Aleks Chapter 17 | CHEM10401
Electrochemistry
Writing and balancing complex half-reactions in acidic solution
Write a balanced half-reaction for the reduction of solid manganese dioxide (MnO2) to manganese ion (Mn2+) in acidic aqueous solution. Be sure to add physical state symbols where appropriate.
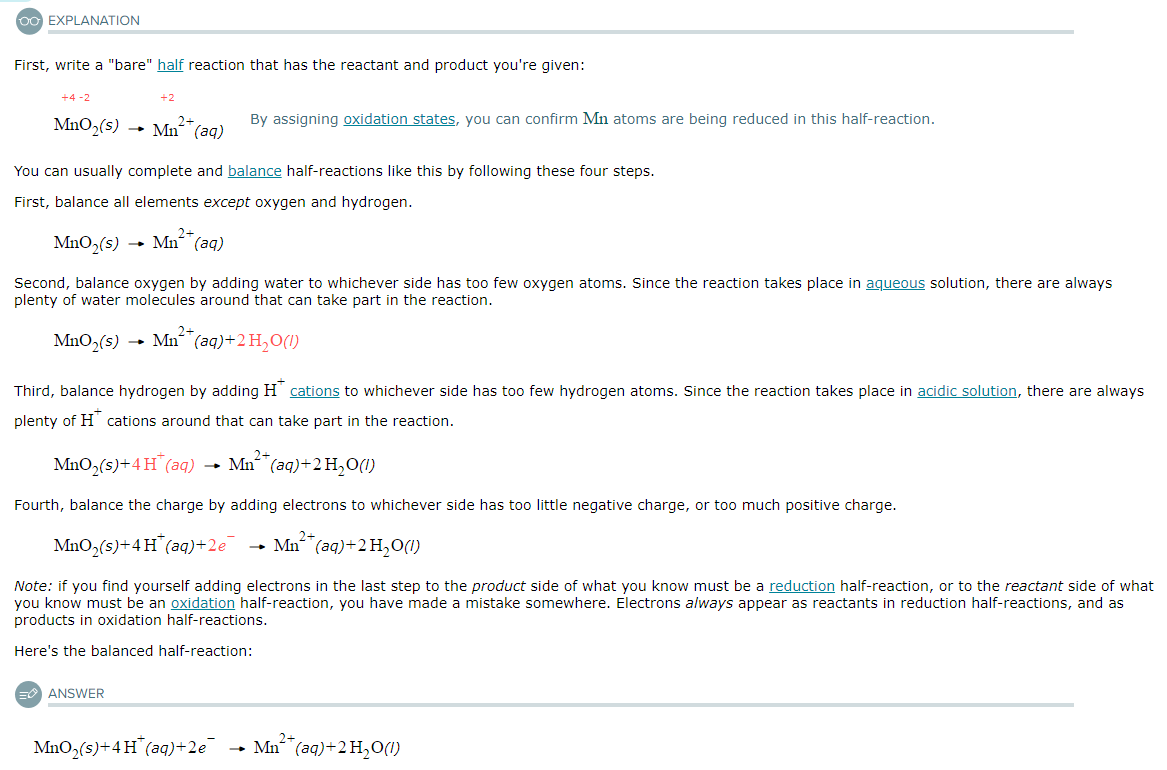
Write a balanced half-reaction for the oxidation of gaseous nitric oxide (NO) to aqueous nitrous acid (HNO2) in acidic aqueous solution. Be sure to add physical state symbols where appropriate.
