Human Pathology Chapter 1. Cell Pathology
Cell Pathology
Cell pathology is the study of cellular abnormalities that can cause disease at any level of biological organization.
Organization of the Body
Cells: The fundamental, basic unit of the body.
Tissues: Groups of similar cells working together to perform similar functions.
Organs: Tissues grouped together in specific proportions to perform complex functions.
Organ System: Groups of organs functioning collaboratively.
Functioning Organism: Integrated organ systems working in harmony.
An abnormality at any level of this organization can lead to disease.
Essential Components of a Typical Cell
Cells are complex structures containing various organelles and components that enable their functions.
Key components include the nucleus, cell membrane, mitochondria, ribosomes, endoplasmic reticulum (RER, SER), Golgi apparatus, lysosomes, microvilli, and desmosomes.
Components of the Nucleus
Nucleus: Contains the genetic information and directs the metabolic functions of the cell.
Content of the Nucleus:
Nucleoli: Involved in ribosome synthesis.
Nucleic Acids Combined with Protein:
DNA (Deoxyribonucleic acid): Contained within chromosomes, carries genetic instructions.
RNA (Ribonucleic acid): Contained in nucleoli and plays various roles in gene expression.
Cell Function, DNA, Genetic Code
Pairing Bases: A DNA molecule consists of two strands held together by weak chemical attractions between complementary bases on adjacent chains.
Specific Base Pairing Rules:
Adenine (A) always pairs with Thymine (T).
Guanine (G) always pairs with Cytosine (C).
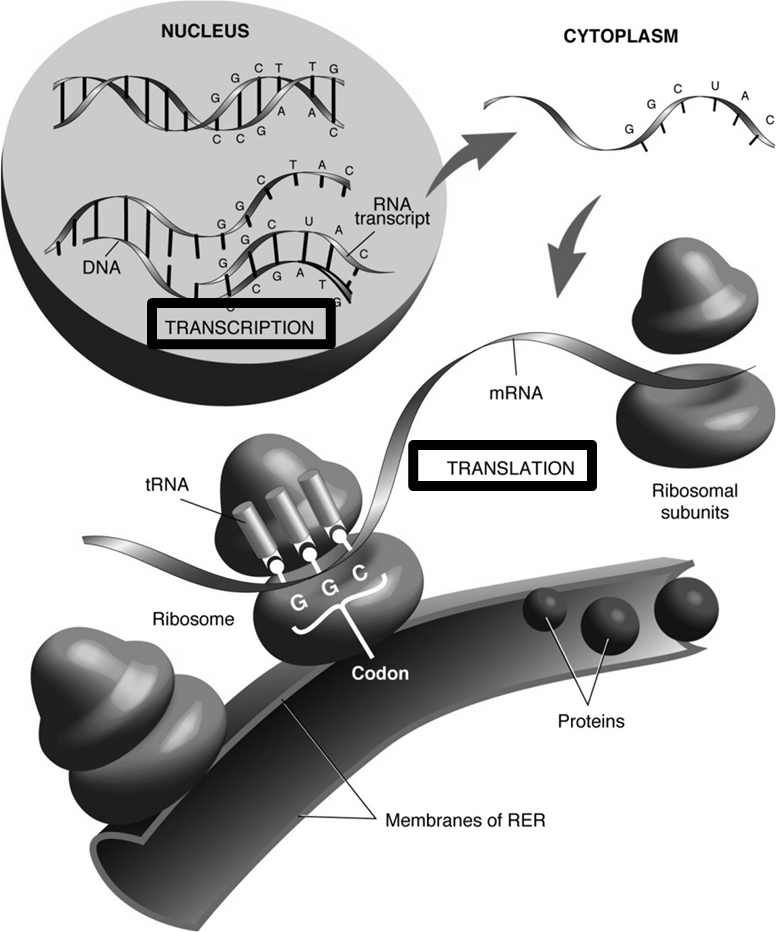
Central Dogma: Gene Expression
This describes the flow of genetic information within a biological system.
NUCLEUS:
Transcription: The process where DNA is used as a template to synthesize an RNA transcript (mRNA). (e.g., DNA sequence TACCCGGG is transcribed into mRNA sequence AUGGGCCC if the template strand is used for the example).
