SCI Q3
ATOMS
they are indivisible particles which cannot be created nor destroyed in chemical reactions
All matter is composed of atoms
Different chemicals have different masses and sizes. Similar chemicals have similar characteristics
In chemical reactions, atoms can only be rearranged
MODELS OF THE ATOM
Democritus
introduced the concept of atoms
Dalton’s model
also known as the “billiard ball” model
John Dalton proposed:
1) Atoms are tiny sphere-shaped particles that can neither be created nor destroyed
2) Atoms of an element are identical in mass and other properties3) Atoms can combine or rearrange during a chemical reaction

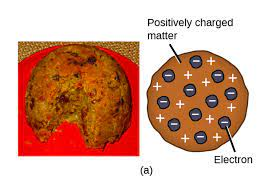
Thomson’s Model
also known as the “plum pudding” model
In 1897, he studied the passage of an electric current through a gas chamber. He discovers the electron
He discovers the negatively charged electron.
Rutherford’s Model
also known as the “nuclear” or “atomic” model
Gold Foil Experiment
Result 1:
Most particles passed undeflected. The atom is mostly empty spaceResult 2:
Some particles were deflected at an angle. The particles traveled near a positive entity.Result 3:
Few particles bounced back. The particles hit a positive entity head-on
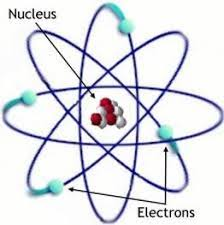
Nucleus: middle, protons are concentrated near the nucleus. Around the nucleus are the electrons.
Protons are positively charged.
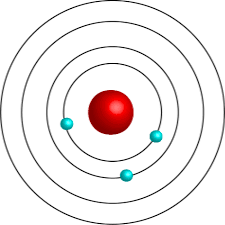
Bohr’s Model
also known as the “planetary” or model
The Danish scientist proposed that the electrons occupy fixed orbits around the nucleus. Each orbit corresponds to a specific amount of energy which increases as the orbit gets farther from the nucleus.
Electron excitation-Moves from a low-energy orbit to a high-energy orbit. It absorbs energy
Electron de-excitation-Moves from a high-energy to a low-energy orbit. It releases energy
Quantum Mechanical Model
also known as the “electron field” or “orbital” model
They follow these principles:
Duality of Matter
Louis De Broglie
All forms of matter have dual nature. A particle and a wave.
Uncertainty Principle
Werner Heisenberg
If electrons have wave properties, their momentum and exact position cannot be determined simultaneously
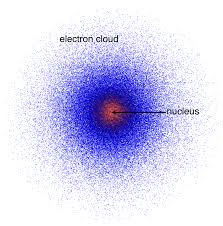
These were used by Austrian physicist Erwin Schrodinger
Quantum Numbers
A set of numbers that were derived from the mathematical Solutions of Schrodinger’s equation.
Principal Quantum Number
Symbol: n
designates the main energy level. It signifies the distance of electrons from the nucleus
Values: 1, 2, 3, 4…
Angular Momentum
Symbol: l
describes the orbital shape. Values 1, 2, 3 correspond to s, p, d, f types of orbital
Values: 1, 2 , 3(n-1)
Magnetic
Symbol: ml
describes orbital orientation in space.
-1 to +1
Spin
Symbol: msElectron spin direction can either be clockwise or counter-clockwise
½ or - ½
Each orbital can only hold 2 electrons
s: 1 (2 electrons)
p: 3 (6 electrons)
d: 5 (10 electrons)
f: 7 (14 electrons)
CHEMICAL BONDING
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures.
Valence Electrons
American Chemist Gilbert Newton Lewis
used to represent the electrons in the outermost energy level/shell of an atom. These electrons are called valence electrons
LEDS or Lewis Electron dot structure
represents the valence electrons as dots around the chemical symbol
Gilbert Newton Lewis
How to determine the LED of an Element?
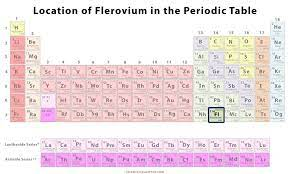
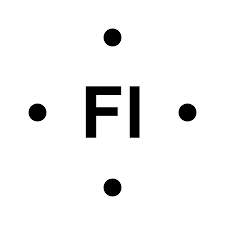
Flerovium is located in group 4A. Therefore, it has 4 electrons. It is represented as:
Group | Dots
1A | 1
2A | 2
3A | 3
4A |4
5A | 5
6A | 6
7A | 7
8A | 8
How to determine the valence configuration of an element?