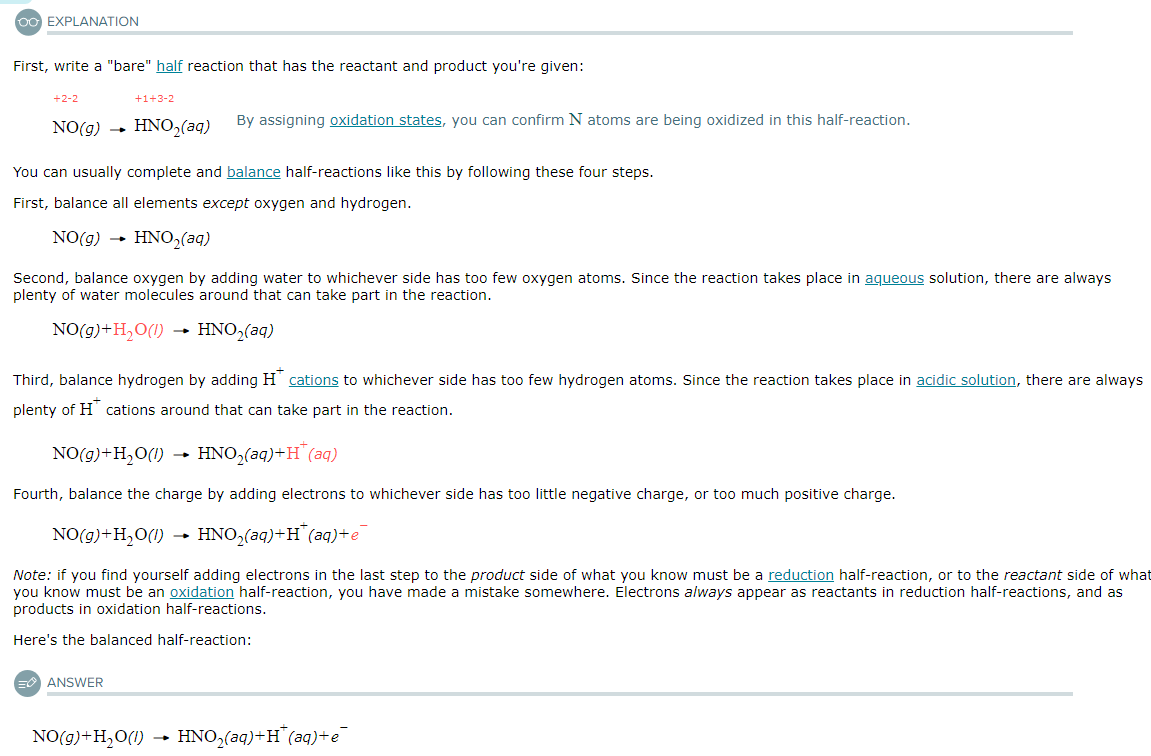
Write a balanced half-reaction for the reduction of gaseous oxygen (O2) to liquid water (H2O) in acidic aqueous solution. Be sure to add physical state symbols where appropriate.
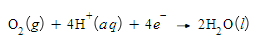
Write a balanced half-reaction for the oxidation of chromium ion (Cr3+) to dichromate ion (Cr2O72-) in acidic aqueous solution. Be sure to add physical state symbols where appropriate.
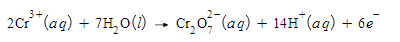
Writing and balancing complex half-reactions in basic solution
Write a balanced half-reaction for the reduction of gaseous nitrogen (N2) to aqueous hydrazine (N2H4) in basic aqueous solution. Be sure to add physical state symbols where appropriate.

Write a balanced half-reaction for the oxidation of chromium ion
(Cr3+) to dichromate ion (Cr2O72-) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
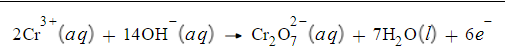
Write a balanced half-reaction for the reduction of permanganate ion (MnO4-) to solid manganese dioxide (MnO2) in basic aqueous solution. Be sure to add physical state symbols where appropriate.