CYTOPLASM:
mRNA (messenger RNA): Carries genetic information from DNA to ribosomes.
Ribosomes: Site of protein synthesis.
tRNA (transfer RNA): Carries specific amino acids guided by codons on the mRNA.
Codon: A sequence of three nucleotides on the mRNA that corresponds to a specific amino acid.
Translation: The process where mRNA sequence is decoded by ribosomes and tRNA to synthesize a protein.
RER (Rough Endoplasmic Reticulum): Membranes of the RER are associated with ribosomes; proteins destined for export or insertion into membranes are synthesized here.
Golgi Apparatus: Further processes and packages proteins for secretion or delivery to other organelles.
Cytoplasm
Cytoplasm: The mass of protoplasm with its various organelles, enclosed by the cell membrane.
Hyaloplasm: The jelly-like substance (cytosol) within the cytoplasm where organelles float; it has no definite shape.
Cytoplasmic Organelles: Key functional structures including mitochondria, ribosomes, endoplasmic reticulum (RER, SER), Golgi apparatus, and lysosomes.
Plasma Membrane: Separates the cytoplasm from the extracellular fluid.
Plasma Membrane
Outer Cell Surface: Forms the boundary of the cell.
Selectively Permeable: Controls the movement of substances into and out of the cell, often maintaining an electric charge.
Lipid Bilayers: Composed primarily of a double layer of lipids with inserted glycolipids and glycoproteins.
Hydrophobic and Hydrophilic Regions: The lipid bilayer has hydrophobic (water-repelling) tails and hydrophilic (water-attracting) heads, which is crucial for its structure and function.
Mitochondria
Structure: Surrounded by a double membrane with inner folds called cristae.
Function: Generate energy in the form of Adenosine Triphosphate (ATP) through cellular respiration.
Content: Full of oxidative enzymes, such as cytochrome oxidase, essential for energy production.
Ribosomes and Rough Endoplasmic Reticulum (RER)
Ribosomes ("polysomes"): Small organelles responsible for the synthesis of proteins, primarily for internal use within the cell.
RER: A network of membranes studded with ribosomes, specialized for the synthesis of proteins destined for export from the cell or insertion into membranes.
Smooth Endoplasmic Reticulum (SER)
Complex Functions: The SER performs a variety of metabolic roles.
Catabolism: Involved in the metabolic degradation of drugs, hormones, and nutrients.
Synthesis: Responsible for the synthesis of steroid hormones, such as female estrogen and male testosterone.
Prominent Locations: Abundant in cells with high metabolic activity related to these functions, such as liver cells, adrenal cells, and Leydig cells.
Lysosomes
Structure: Membrane-bound digestive organelles.
Content: Contain various acidic enzymes capable of breaking down cellular waste and foreign materials.
Types:
Primary Lysosome: Newly formed lysosome containing inactive enzymes.
Secondary Lysosomes: Formed when a primary lysosome fuses with a phagosome or autophagosome, activating its enzymes to digest contents.
Residual Bodies ("lipofuscin"): Undigested materials that remain in lysosomes after digestion.
Function: Act as the cell's "trashcan" or recycling center, breaking down macromolecules, old organelles, and foreign substances.
Lipofuscin
Description: A brown pigment composed of oxidized lipids.
Origin: Represents the undigested contents of autophagosomes (cellular components) and heterophagosomes (external components).
Accumulation: Tends to accumulate in aging tissues; its presence can be used to estimate cellular age or the age of an organ based on visual appearance and coloring.
Movement of Materials Into and Out of Cells
Diffusion: The passive movement of dissolved particles (solute) from an area of higher concentration to an area of lower concentration, down their concentration gradient.
Osmosis: The passive movement of water molecules from a more dilute solution (higher water concentration) to a more concentrated solution (lower water concentration) across a semi-permeable membrane.
Active Transport: The transfer of a substance across the cell membrane from a region of low concentration to one of higher concentration (against its concentration gradient).
This process requires the cell to expend energy, typically in the form of ATP, because the substance is moving uphill against its gradient.
