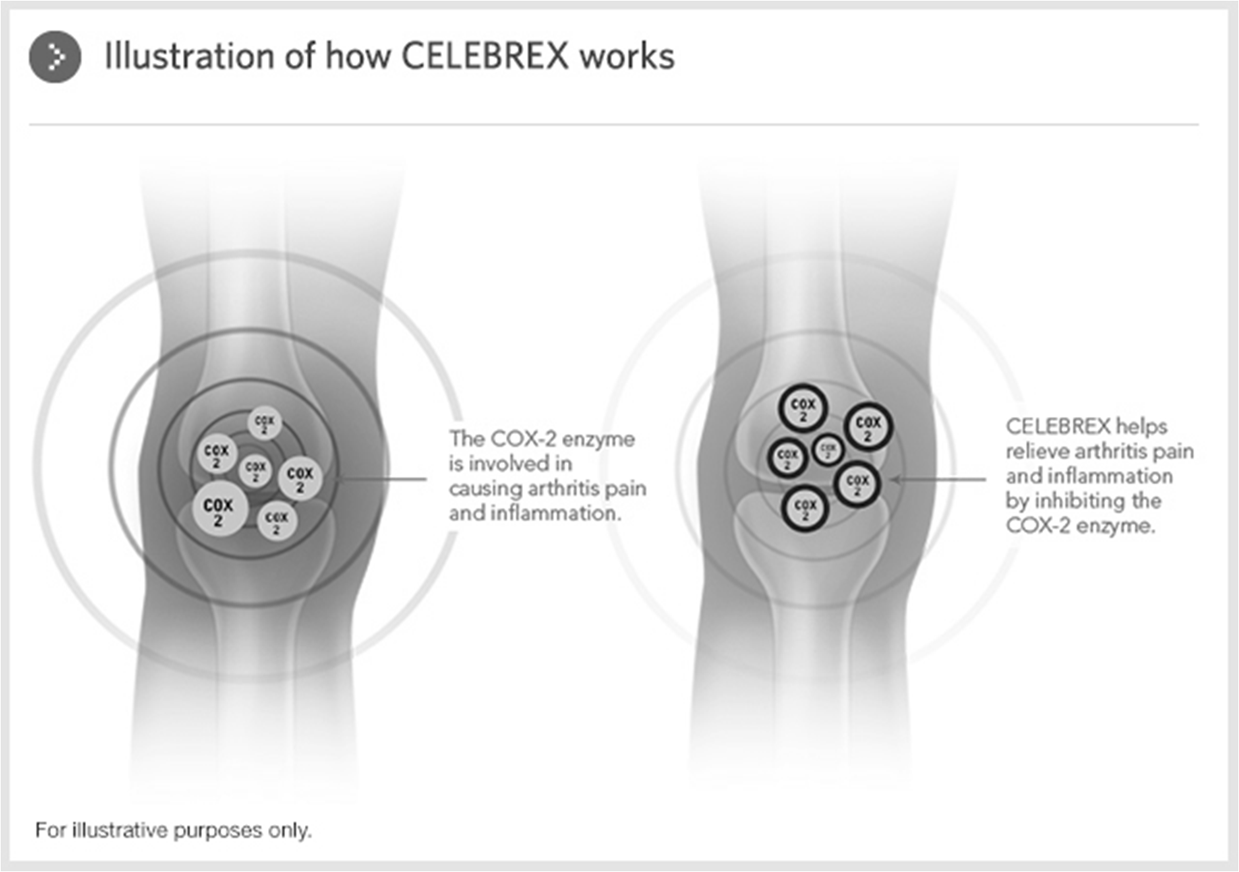
Examples: Celecoxib (Celebrex). Rofecoxib and Valdecoxib (Viaxx) were removed from the market due to cardiovascular concerns.
Celebrex (Celecoxib): Helps relieve arthritis pain and inflammation by inhibiting the COX-2 enzyme.
Nonopioid and Non-NSAID Analgesics:
Example: Acetaminophen (e.g., Tylenol). Its anti-inflammatory mechanism is debated, but it primarily acts as an analgesic and antipyretic.
Misoprostol (Cytotec)
Important Warning: Contraindicated in pregnancy. It can cause abortion, premature birth, or birth defects. Patients must avoid pregnancy while taking it and for at least one month or one completed menstrual cycle after stopping treatment. Female patients of childbearing age must meet specific requirements before use (negative pregnancy test, effective birth control, warnings received, initiation on days 2-3 of menstrual period).
Uses:
Abortion: Used in combination with mifepristone to terminate pregnancy.
Childbirth: Can be used vaginally at the time of delivery for cervical ripening, induction of labor, and treatment of severe postpartum bleeding, by causing uterine muscle contractions.