Write a balanced half-reaction for the oxidation of manganese ion (Mn2+) to permanganate ion (MnO4-) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
Write a balanced half-reaction for the reduction of solid manganese dioxide
(MnO2) to manganese ion (Mn2+) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
[#2 in workbook]
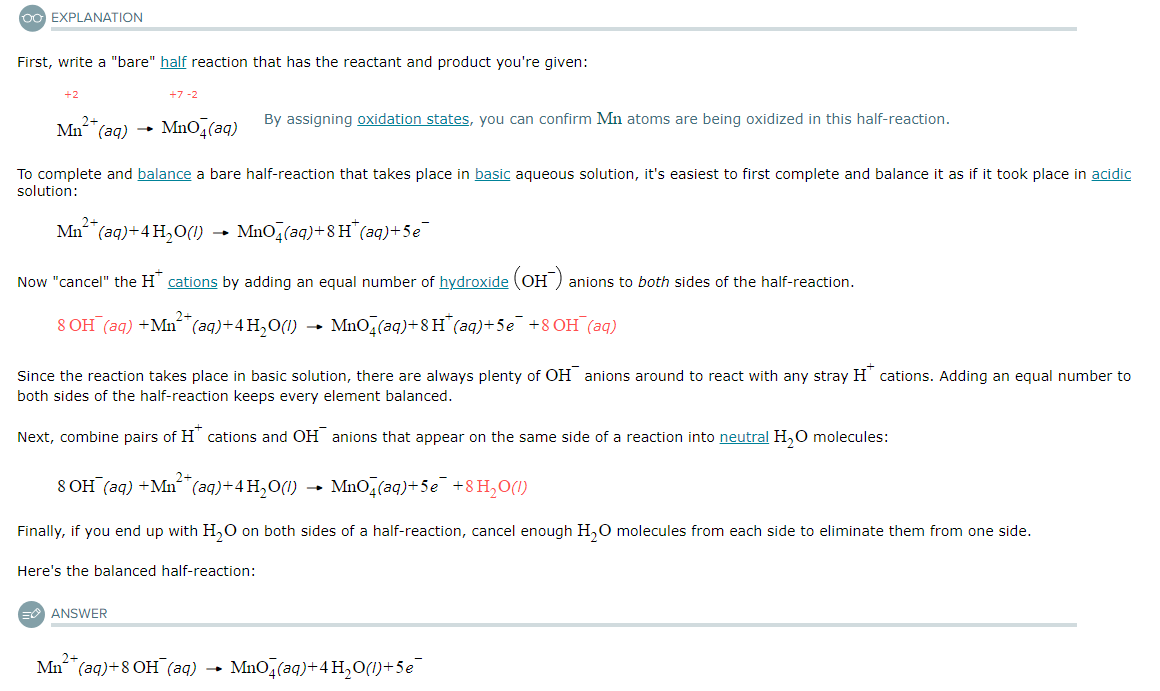
Write a balanced half-reaction for the oxidation of liquid water (H2O) to aqueous hydrogen peroxide (H2O2) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
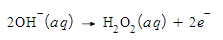
Write a balanced half-reaction for the reduction of bismuth oxide ion (BiO3-) to bismuth ion (Bi3+) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
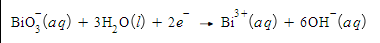
Write a balanced half-reaction for the oxidation of gaseous nitric oxide (NO) to aqueous nitrous acid (HNO2) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
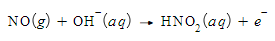
Using the relationship between charge, current and time