Example:
Flerovium’s electron is 114
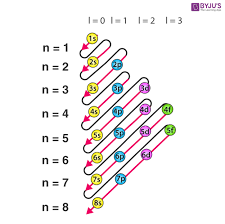
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 5f14 6s2 6p6 6d10 7s2 7p2
2+2+6+2+6+10+2+6+10+14+2+6+10+14+2+6+10+2+2 = 114
To find the valence configuration, you must find the highest number. The highest numbers there are 7s2 7p2
The electrons are 2 and 2 so you must add them. Therefore, the valence configuration is 4.
It has 4 valence electrons and 4 dots in the LEDS.
Chemical Bonds
Atoms share, lose, and gain valence electrons to achieve a noble gas electron configuration with eight electrons in the outer shell. This is called an octet.
Ionic Bonding
a metal and nonmetal atom form an ionic bond. The atoms become electrically charged particles
The metal loses electrons and becomes a positive ion called a cation
the nonmetal accepts the electrons and becomes a negative ion and becomes an anion. A pair of cation and anion is called a formula unit.
Covalent Bonding
2 nonmetal atoms can share valence electrons and form a covalent bond. Covalently bonded atoms are called molecules
Covalent bonds can be single, double, or triple.
One pair of electrons forms a single bond, two pairs form a double, and three pairs form a triple bond.
Polar Covalent Bonds
2 different nonmetal atoms form a polar bond
This creates an uneven electron distribution in the bond.
Electronegativity 0.5-1.7
Nonpolar Covalent Bonds
2 identical nonmetal atoms form a nonpolar bond
The atoms equally share the electrons
Electron density is the probable volume of space occupied by electrons
the charged ends make up a dipole.
Electronegativity 0-0.4
The polarity of Molecules
defines the polarity(or nonpolarity) of the molecule.
Electronegativity
measure of the ability of an atom in a chemical bond to attract electrons to itself. The more electronegative an atom is, the greater its ability to attract electrons in a bond.
EN = electronegativity
(You can see the electronegativity in the periodic table)
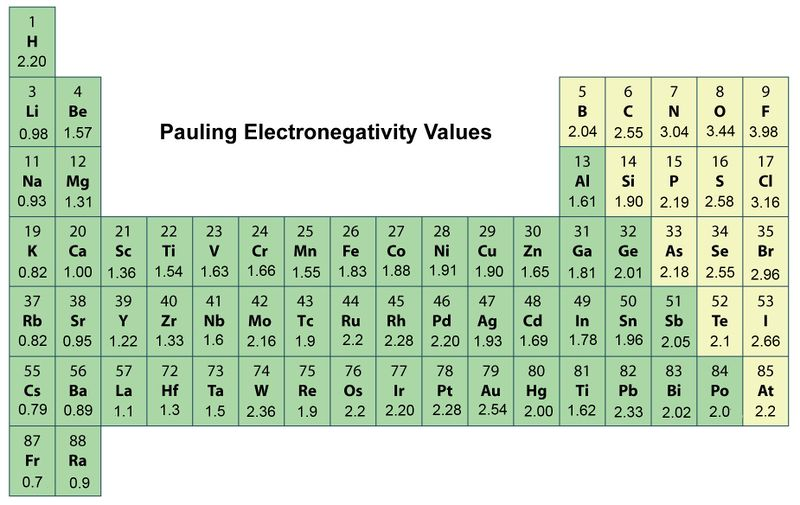
(round up ex: 0.79 - 8)
Example of Ionic
Na-Cl
3-0.9=2.1
Therefore EN>1.8
K-I
2.5-0.8=1.7
Therefore 0.4<EN<1.8
Example of Polar
C-O
3.5-2.5=1
Therefore 0.4<EN<1.8
Example of Nonpolar
H-H
2.1-2.1=0
Therefore EN<0.4
Ionic compounds
compounds formed during ionic bonding
Covalent compounds
compounds formed during ionic bonding
Metallic Bonding
-it only happens between metals. It occurs when metal atoms share their pooled valence electrons.
