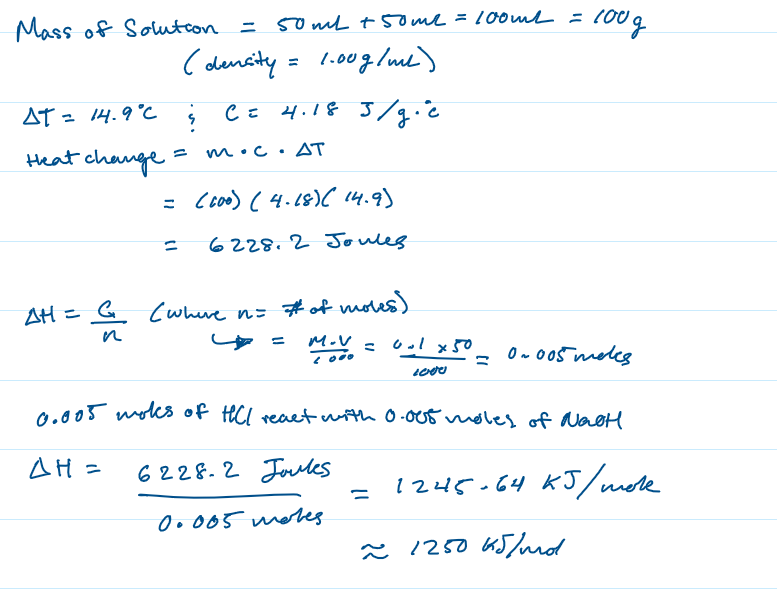Lab 2 - Calorimetry
Enthalpy (H) - The amount of heat absorbed/evolved in the reaction under conditions of constant pressure
Endothermic Reaction - Reaction has a positive ΔH and system absorbs heat from surroundings. Cold to the touch.
Exothermic Reaction - Reaction has a negative ΔH and system releases heat into surroundings. Warm to the touch.
Heat Capacity - Measure of how much heat an object can absorb before its temperature increases by a certain amount
Greater heat capacity results in smaller temperature rise for a given amount of heat
The heat capacity of an object is proportional to its mass and the specific heat of the material
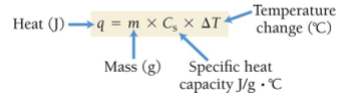 q = Heat (J) absorbed or released by system; m= mass (g) of substance;
q = Heat (J) absorbed or released by system; m= mass (g) of substance;
Cs or s = specific heat cap. of substance; ΔT = change in temperature (Final-Initial)
Things that Affect Heat Capacity
Specific heat capacity
Mass of substance/Amount of matter
Temperature change
Type of substance
Specific Heat Capacity (Cs) - The amount of heat energy required to raise the temperature of one gram of a substance by 1°C
Units: J / (g ⋅ °Celsius)
Thermal Energy Transfer
The amount of energy/heat lost by the hot material equals the amount of heat gained by the cold material
q of system = = -q of surrounding
Transfer of heat is always from the hot object to the cold object
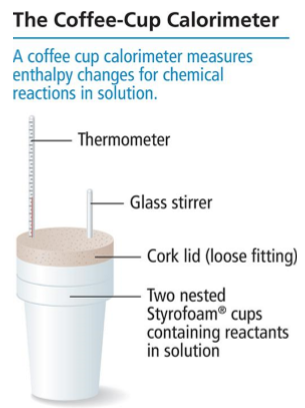
Calorimetry - The process of transferring heat (q) from a hot substance (usually a metal) to a cool substance (usually water) until both are at the same temperature. A calorimeter (usually a foam cup) is used to measure the change in temperature.
Reactions carried out in aqueous solutions are at constant pressure
Coffee-Cup Calorimeter - Most common calorimeter used to measure enthalpy changes; Involves two nested foam cups, thermometer, glass stir, and a cork lid.
Practice Problem 1
You submerge a hot alloy (120 grams & 150°C) into a coffee cup calorimeter containing a coolant fluid (180 grams & 18°C). When the system reaches thermal equilibrium, the final temperature is 22°C. The specific heat capacity of the coolant is 3.9 J/g⋅K . Calculate the specific heat capacity of the alloy.
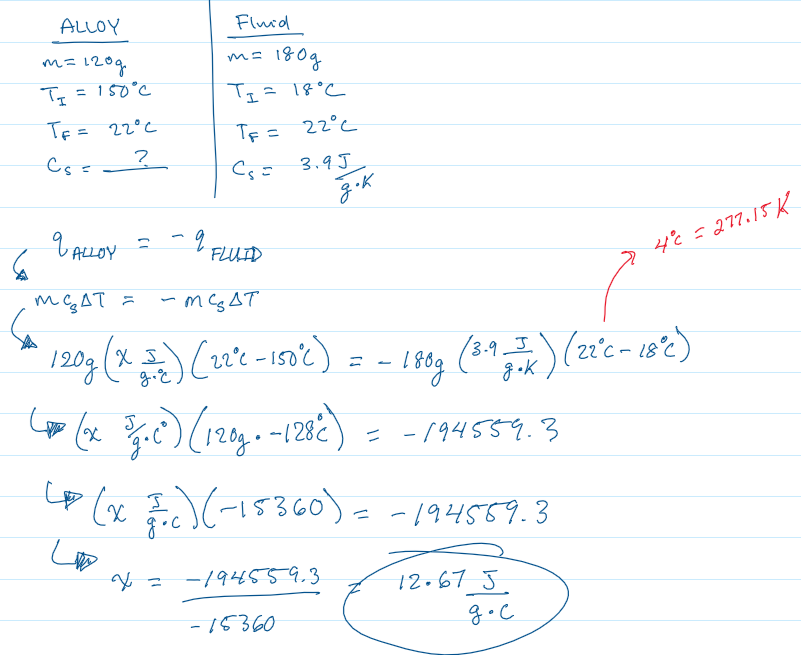 Practice Problem 2
Practice Problem 2
In a coffee cup calorimetry experiment, 50.0 mL of 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl. The temperature of the solution increased by 14.9°C. Calculate ΔH for the reaction. Use 1.00 g/mL as the density of the solution and 4.18 J/ g °C as the specific heat capacity.