An electric current of 29.00A flows for 19.0 seconds. Calculate the amount of electric charge transported.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
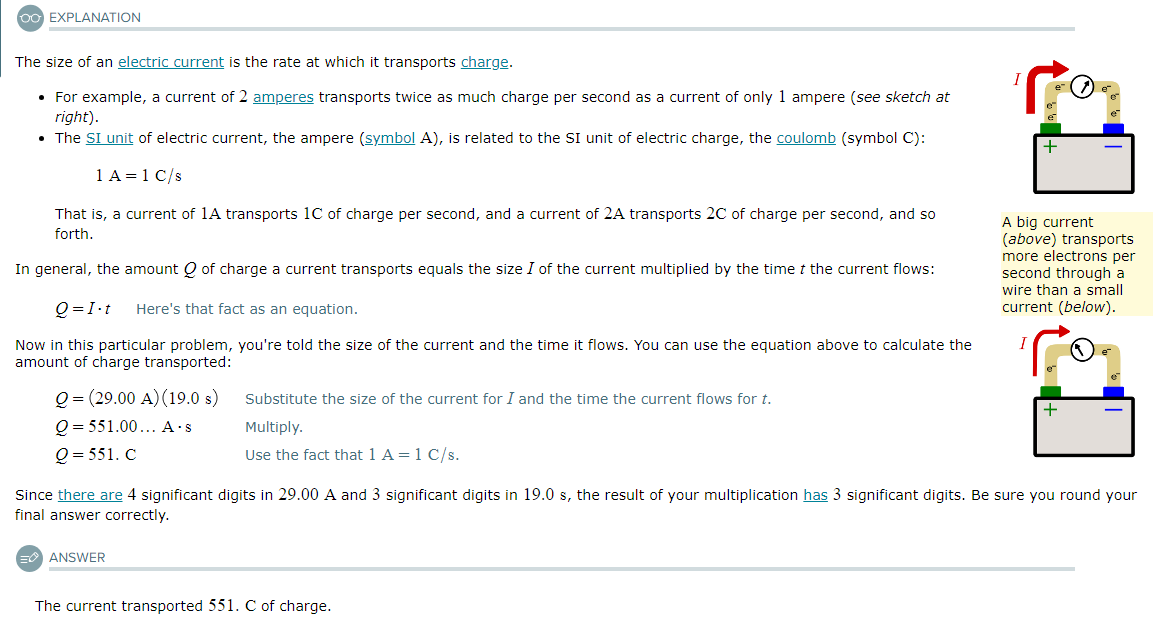
An electric current of 0.550A transports 0.40kC of charge. Calculate the time this took.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
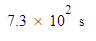
An electric current transports 160.μC of charge in 15.0 minutes. Calculate the size of the electric current.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
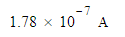
An electric current of 1.000A flows for 370. milliseconds. Calculate the amount of electric charge transported.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
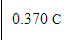
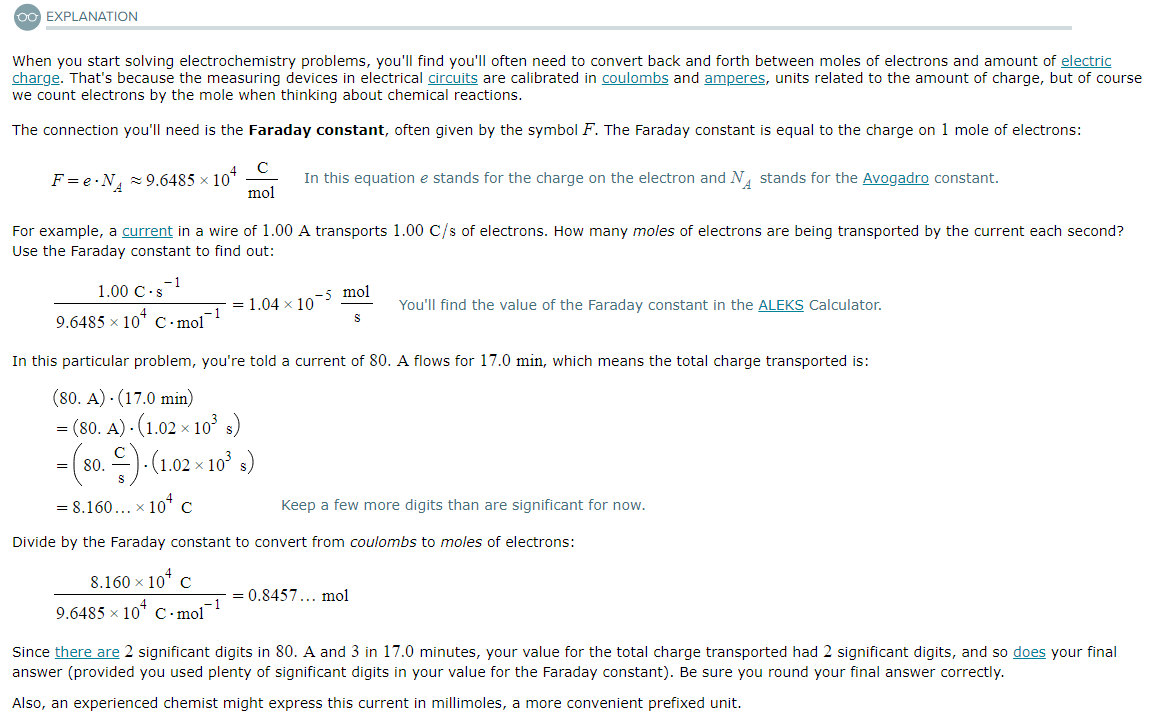
Using the Faraday constant
Suppose a current of 80.A flows through a copper wire for 17.0 minutes. Calculate how many moles of electrons travel through the wire.