Many vital metabolic processes, such as ion and molecule regulation, depend on active transport.
Phagocytosis: The ingestion of large particles that are too big to pass through the cell membrane.
The cytoplasm flows around the particle, and cytoplasmic processes fuse, engulfing the particle within a vacuole inside the cell.
Pinocytosis: The ingestion of fluid (rather than solid material) by the cell, often referred to as "cell drinking."
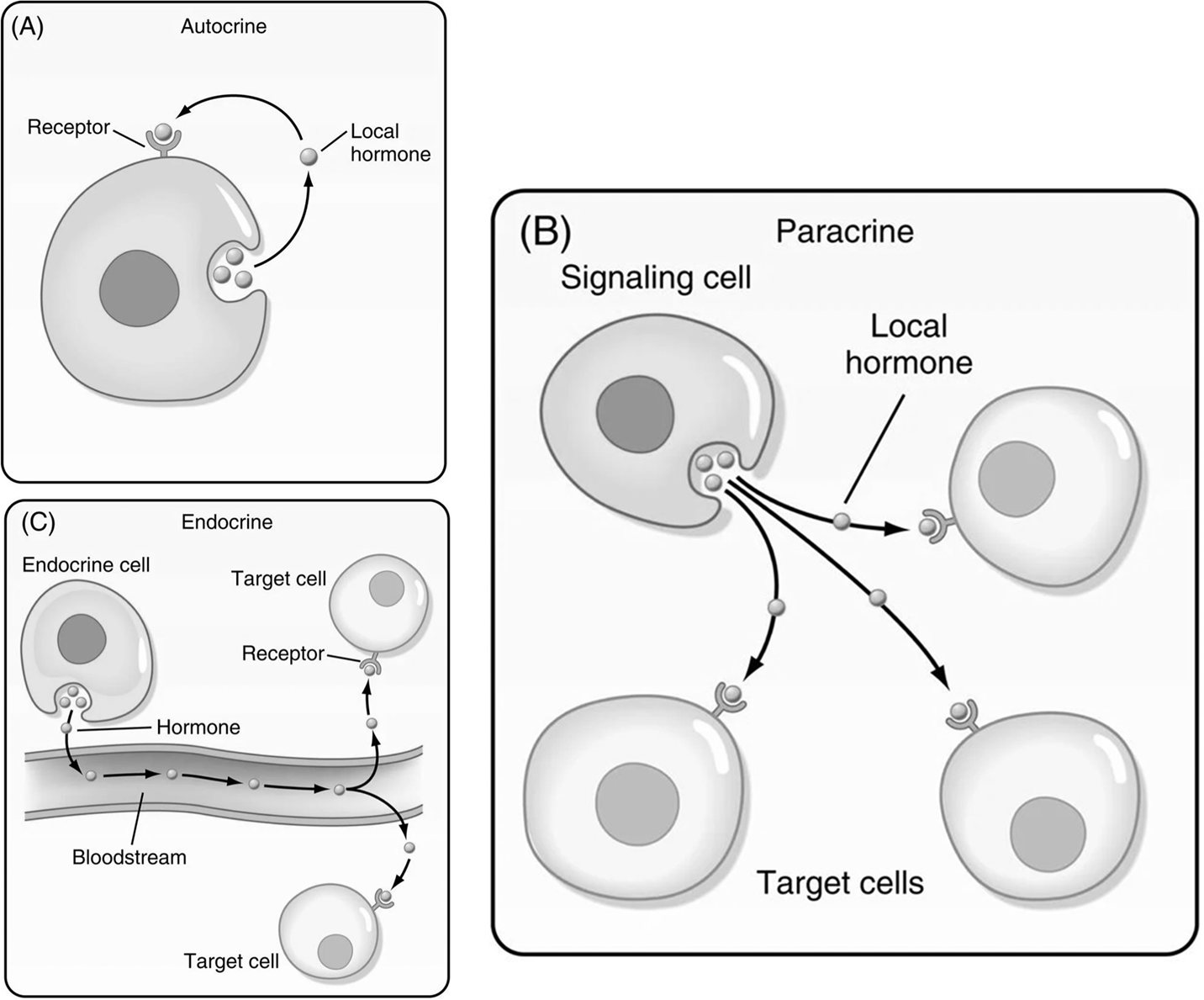
Cellular Communication
Autocrine Signaling: A form of cell-to-cell communication where a cell secretes a signal molecule that binds to receptors on the very same cell, triggering a response within itself.
