Moles of gas and solution
Key Vocabulary
Species – molecule, atom, ion, or compound in an equation
Mole – amount of substance, unit is mole (mol)
Relative formula mass (Mr) – sum of atomic masses in a chemical formula
Relative molecular mass (Mr) – formula mass used for molecules
Concentration – amount of solute in a solution, usually in mol/dm³ or M
Solute – substance dissolved in a solvent
Solvent – liquid in which a substance dissolves
Solution – mixture of solute and solvent
Decimetre cubed (dm³) – unit of volume, 1 dm³ = 1 litre = 1000 cm³
Molar volume of a gas – volume occupied by one mole of gas
Moles of Gases
Moles of a gas can be related to its volume, similar to solids and masses.
1 mole of gas occupies 24 dm³ (24,000 cm³) at room temperature and pressure (r.t.p, 25°C/298 K, 1 atm).
The gas identity does not affect this; 1 mole of any gas occupies the same volume.
Equation:
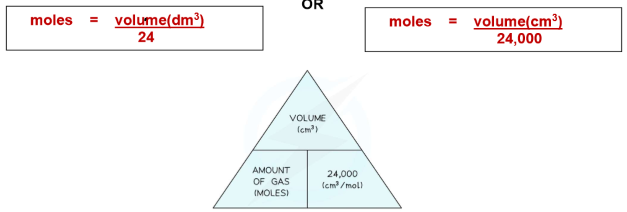
Moles = Volume (dm³) ÷ 24
Or, Moles = Volume (cm³) ÷ 24,000
Examples:
Moles in 300 cm³ of oxygen gas:
Moles = 300 ÷ 24,000 = 0.0125 mol
Volume of chlorine gas in 0.4 moles:
Volume = 0.4 × 24 dm³ = 9.6 dm³
Volume occupied by 0.10 g of hydrogen at r.t.p:
Moles of H₂ = mass ÷ Mr = 0.10 ÷ 2 = 0.05 mol
Volume = 0.05 × 24 dm³ = 1.2 dm³
Mass of 12.00 dm³ of oxygen at r.t.p:
Moles = 12 ÷ 24 = 0.5 mol
Mass = moles × Mr = 0.5 × 32 = 16 g
Reactions Involving Gases
Using the balanced equation, the volume of a gas can be calculated from the moles of another species.
Example:
2.00 g CaCO₃ reacts with excess HCl:
Equation: CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
Steps:
Calculate moles of CaCO₃:
moles = mass ÷ Mr = 2.00 ÷ 100 = 0.02 mol
Use stoichiometric ratio (CaCO₃:CO₂ = 1:1) to find moles of CO₂:
0.02 mol CO₂ expected
Calculate volume of CO₂:
volume = moles × molar gas volume = 0.02 × 24 = 0.48 dm³ = 4.80 dm³
Using a table:
Species | CaCO₃ | CO₂ |
|---|---|---|
Mole ratio | 1 | 1 |
Mass (g) | 2.00 | – |
Mr (g/mol) | 100 | 24 |
Moles | 0.02 | 0.02 |
Volume (dm³) | – | 0.48 |
Percentage Yield:
Actual volume collected = 4000 cm³ = 4.00 dm³