Be sure your answer has the correct unit symbol and the correct number of significant digits.

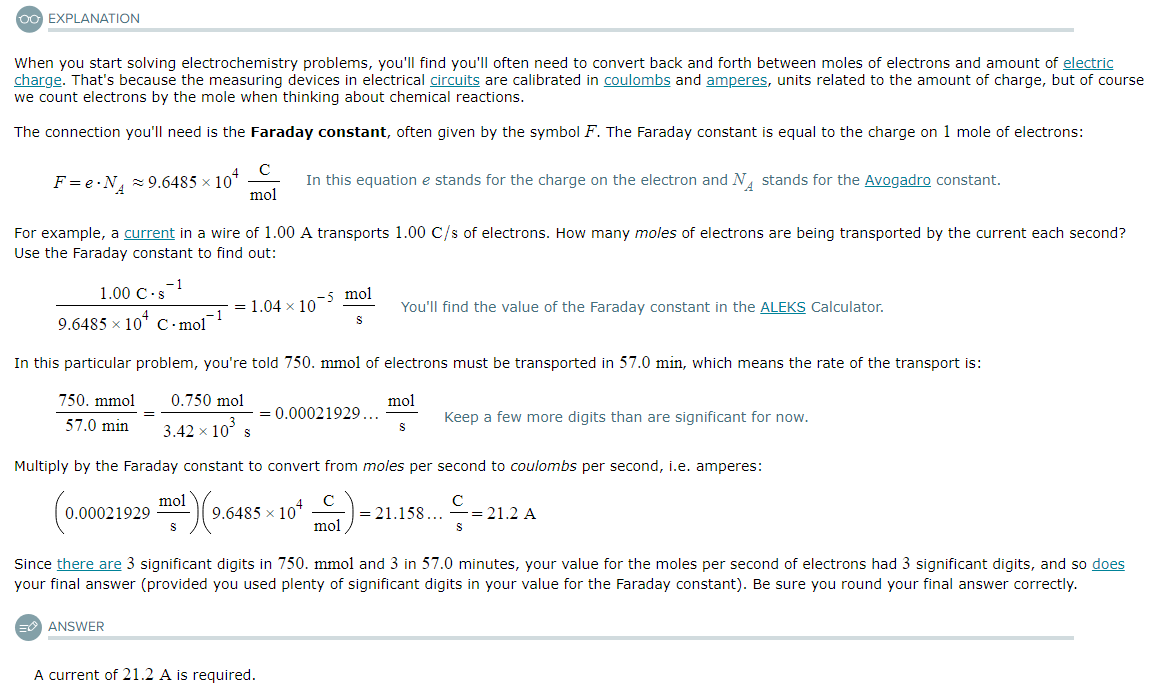
Suppose 750.mmol of electrons must be transported from one side of an electrochemical cell to another in 57.0 minutes. Calculate the size of electric current that must flow.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
Suppose a current of 60. A flows through a copper wire for 43.0 minutes. Calculate how many moles of electrons travel through the wire.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
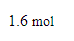
Suppose 0.430mol of electrons must be transported from one side of an electrochemical cell to another in 195. seconds. Calculate the size of electric current that must flow.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
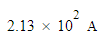
Suppose a current of 70.A flows through a copper wire for 23.0 minutes. Calculate how many moles of electrons travel through the wire.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