Classification of Inflammations
Acute Inflammation:
Characteristics: Increased blood flow, increased permeability of vessels allowing cells, proteins, fluids, and inflammatory cells (neutrophils or PMN) to exit into the tissue.
Occurrence: Usually a natural homeostatic process, a rapid and short-lived response to injury.
Chronic Inflammation:
Characteristics: Characterized by the prolonged presence of macrophages, lymphocytes, and plasma cells.
Associated Features: Involves associated tissue destruction, scarring, and granulation tissue formation.
Duration: Persists over a longer period of time.
Prognosis: Always considered a negative or detrimental process, indicating ongoing tissue damage and potential dysfunction.
Pathologic Forms of Inflammation
Serous Inflammation: Represents an early stage of most inflammations, characterized by the production of a transudate, usually with low cellular content.
Fibrinous Inflammation: Characterized by an exudate rich in coagulated fibrin, often seen on serosal surfaces like the pericardium or peritoneum.
Purulent Inflammation: An acute form of exudative inflammation where enzymes produced by white blood cells cause liquefaction of affected tissues, leading to the formation of pus.
Pus: An inflammatory exudate composed of polymorphonuclear leukocytes (PMN), necrotic tissues, microorganisms, and tissue fluids. It typically ranges from pale yellow to yellow-green, sometimes whitish, and occasionally bloody. Bacterial infection (
gram-positive or gram-negative) is its most common cause.Abscess: A localized collection of pus, often encapsulated by a fibrous wall, as seen in lung tissue (Figure 4-18) or the brain (Figure 2-13).
Ulcerative Inflammation: Occurs when necrosis on or near the surface of an organ or tissue leads to the loss of tissue and the creation of a local defect (an ulcer), such as a peptic ulcer (Figure 2-15).
Pseudomembranous Inflammation: An acute inflammatory response to a powerful necrotizing toxin (e.g., diphtheria toxin), characterized by the formation of a false membrane on a mucosal surface, composed of precipitated fibrin, necrotic epithelium, and inflammatory leukocytes.
Granulomatous Inflammation: A form, usually chronic, attended by the formation of granulomas.
Granuloma Components: Includes various immune cells such as giant cells, epithelioid macrophages, lymphocytes, and macrophages, often surrounding an area of necrosis (e.g., caseous necrosis).
Healing and Repair
Outcomes: Tissue can heal through regeneration (restoration of native tissue) or repair (formation of scar tissue).
Determinants of Outcome: The type of cells forming the tissue greatly influences the healing process:
Continuously Dividing Cells (Labile Cells): Cells that constantly proliferate throughout life (e.g., epidermal cells, hematopoietic cells). These tissues can regenerate extensively.
Quiescent Facultative Mitotic Cells (Stable Cells): Cells that are typically in G_0 (resting phase) but can re-enter the cell cycle and divide when stimulated (e.g., muscle cells, liver cells). These tissues have regenerative capacity.
Nondividing Postmitotic Cells (Permanent Cells): Cells that have exited the cell cycle permanently and cannot divide (e.g., brain neurons, heart muscle cells). Injury to these tissues primarily results in scarring.
Cell Cycle (Mitosis):
G_0: Resting phase.
G_1: Growth phase.
S: DNA replication phase.
G_2: Growth and final preparations for division.
M (Mitosis): Prophase, Prometaphase, Metaphase, Anaphase, Telophase.
Fibroblasts and Collagen Production
Fibroblasts: Key cells in connective tissue repair, responsible for synthesizing and secreting components of the extracellular matrix, most notably collagen.
Process: Inside the fibroblast (in the rough endoplasmic reticulum and Golgi apparatus), procollagen molecules are synthesized. These are then secreted and undergo extracellular processing, including cleavage of propeptides and hydroxylation, to form collagen fibrils, which then assemble into robust collagen fibers.
Wound Healing Process
General Stages:
A. Incision: Initial injury creates a defect in the epidermis, often covered by a scab.
B. Hours Post-Injury: Leukocytes migrate to the site to initiate inflammatory cleansing.
C. Days Post-Injury: Granulation tissue forms, composed of macrophages, fibroblasts, and new blood vessels.
D. Weeks Post-Injury: The wound contracts, and granulation tissue is replaced by a scar.
Detailed Steps of Wound Repair:
1. Inflammatory Process: Injury immediately triggers an inflammatory response to clear debris and pathogens.
2. Wound Contraction: Myofibroblasts (hybrid cells with characteristics of both smooth muscle cells and fibroblasts) begin to reduce the defect size and pull the tissue margins closer together.
3. Angiogenesis: Angioblasts start producing new blood vessels (neovascularization) to the damaged area. This is crucial for supplying oxygen and nutrients necessary for healing.
4. Extracellular Matrix (ECM) Production: Fibroblasts produce components for the ECM:
Fibronectin: glues the substances together) and collagen-III (immature) and it is replaced by collagen-I (mature) which leads to scar tissue and final stages of healing.
