Tricarboxylic acid cycle KREBS
1. Introduction to the Krebs Cycle
Definition: The final oxidative pathway where carbohydrates, fats, and proteins converge, converting carbon skeletons into CO₂ and generating energy.
Key Features:
Occurs in the mitochondria.
Produces NADH and FADH₂ for oxidative phosphorylation.
An aerobic process, as it requires oxygen indirectly for ETC.
Overall Reaction:
Acetyl-CoA + 3 NAD⁺ + FAD + GDP + Pi → 2 CO₂ + 3 NADH + FADH₂ + GTP + CoA
2. Entry Point: Oxidative Decarboxylation of Pyruvate to Acetyl CoA
Enzyme: Pyruvate dehydrogenase complex (PDH).
(aggregate of 3 enzymes)
Pyruvate dehydrogenase (PDH or E1, also called a decarboxylase)
Dihydrolipoyl transacetylase (E2)
Dihydrolipoyl dehydrogenase (E3)
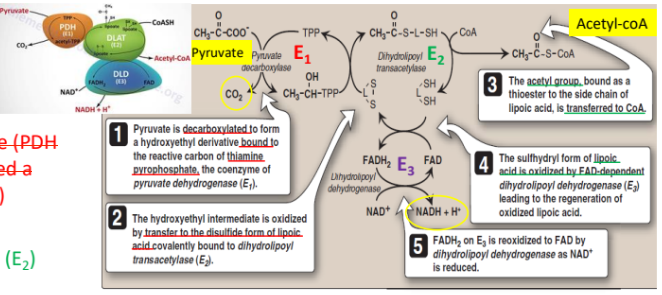
Key Reaction: Pyruvate (3C) → Acetyl-CoA (2C) + NADH + CO₂
Coenzymes ( acts as carriers or oxidants) : Thiamine pyrophosphate (TPP) for E1 , lipoic acid, CoA for E2 , NAD⁺, FAD for E3.
Deficiencies of thiamine (vitamin B1) can cause serious CNS problems → brain cells are unable to produce sufficient ATP (via TCA cycle) if PDH complex is inactive.
Regulation of PDH Complex :
alternately activate & inactivate E1 of PDH complex
Pyruvate dehydrogenase (PDH) kinase
Phosphorylates → Inhibits PDH complex
Pyruvate dehydrogenase (PDH) phosphatase:
Dephosphorylates → Activates PDH complex
The kinase itself can be activated by high-energy signals e.g. ATP, acetyl CoA & NADH → PDH complex is turned off.
The complex is also subject to product (NADH, acetyl CoA) inhibition.
Pyruvate - potent inhibitor of PDH kinase. If [pyruvate] elevated → E1 will be maximally active.
Calcium - strong activator of PDH phosphatase, stimulating E1 activity. Important in skeletal muscle where release of Ca2+ during muscle contraction stimulates PDH complex to drive energy production.
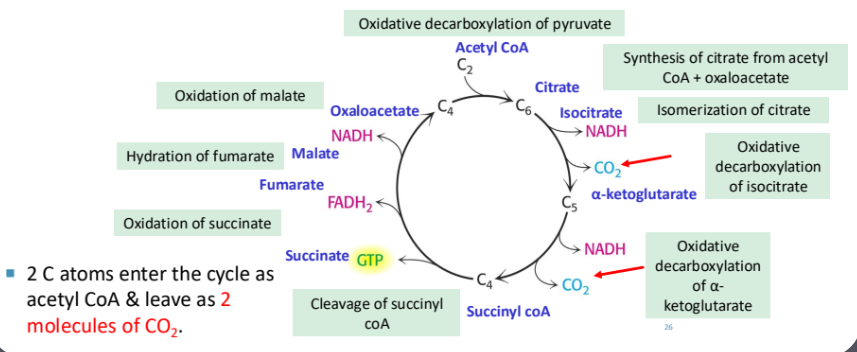
3. Steps of the Krebs Cycle
Synthesis of Citrate
Reaction: Acetyl-CoA (2C) + Oxaloacetate (4C) → Citrate (6C)
Enzyme: Citrate synthase
Isomerization of Citrate
Reaction: Citrate → Isocitrate (6C)
Enzyme: Aconitase
Inhibition: Fluoroacetate (toxic).
Oxidative Decarboxylation of Isocitrate
Reaction: Isocitrate (6C) → α-Ketoglutarate (5C) + NADH + CO₂
Enzyme: Isocitrate dehydrogenase
Regulation: Activated by ADP, Ca²⁺; inhibited by ATP, NADH.
Oxidative Decarboxylation of α-Ketoglutarate
Reaction: α-Ketoglutarate (5C) → Succinyl-CoA (4C) + NADH + CO₂
Enzyme: α-Ketoglutarate dehydrogenase complex
Coenzymes: Same as PDH.
Cleavage of Succinyl-CoA
Reaction: Succinyl-CoA → Succinate + GTP
Enzyme: Succinyl-CoA synthetase
Note: GTP is equivalent to ATP.
Oxidation of Succinate
Reaction: Succinate → Fumarate + FADH₂
Enzyme: Succinate dehydrogenase (also part of ETC).
Hydration of Fumarate (reversible)
Reaction: Fumarate → Malate
Enzyme: Fumarase
Oxidation of Malate
Reaction: Malate → Oxaloacetate + NADH
Enzyme: Malate dehydrogenase
4. Energy Yield
Per Turn of the Cycle:
3 NADH → 9 ATP
1 FADH₂ → 2 ATP
1 GTP → 1 ATP
Total: 12 ATP per cycleEach molecule glucose produce 2 pyruvate.
Total: 24 ATP + 6 ATP from each oxidative decarboxylation of pyruvate into acetyl CoA as 1 NADH is produced.
5. Regulation of the TCA Cycle
Key Enzymes:
Citrate synthase.
Isocitrate dehydrogenase (rate-limiting).
α-Ketoglutarate dehydrogenase.
Activators: ADP, Ca²⁺ (low energy demand).
Inhibitors: ATP, NADH (high energy availability).