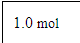
Recognizing consistency among equilibrium constant, free energy, and cell potential
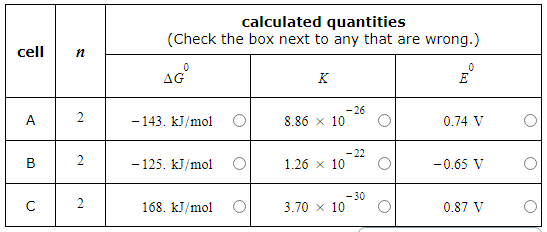
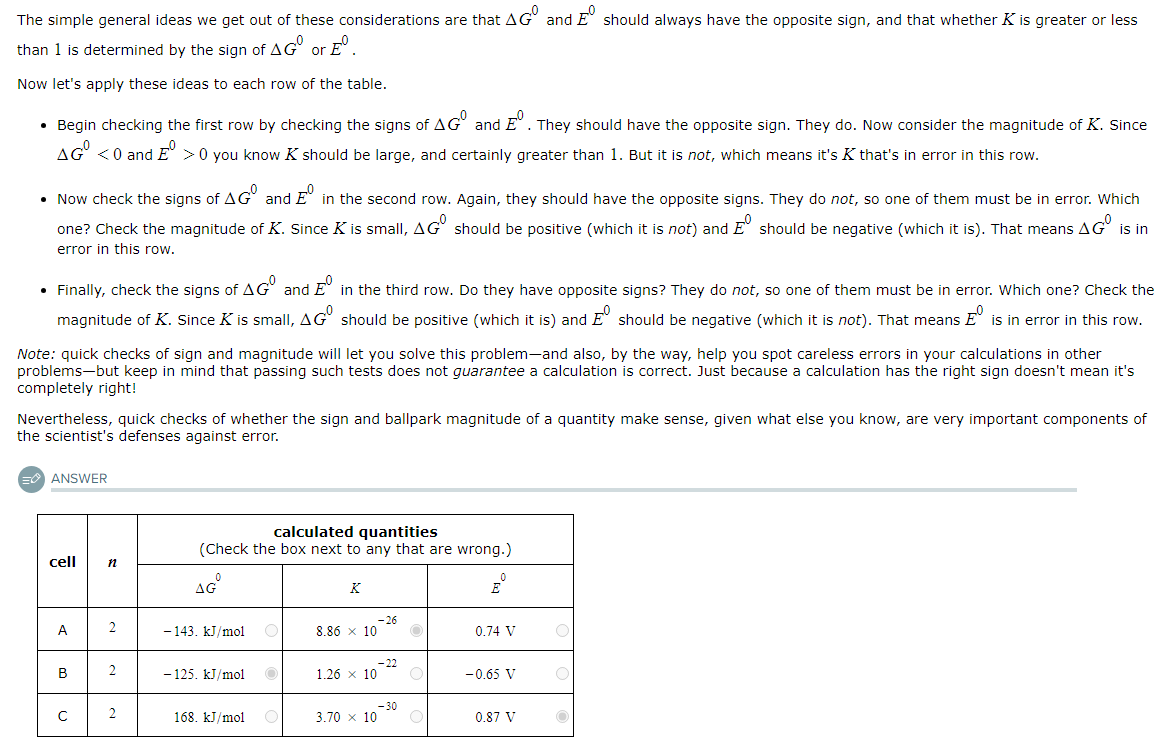
A student made measurements on some electrochemical cells and calculated three quantities:
The standard reaction free energy ΔG0.
The equilibrium constant K at 25.0°C.
The cell potential under standard conditions E0.
His results are listed below.
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any.
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct.
Also note: for each cell, the number n of electrons transferred per redox reaction is 2.
A student made measurements on some electrochemical cells and calculated three quantities:
The standard reaction free energy ΔG0.
The equilibrium constant K at 25.0°C.
The cell potential under standard conditions E0.
His results are listed below.
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any.
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct.