Theoretical = 4.80 dm³
% Yield = (actual ÷ theoretical) × 100 = (4.00 ÷ 4.80) × 100 = 83%
Gas Calculations Questions
Volume of O₂ to oxidise 0.4 g Mg
Equation: 2Mg + O₂ → 2MgO
Mr Mg = 24 g/mol
Moles Mg = 0.4 ÷ 24 = 0.0167 mol
Mole ratio Mg:O₂ = 2:1 → moles O₂ = 0.0167 ÷ 2 = 0.00835 mol
Volume O₂ = 0.00835 × 24,000 cm³ = 200.4 cm³
Mg | O₂ |
|---|---|
Mole ratio | 2 |
Mass/g | 0.4 |
Mr / g/mol | 24 |
Moles | 0.0167 |
Volume/cm³ | – |
Volume of CO₂ from 20 g CuCO₃
Equation: CuCO₃ → CuO + CO₂
Mr CuCO₃ = 123.5 g/mol
Moles CuCO₃ = 20 ÷ 123.5 = 0.162 mol
Mole ratio = 1:1 → moles CO₂ = 0.162 mol
Volume CO₂ = 0.162 × 24 dm³ = 3.89 dm³
CuCO₃ | CO₂ |
|---|---|
Mole ratio | 1 |
Mass/g | 20 |
Mr / g/mol | 123.5 |
Moles | 0.162 |
Volume/dm³ | – |
Volume of Cl₂ to form 27 g FeCl₃
Equation: 2Fe + 3Cl₂ → 2FeCl₃
Mr FeCl₃ = 162.5 g/mol
Moles FeCl₃ = 27 ÷ 162.5 = 0.166 mol
Mole ratio Cl₂:FeCl₃ = 3:2 → moles Cl₂ = 0.166 × (3/2) = 0.249 mol
Volume Cl₂ = 0.249 × 24,000 cm³ = 5,976 cm³ ≈ 5.98 dm³
Cl₂ | FeCl₃ |
|---|---|
Mole ratio | 3 |
Volume/cm³ | – |
Mr / g/mol | 71 |
Moles | 0.249 |
Volume of CO₂ from 25 cm³ C₂H₆
Equation: C₂H₆ + 3½O₂ → 2CO₂ + 3H₂O
Mr not needed for gas volume
Moles C₂H₆ = 25 ÷ 24,000 = 0.0010417 mol
Mole ratio C₂H₆:CO₂ = 1:2 → moles CO₂ = 0.0010417 × 2 = 0.002083 mol
Volume CO₂ = 0.002083 × 24,000 cm³ = 50 cm³
C₂H₆ | CO₂ |
|---|---|
Mole ratio | 1 |
Volume/cm³ | 25 |
Molar gas volume/cm³ | 24,000 |
Moles | 0.001042 |
Maximum volume of butane from 13.5 g C₄H₆ and 18 dm³ H₂
Equation: C₄H₆ + 2H₂ → C₄H₁₀
Mr C₄H₆ = 54 g/mol
Moles C₄H₆ = 13.5 ÷ 54 = 0.25 mol
Moles H₂ = 18 ÷ 24 = 0.75 mol
Mole ratio C₄H₆:H₂ = 1:2 → 0.25 × 2 = 0.5 mol H₂ needed → H₂ in excess, C₄H₆ is limiting
Moles C₄H₁₀ = 0.25 mol
Volume C₄H₁₀ = 0.25 × 24 dm³ = 6 dm³
C₄H₆ | H₂ |
|---|---|
Mole ratio | 1 |
Mass/g or volume | 13.5 g |
Mr / g/mol | 54 |
Moles | 0.25 |
C₄H₆ | C₄H₁₀ |
|---|---|
Mole ratio | 1 |
Moles | 0.25 |
Volume/dm³ | – |
Gas Volume Calculations Using Avogadro’s Law
Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules and moles.
Therefore, for gases, mole ratio = volume ratio.