Properties of Metallic Bonds
Lusterous - shiny
Heat conductive
Ductile - can be stretched, broken, and reformed
Malleable - compressed into thin sheets
Strong
Electrical Conductivity
London Dispersion Force
a temporary attractive force occurs when 2 adjacent atoms form temporary dipoles as a result of the positions occupied by their electrons
Dipole-Dipole Interaction
attractive forces between the positive end of one polar molecule and the negative end of another polar molecule
Hydrogen Bonding
strong dipole-dipole interaction between a hydrogen atom bonded to a high electronegative atom
ORGANIC COMPOUNDS
Compounds
2 or more elements combined
Carbon
all organic compounds contain carbon but not every compound contains carbon
has the ability to join with several chemicals at the same time.
Examples of Organic Compounds
carbon
hydrogen
nitrogen
oxygen
phosphorous
sulfur
Hydrocarbons
compounds containing carbon and hydrogen only
Aliphatic
present in food
Dark choco- phenethylamine
Milk choco- butyric acid
White choco-palmitic acid
Aromatic
smell(ex. TNT or Trinitoluene)
used in plastic and petrochemical industries
present in chlorophyll
Root Words for Hydrocarbon Nomenclature
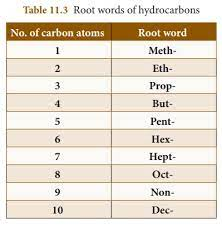
Nomenclature
chemical nomenclature and the names that we use for chemicals
Examples:
Systematic Common
H20 Water
Single Bond -
Double Bond =
Triple Bond 3-
C5H2(5)+2 - equation
C5H12 - molecular Formula
Classification of Hydrocarbon
Saturated Hydrocarbons
Unsaturated Hydrocarbons
Aromatic Hydrocarbon
Structural Formula:
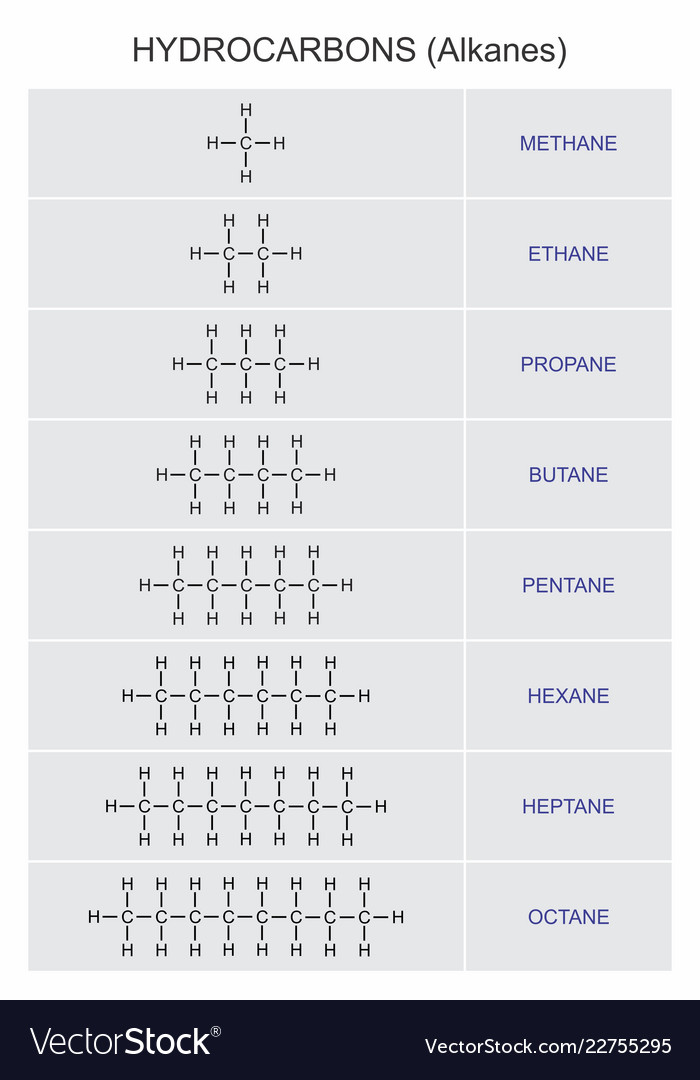
These are Saturated Hydrocarbons
Saturated Hydrocarbon
can contain only carbon-carbon single bonds
they are saturated because they have the maximum number of bonded hydrogen
Unsaturated Hydrocarbon
contains carbon-carbon double/triple bond
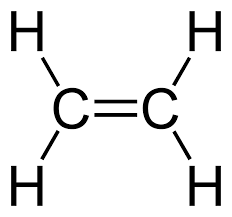
Alkenes
-contain at least one carbon-carbon double bond
Ex. Ethene
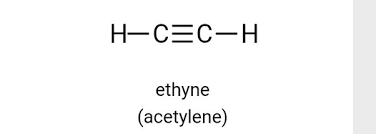
Alkynes
contain at least one carbon-carbon triple bond
Ex. Ethylene
Aromatic Hydrocarbon
contains at least one special type of hexagonal ring of carbon atoms with three double bond in the alternate positions.