Also note: for each cell, the number n of electrons transferred per redox reaction is 1.
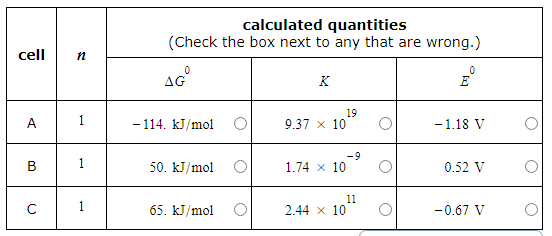
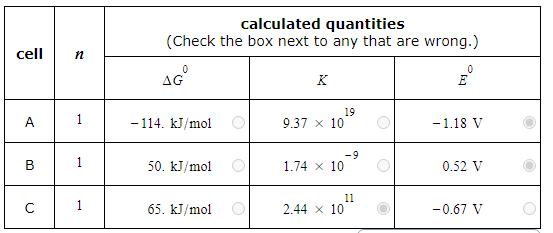
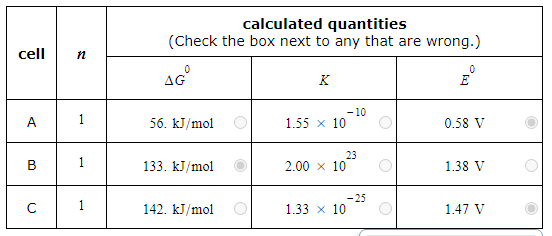
A student made measurements on some electrochemical cells and calculated three quantities:
The standard reaction free energy ΔG0.
The equilibrium constant K at 25.0°C.
The cell potential under standard conditions E0.
His results are listed below.
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any.
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct.
Also note: for each cell, the number n of electrons transferred per redox reaction is 1.
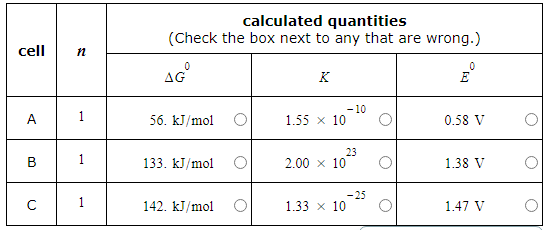
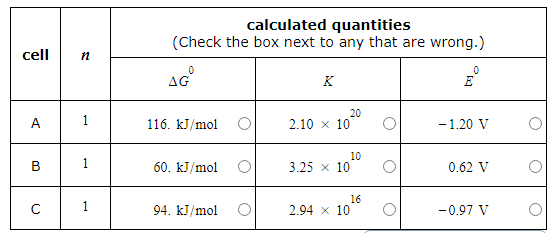
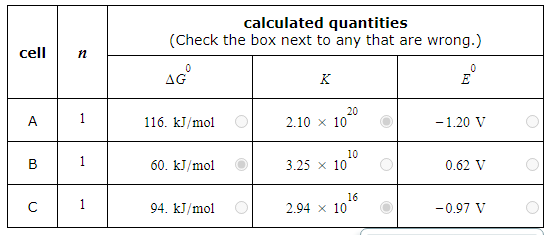
A student made measurements on some electrochemical cells and calculated three quantities:
The standard reaction free energy ΔG0.
The equilibrium constant K at 25.0°C.
The cell potential under standard conditions E0.
His results are listed below.
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any.
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct.
Also note: for each cell, the number n of electrons transferred per redox reaction is 1.