Examples:
Volume of O₂ to oxidise 10 cm³ CH₄
Equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Mole ratio CH₄:O₂ = 1:2 → Volume ratio = 10 : 20 cm³
Answer: 20 cm³ O₂
Volume of CO₂ from 25 cm³ C₂H₆
Equation: C₂H₆ + 3½O₂ → 2CO₂ + 3H₂O
Mole ratio C₂H₆:CO₂ = 1:2 → Volume ratio = 25 : 50 cm³
Answer: 50 cm³ CO₂
Volume of O₂ for 40 cm³ CO
Equation: CO + ½O₂ → CO₂
Mole ratio CO:O₂ = 1:½ → Volume ratio = 40 : 20 cm³
Answer: 20 cm³ O₂
Hydrogenation of 100 cm³ C₄H₆
Equation: C₄H₆ + 2H₂ → C₄H₁₀
Mole ratio C₄H₆:H₂ = 1:2 → Volume H₂ = 100 × 2 = 200 cm³
Volume of C₄H₁₀ produced = 100 cm³ (mole ratio 1:1)
Chlorination of 20 cm³ C₂H₆
Equation: C₂H₆ + 6Cl₂ → 6HCl + C₂Cl₆
Mole ratio C₂H₆:Cl₂ = 1:6 → Volume Cl₂ = 20 × 6 = 120 cm³
Volume HCl produced = 20 × 6 = 120 cm³
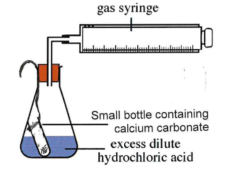
Predicting the Volume of CO₂ Produced
Equation: CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂
Method:
50 cm³ of 2 M HCl is measured into a conical flask (excess).
0.4 g CaCO₃ is weighed into a phial and placed in the flask.
A bung with delivery tube is fitted to a gas syringe.
Flask is tilted to mix reactants; volume of CO₂ collected is recorded.
Experiment is repeated and average volume of CO₂ calculated.
Calculations:
Mr CaCO₃ = 100 g/mol
Moles CaCO₃ = 0.4 ÷ 100 = 0.004 mol
Mole ratio CaCO₃:CO₂ = 1:1 → moles CO₂ = 0.004 mol
Volume of CO₂ at r.t.p = 0.004 × 24 dm³ = 0.096 dm³ = 96 cm³
Percentage yield:
If actual CO₂ collected = 85 cm³,
% Yield = (85 ÷ 96) × 100 ≈ 88.5%
Reason % yield < 100%:
Gas may escape before collection.
Some CaCO₃ may not fully react.
Measurement inaccuracies in gas syringe.
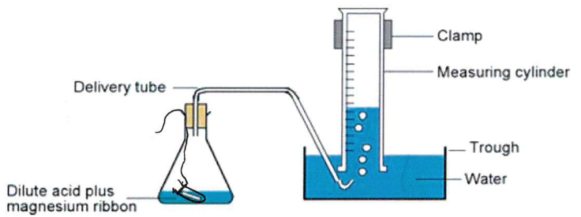
Calculation of the Volume of H₂ Produced
Equation: Mg + 2HCl → MgCl₂ + H₂
Method:
50 cm³ of 2 M HCl is added to a conical flask (excess).
0.04 g magnesium is placed in a phial in the flask.
A bung with delivery tube is fitted; delivery tube end is in water trough.
100 cm³ measuring cylinder is filled with water and inverted over the tube.
Flask is tilted to mix reactants; volume of H₂ collected by downward water displacement is recorded.
Calculations:
Mr Mg = 24 g/mol
Moles Mg = 0.04 ÷ 24 ≈ 0.00167 mol
Mole ratio Mg:H₂ = 1:1 → moles H₂ = 0.00167 mol
Volume of H₂ at r.t.p = 0.00167 × 24 dm³ ≈ 0.0401 dm³ = 40.1 cm³
Percentage yield:
If actual H₂ collected = 36 cm³,
% Yield = (36 ÷ 40.1) × 100 ≈ 89.8%
Reason % yield < 100%:
Gas may escape during mixing.
Magnesium may not fully react.
Measurement errors in collecting gas.
Concentrations of Solutions
Moles of solute can be related to solution volume and concentration.
Concentration is measured in mol/dm³ or M (1 dm³ = 1000 cm³).
A 1 M solution contains 1 mole of solute in 1 dm³ of solution.