Paracrine Signaling: A local form of cell communication where a cell releases signaling molecules that diffuse through the intercellular fluid to affect nearby, neighboring target cells.
Often described as "local signaling," it allows cells to communicate over short distances.
Signals are absorbed or degraded quickly due to the short distances, preventing widespread effects.
Heavily involved in the action of many localized hormones.
Endocrine Signaling: A form of long-distance cell communication where specialized endocrine cells release chemical messengers, known as hormones, into the bloodstream.
These hormones then travel through the circulatory system to target cells located throughout the body, eliciting a specific response.
Cell Injury
Reversible Cell Injury: Cellular damage that can be healed, allowing the cell to recover its normal function.
A typical sign is cellular swelling.
The cell can recover by itself once the injurious stimulus is removed.
Irreversible Cell Injury: Damage that leads to permanent alteration and ultimately cell death.
Examples include stroke or heart attack, which result in the irreversible loss of brain or heart cells.
The loss of these cells cannot be recovered.
Causes of Cell Injury
Hypoxia/Anoxia: Partial (hypo) or complete absence (an) of oxygen supply to cells, impairing cellular respiration.
Toxins: Chemical substances that cause damage to cells.
Microbes: Pathogenic microorganisms (e.g., bacteria, viruses) that can infect and damage cells.
Inflammation and Immune Reactions: The body's own defense mechanisms can sometimes cause collateral damage to healthy cells and tissues.
Oxygen Radicals: Highly reactive molecules (e.g., from UV particles or sunlight) that can cause oxidative stress and damage cellular components, leading to conditions like cancer.
Toxic Cell Injury
Direct Toxin: A substance that directly damages cells.
Example: Heavy metals like mercury or lead, which disrupt disulfide (S-S) bonds in proteins, altering their structure and function.
Indirect Toxin: A substance that becomes toxic only after it is metabolized by the body into a more harmful compound.
Example: Carbon tetrachloride (CCl4), is metabolized in the liver to become carbon trichloride (CCl3)which is significantly more toxic and causes cellular damage.
Microbial Pathogens
Bacteria: Produce toxins that can directly damage cells and cause infection.
Example: Food poisoning, where symptoms might appear 8 hours after ingestion and resolve once the bacteria or their toxins are cleared.
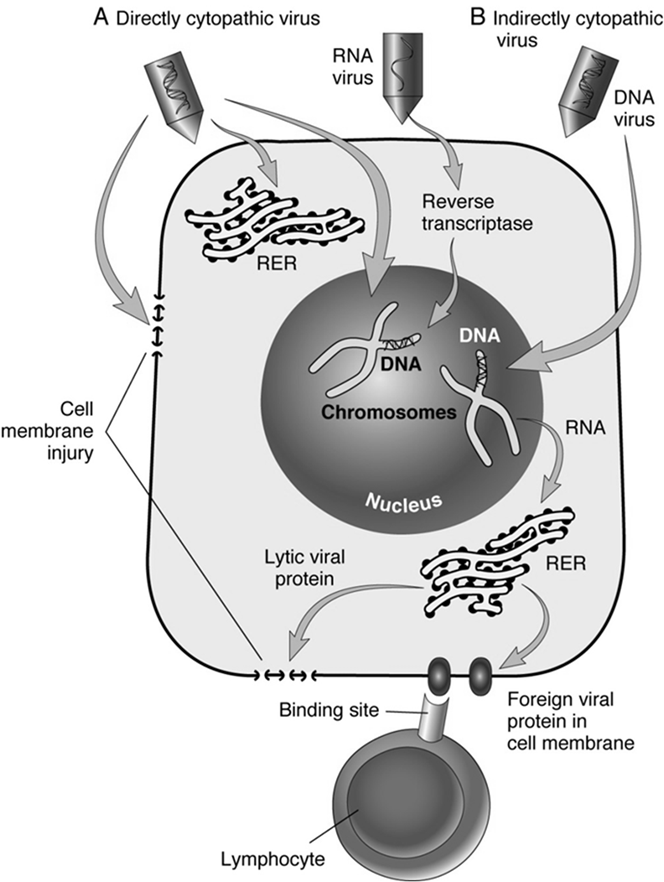
Viruses: "Kill cells from within" by hijacking the host cell's machinery.