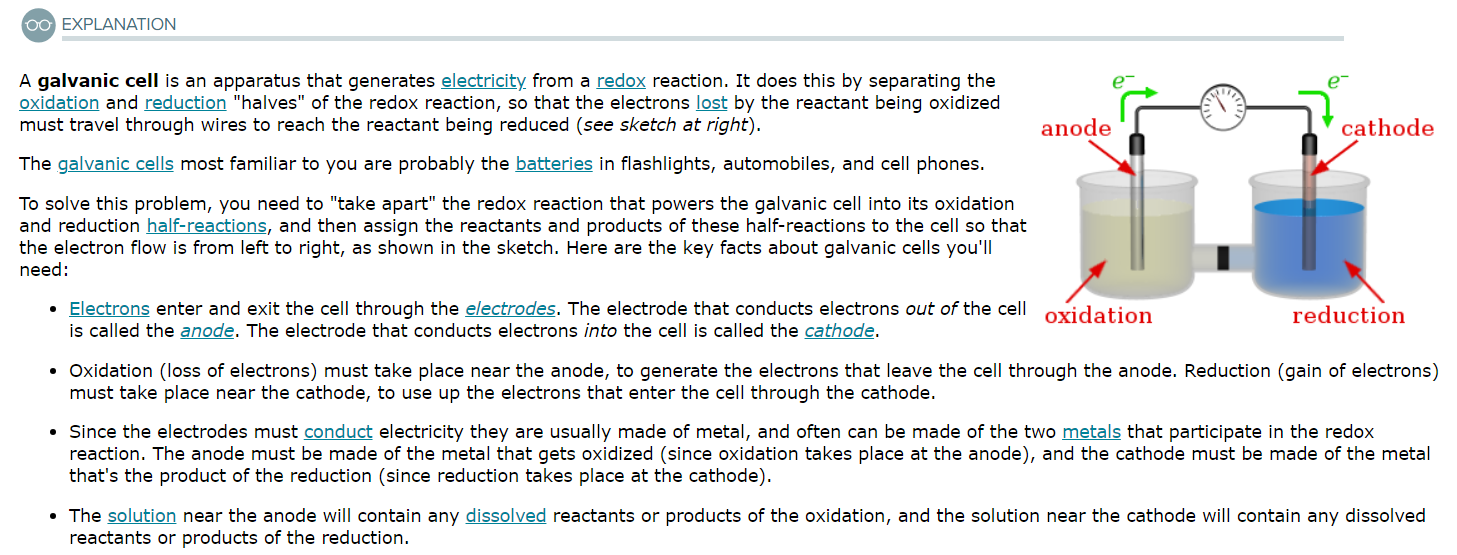
Designing a galvanic cell from a single-displacement redox reaction
Suppose the galvanic cell sketched below is powered by the following reaction:
Mn(s) + ZnCl2(aq) → MnCl2(aq) + Zn(s)
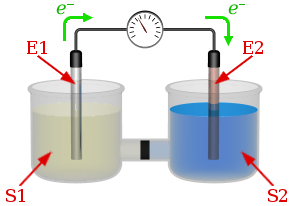
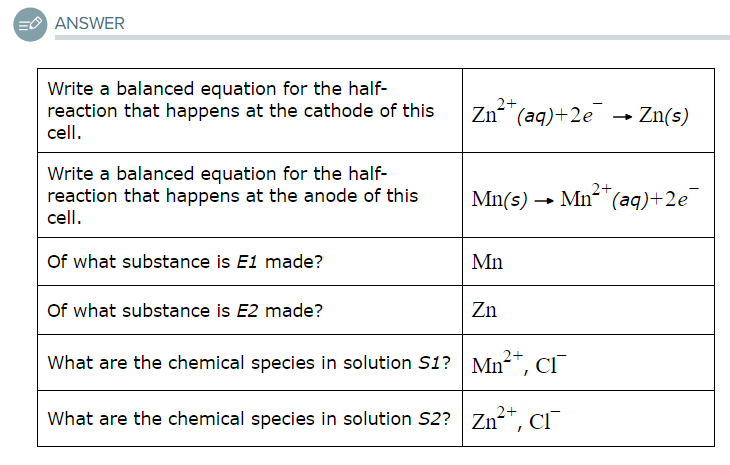
Write a balanced equation for the half-reaction that happens at the cathode of this cell.
Write a balanced equation for the half-reaction that happens at the anode of this cell.
Of what substance is E1 made?
Of what substance is E2 made?
What are the chemical species in solution S1?
What are the chemical species in solution S2?

 Knowt
Knowt