Complications of Wound Healing
Deficient scar formation
Separation of tissue margins
Excess scar formation may lead to Keloid (Collagen- III)
Large scars in burn patients
Keloids
Characterized by an overproduction of collagen that extends beyond the original boundaries of the wound. They are often raised, thick, and can cause discomfort or itching, presenting a challenge for both cosmetic and therapeutic management.
A genetic condition where collegen-3 is produced in excess and is not converted into collegen-1 resulting in excess scar tissue
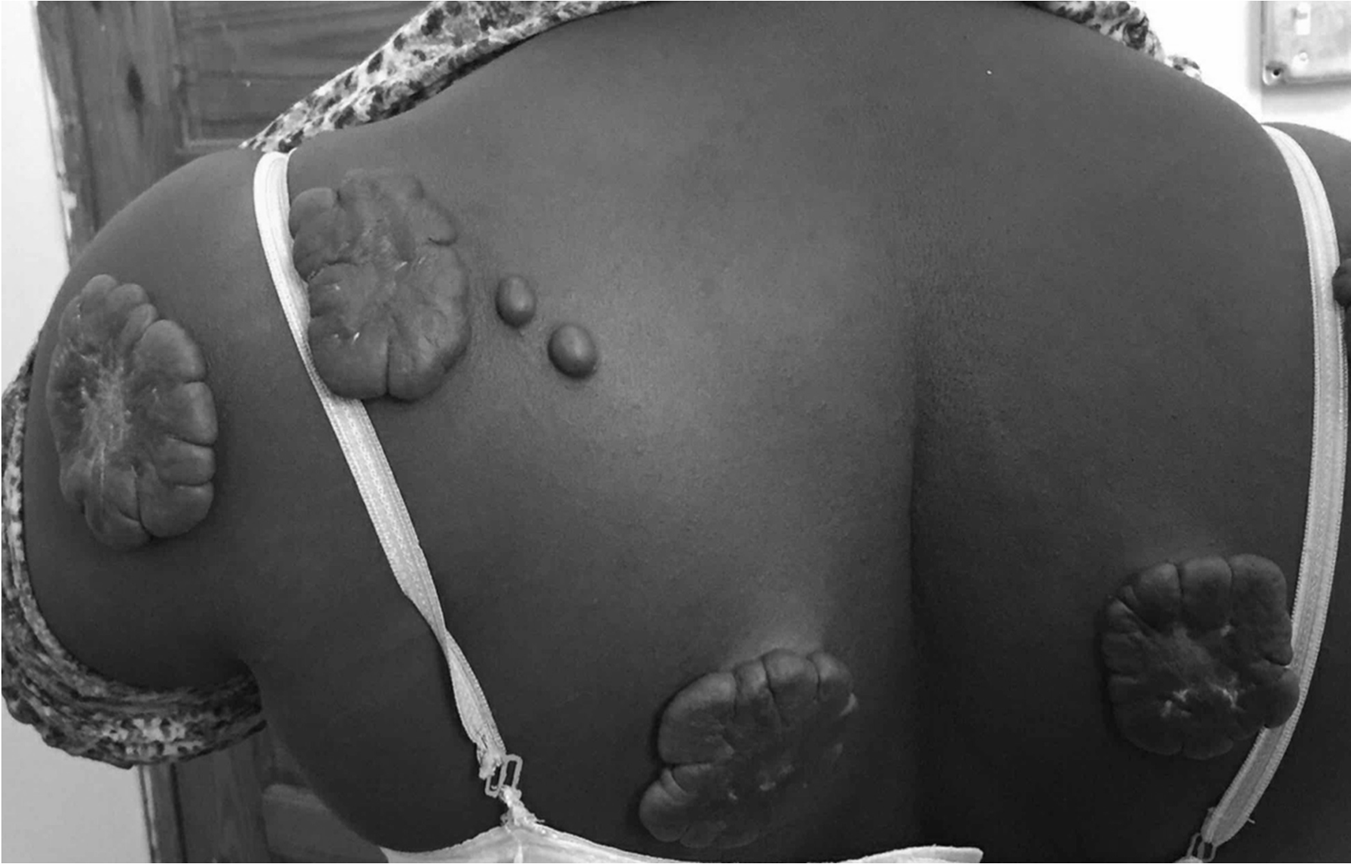
Case study: Ehlers-Danlos Syndrome
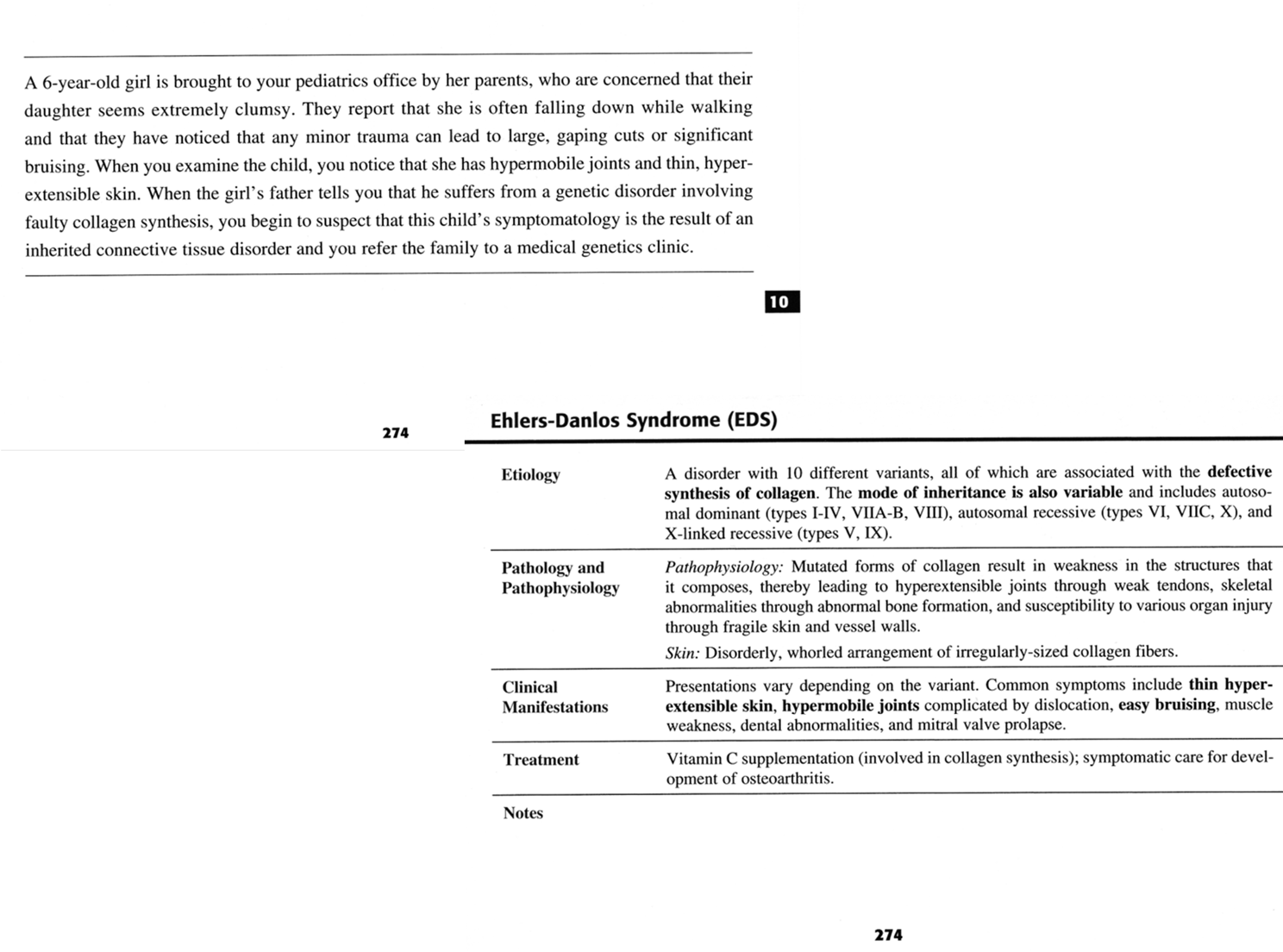
It is associated with hyper extensive joints, hyper elasticity of skin, dissecting aortic aneurysms, rupture of the colon, and vessel instability resulting in skin hemorrhages. It is caused by mutations which affects the collagen structure and synthesis. Transmission based on Mendelian genetics.
Types I and III collagen are most often affected.
Hyper extensive joints with hyper elasticity of the skin is a hallmark sign of Ehlers-Danlos syndrome
Genetically inherited from mom or dad
Infections
•An inflammatory process caused by disease- producing organisms
•“itis” is the suffix used with the name of the tissue or organ to indicate an infection or inflammatory process
•Examples: appendicitis, hepatitis, colitis
•Cellulitis: acute spreading infection at any site
•Abscess: infection associated with breakdown of tissues and formation of pus
•Septicemia or septic shock: overwhelming infection where pathogenic bacteria gain access to bloodstream. Body’s response to septic shock usually is fever
Infection Virulence
Virulence pathogenicity how badly it can create disease. covid was a highly virulence organism
Virulence of organism: ease with which a pathogenic organism can overcome the defenses of the body
Highly virulent organism: one that produces disease in the majority of individuals
Low virulence organism: one that produces disease only in highly susceptible individuals under favorable conditions