Ex.
Toluene
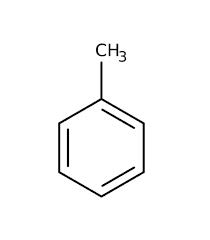
the aromatic compounds may also contain more than one benzene rings.
Ex. Naphthalene
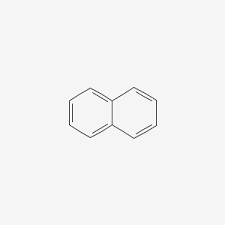
Hydrocarbon Types
1) Saturated(Alkanes)
2) Unsaturated(Alkynes, Alkenes)
3) Aromatic
Characteristics
1) Single Bond
2) double and triple bond
3) Benzene ring
Example
1)CH3CH2CH3 - Propane
2)CH3-C-CH - Propyne
3) Methyl Benzene
Aufbao Principle
electrons fill lower-energy atomic orbitals before filling higher-energy ones.
was initially proposed by Niels Bohr
Alkanes
saturated hydrocarbon containing only a carbon-carbon single bond in their molecule. They are also called Paraffins
Undergoes reactions at high temperatures and pressure
They may be divided as:
Open Chain or acylic alkanes (CnH2n+2)
Cycloalkanes or cyclic alkanes(C2H2n)
Example of Open Chain:
C1h4 (Molecular formula)
Expanded structural formula:
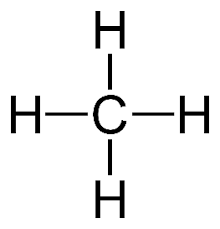 Name: Methane
Name: Methane
Nomenclature of Alkanes
implies assigning a proper name
Longest chain rule
Select the longest continuous chain or parent chain of carbon atoms
Position of the substituent
The substituent is the branch of carbon atoms
For substituents, you change the bond(ex. ane, ene, etc.) into yl.
Ex.
Butane (remove ane)
Butyl (replace with yl)
carbon atoms of the parent chain are numbered to identify the parent alkane
Ex.
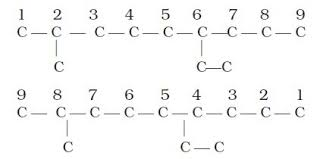
Lowest set of locants
Locants refer to the position of the substituents
Depending on the location of the substituent, the numbering of the parent chain differs.
Presence of more than one substituent
Number to number (,)
Number to letter (-)

If the substituents are similar:
Number, number-prefix/substituent/parent chain
Example:
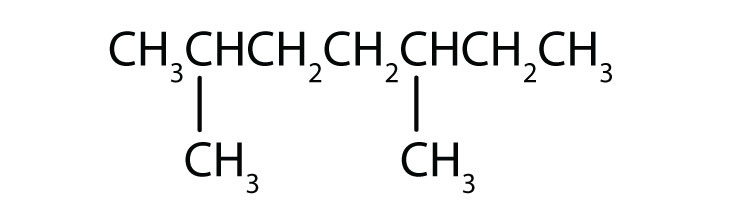 Number:2, 4
Number:2, 4Prefix: di
Substituent: Methyl
Parent Chain: Pentane
The name is: 2,4-dimethylpentane
If the substituents are not similar:
Alphabetical order
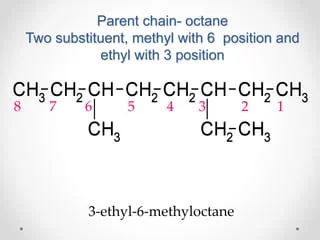
Number: 3, 6Substituents: Ethyl and methyl
Parent Chain: Octane
Alphabetical Order: Ethyl first before methyl
The name is: 3-ethyl-6-methyloctane