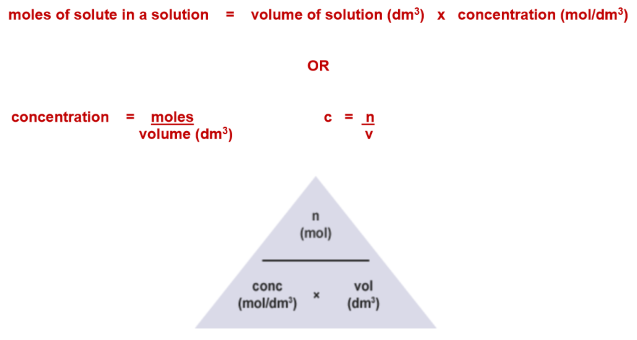
Equations:
Moles: n = c × V (V in dm³)
Concentration: c = n ÷ V
Examples:
Moles in 25 cm³ of 0.5 M NaOH:
Convert volume: 25 ÷ 1000 = 0.025 dm³
Moles = 0.025 × 0.5 = 0.0125 mol
Concentration of 50 cm³ containing 0.01 mol NH₃:
Volume in dm³: 50 ÷ 1000 = 0.05 dm³
Concentration: c = 0.01 ÷ 0.05 = 0.2 M
Concentration of 80 g NaOH in 800 cm³:
Mr NaOH = 40 + 16 + 1 = 57 g/mol
Moles NaOH = 80 ÷ 40 ≈ 2 mol (correct: 80 ÷ 40? Wait step by step)
Na = 23, O = 16, H = 1 → Mr = 23 + 16 + 1 = 40 g/mol
Moles = 80 ÷ 40 = 2 mol
Volume = 800 ÷ 1000 = 0.8 dm³
Concentration = 2 ÷ 0.8 = 2.5 M
Moles of Solute in Solution
1. Moles of solute:
Formula: n = c × V (V in dm³)
a. 50 cm³ of 2.00 M solution:
V = 50 ÷ 1000 = 0.050 dm³
n = 2 × 0.050 = 0.100 mol
b. 20 cm³ of 0.50 M solution:
V = 20 ÷ 1000 = 0.020 dm³
n = 0.50 × 0.020 = 0.010 mol
c. 20.0 cm³ of 0.10 M solution:
V = 20 ÷ 1000 = 0.020 dm³
n = 0.10 × 0.020 = 0.002 mol
2. Concentration of solution:
Formula: c = n ÷ V (V in dm³)
a. 0.05 mol in 200 cm³:
V = 200 ÷ 1000 = 0.200 dm³
c = 0.05 ÷ 0.200 = 0.25 M
b. 0.003 mol in 25 cm³:
V = 25 ÷ 1000 = 0.025 dm³
c = 0.003 ÷ 0.025 = 0.12 M
c. 0.125 mol in 500 cm³:
V = 500 ÷ 1000 = 0.500 dm³
c = 0.125 ÷ 0.500 = 0.25 M
3. Volume of solution:
Formula: V = n ÷ c (V in dm³, then convert to cm³)
a. 2.00 M solution containing 0.5 mol:
V = 0.5 ÷ 2 = 0.25 dm³ = 250 cm³
b. 0.500 M solution containing 0.0300 mol:
V = 0.0300 ÷ 0.500 = 0.06 dm³ = 60 cm³
4. Mass of solute:
Formula: mass = n × Mr
a. 500 cm³ of 1 M NaOH:
V = 500 ÷ 1000 = 0.5 dm³
n = 1 × 0.5 = 0.5 mol
mass = 0.5 × 40 = 20 g
b. 25.0 cm³ of 0.5 M NaOH:
V = 25 ÷ 1000 = 0.025 dm³
n = 0.5 × 0.025 = 0.0125 mol
mass = 0.0125 × 40 = 0.50 g
5. Concentration of solution by mass:
a. 26.5 g Na2CO3 in 250 cm³:
Mr Na2CO3 = 23×2 + 12 + 16×3 = 106 g/mol
moles = 26.5 ÷ 106 ≈ 0.25 mol
V = 250 ÷ 1000 = 0.25 dm³
c = 0.25 ÷ 0.25 = 1.0 M
b. 62.5 g CuSO4·5H2O in 1 L:
Mr CuSO4·5H2O = 63.5 + 32 + 16×4 + 5×18 = 63.5 + 32 + 64 + 90 = 249.5 g/mol
moles = 62.5 ÷ 249.5 ≈ 0.25 mol
V = 1 dm³
c = 0.25 ÷ 1 = 0.25 M
6. Mass of AgNO3 for 50.0 cm³ of 0.2 M solution:
V = 50 ÷ 1000 = 0.050 dm³
n = 0.2 × 0.050 = 0.010 mol
Mr AgNO3 = 108 + 14 + 16×3 = 108 + 14 + 48 = 170 g/mol
mass = 0.010 × 170 = 1.70 g
Titration Calculations
Titration involves reacting an acid with an alkali and measuring the exact volumes needed for neutralisation.