They have an incubation period, leading to a delayed reaction, as they use the cell's central dogma (DNA replication, transcription, translation) to replicate themselves.
Direct Cytopathic Effect: Viruses directly damage cells during replication.
Indirect Cytopathic Effect: Host immune response triggered by the viral infection damages infected cells.
Cell Adaptations
Definition: Changes that cells undergo after prolonged exposure to adverse or even normal stimuli, allowing them to survive or function under new conditions.
These are cellular alterations in size, number, or form.
Main Forms of Adaptation:
Atrophy: Decrease in cell size.
Hypertrophy: Increase in cell size.
Hyperplasia: Increase in cell number.
Metaplasia: Change from one mature cell type to another mature cell type.
Dysplasia: Disordered growth and maturation of cells, often a precursor to neoplasia.
Atrophy
Definition: A decrease in the size of a cell, tissue, organ, or even the entire body.
Types:
Physiologic and Predictable Atrophy: Occurs as a normal part of development or aging.
Examples: Atrophy of the thymus gland after puberty, general aging processes.
Pathologic Atrophy: Caused by disease or detrimental conditions.
Causes: Lack of nutrition, chronic ischemia (reduced blood supply), denervation (loss of nerve supply), prolonged inactivity (disuse atrophy).
Example: Dementia, which is characterized by the atrophy of neurons, leading to shrinkage of the cerebral gyri and widening of sulci, impacting memory and cognitive function as part of the aging process.
Hypertrophy
Definition: The enlargement of individual cells, which leads to an increase in the overall size of the tissue or organ.
Examples:
Normal Hypertrophy: Enlargement of skeletal muscles in bodybuilders due to increased workload, considered a normal adaptive response.
Abnormal Hypertrophy: Hypertrophy of the heart, specifically the left ventricle, in response to chronic hypertension (high blood pressure). This is an abnormal adaptation where heart tissue grows due to overworking to pump against increased resistance, eventually leading to cardiac dysfunction.
Hyperplasia
Definition: An increase in the number of cells in a tissue or organ, leading to its enlargement.
Examples:
Normal Hyperplasia: The growth from adolescence to adulthood involves a normal increase in cell numbers in many tissues throughout the body.
Abnormal Hyperplasia:
Endometrial Hyperplasia: Increased number of endometrial cells in the uterus, often caused by excessive estrogen stimulation.
Benign Prostatic Hyperplasia (BPH): Enlargement of the prostate gland due to an increased number of stromal and epithelial cells, common in elderly men.
Callus Formation: Increased number of epidermal cells in response to chronic irritation or pressure, leading to thickened skin (e.g., corn callus).
Metaplasia & Dysplasia
Metaplasia: A reversible change where one mature differentiated cell type is replaced by another mature differentiated cell type that is better able to withstand a specific stress.
Example: Chronic irritation from cigarette smoke can cause the normal ciliated pseudostratified columnar epithelium lining the bronchi to be replaced by more resilient squamous epithelium.
This is considered a "middle phase" in the progression towards more severe cellular changes.
It is reversible if the causative factors (e.g., smoking) are removed; lung cells can revert to normal at this stage.
The natural progression sequence in certain tissues (e.g., uterine cervix, respiratory tract) can be metaplasia \rightarrow dysplasia \rightarrow neoplasia (cancer).
Dysplasia: An increasing degree of disordered growth or maturation of the tissue, characterized by variations in cell size, shape, and organization.
It is considered a pre-neoplastic lesion, meaning it precedes the development of true cancer (neoplasia).
Example: Cervical dysplasia resulting from human papillomavirus (HPV) infection.
At this stage, the tissue becomes permanently damaged if the causative factors persist and progression continues, making it a critical stage for intervention.