If the concentration of one solution is known, the other can be calculated.
Example:
25 cm³ of 0.200 M NaOH required 40 cm³ of HCl to neutralise. Find the concentration of HCl.
Steps:
Balanced equation (underline reactants used):
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)Mole ratio:
1 : 1Volumes (dm³) and concentrations:
NaOH: 25/1000 = 0.025 dm³, 0.200 M
HCl: 40/1000 = 0.040 dm³, unknown
Moles of known substance (NaOH):
n = V × c = 0.025 × 0.2 = 0.005 mol
Moles of HCl (from mole ratio 1:1):
n = 0.005 mol
Concentration of HCl:
c = n / V = 0.005 / 0.040 = 0.125 M
1. Calculate the concentration of potassium hydroxide solution used when 25cm³ was completely neutralised by 26.50cm³ of 0.10 moldm⁻³ hydrochloric acid?
KOH(aq) + HCl(aq) → KCl(aq) + H₂O(l)
Substance | Mole ratio | Volume / dm³ | Concentration / M | Moles (mol) |
|---|---|---|---|---|
KOH | 1 | 25/1000 = 0.025 | ? | 0.00265 |
HCl | 1 | 26.50/1000 = 0.0265 | 0.10 | 0.00265 |
Concentration of KOH: c = n / V = 0.00265 / 0.025 = 0.106 M
2. Calculate the concentration of sulfuric acid if 17.40cm³ was required to completely neutralise 25cm³ of a 0.50 moldm⁻³ potassium hydroxide solution.
2KOH(aq) + H₂SO₄(aq) → K₂SO₄(aq) + 2H₂O(l)
Substance | Mole ratio | Volume / dm³ | Concentration / M | Moles (mol) |
|---|---|---|---|---|
KOH | 2 | 25/1000 = 0.025 | 0.50 | 0.0125 |
H₂SO₄ | 1 | 17.40/1000 = 0.0174 | ? | 0.00625 |
Concentration of H₂SO₄: c = n / V = 0.00625 / 0.0174 ≈ 0.359 M
3. In a titration, 10cm³ of a 1.0 moldm⁻³ solution of sulphuric acid was neutralised by 36.75cm³ of a sodium hydroxide solution. Calculate the concentration of the sodium hydroxide solution.
2NaOH(aq) + H₂SO₄(aq) → Na₂SO₄(aq) + 2H₂O(l)
Substance | Mole ratio | Volume / dm³ | Concentration / M | Moles (mol) |
|---|---|---|---|---|
NaOH | 2 | 36.75/1000 = 0.03675 | ? | 0.020 |
H₂SO₄ | 1 | 10/1000 = 0.010 | 1.0 | 0.010 |
Concentration of NaOH: c = n / V = 0.020 / 0.03675 ≈ 0.544 M
4. Calculate the concentration of hydrochloric acid when 16.85cm³ was required to completely neutralise 25cm³ of a 1.00 moldm⁻³ solution of sodium hydroxide.
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
Substance | Mole ratio | Volume / dm³ | Concentration / M | Moles (mol) |
|---|---|---|---|---|
NaOH | 1 | 25/1000 = 0.025 | 1.00 | 0.025 |
HCl | 1 | 16.85/1000 = 0.01685 | ? | 0.025 |
Concentration of HCl: c = n / V = 0.025 / 0.01685 ≈ 1.48 M