Death
Somatic Death: Clinically defined as "brain death," referring to the death of the entire organism.
After somatic death, tissues undergo autolysis (self-digestion by the cell's own enzymes).
Cell Death: Occurs in two primary forms within a living organism:
Necrosis: Localized death of cells or tissues in a living organism, typically due to external injury or pathology.
Apoptosis: Programmed death of single cells within a living organism, an active and regulated process.
Comparison of Necrosis and Apoptosis
Feature | Necrosis | Apoptosis |
|---|---|---|
Cause | Exogenous injury | May be exogenous or endogenous |
Mechanisms | Vital processes inhibited | Energy dependent, vital processes active |
Cells Affected | Multiple cells | Single cells |
Cell Morphology | Swollen, ruptured | Rounded up, fragmented (apoptotic bodies) |
Cell Membrane | Ruptured, loss of integrity | Functionally intact, but altered |
Outcome | Phagocytosis by neutrophils | Phagocytosis by macrophages and "nonprofessional" macrophages |
Apoptosis: A viable cell (e.g., leukemic cell) undergoes condensation and fragmentation of its nucleus into apoptotic bodies, which are then quickly cleared by phagocytes.
Necrosis: Cells swell, mitochondria and RER become swollen, nuclear clumping occurs, and eventually the cell membrane ruptures, leading to inflammation.
Types of Necrosis
Coagulative Necrosis: Refers to light microscopic alterations in a dead cell, where the cellular architecture is preserved for a period, resembling coagulated proteins.
Morphologic Changes (occur during coagulative necrosis):
Pyknosis: The nucleus becomes smaller, condenses, and stains deeply basophilic due to chromatin clumping.
Karyorrhexis: The pyknotic nucleus breaks up into many smaller fragments, scattered as "nuclear dust."
Karyolysis: The pyknotic nucleus may be extracted from the cell due to enzymatic digestion, leading to a faded or absent nucleus.
Gangrene: A macroscopic form of coagulative necrosis, typically arising from ischemic conditions of a limb.
Dry Gangrene: Characterized by no blood flow. The affected tissue is hard, dry, shrunken, and discolored (often black), with a clear line of demarcation between viable and necrotic tissue (e.g., involving toes or heels due to constant pressure).
Wet Gangrene: Involves the presence of bacterial infection, blood, and pus. The tissue is typically soft, swollen, putrid, and dark, with less clear demarcation due to liquefaction by bacterial enzymes.
Liquefactive Necrosis: Occurs when the rate of dissolution of necrotic cells is faster than the rate of repair.
The tissue becomes soft and liquidy, often forming a viscous, pus-filled cavity (e.g., an abscess).
Commonly seen in the brain after an ischemic injury, where the lipid-rich tissue undergoes autolysis.
Caseous Necrosis: A distinct form of necrosis characteristic of tuberculosis (TB).
Lesions of TB are compact aggregates of macrophages and other inflammatory cells known as granulomas.
The debris from dead cells is grayish-white and soft, with a texture resembling "clumpy cheese" (hence "caseous," meaning cheese-like).
Fat Necrosis: Specifically affects adipose (fat) tissue.
Most commonly results from pancreatitis (inflammation of the pancreas, releasing lipolytic enzymes) or trauma (e.g., in the breast).
Visually appears as irregular, chalky white areas embedded in otherwise normal adipose tissue, due to the release of fatty acids which combine with calcium to form soaps (saponification).
Traumatic fat necrosis in the breast can sometimes be mistaken for cancer due to its palpable lumpiness.
Calcification
Dystrophic Calcification: Occurs when necrotic or degenerating tissue attracts calcium salts, leading to its calcification.
This happens despite normal serum calcium levels.
Examples: Calcification in atherosclerotic plaques in arteries (atherosclerosis), or calcified damaged heart valves (e.g., calcified aortic valve).
Metastatic Calcification: Involves the deposition of calcium salts in normal, healthy tissues.
This occurs due to hypercalcemia (abnormally high levels of calcium in the blood).
The most common cause of hypercalcemia is hyperparathyroidism (overactivity of the parathyroid glands, which regulate calcium levels).