Test 1 Pathopharm (Hematololgy, Fluid and Electrolyte)
Lecture 1
DRUG TABLE
HEMATOLOGY:
F&E
Fluid & Electrolytes Drug Table (1)
Orientation to Pharmacology
Objectives
- Formulate a beginning understanding of factors relating to drug administration
- Describe desirable properties of a drug
- Describe the nurse’s role in drug administration
Some terms and Conversions to know:
Liquids | Solids |
|---|---|
1 mL = 1 cm3 | 1,000 mcg = 1 mg |
1,000 mL = 1 L | 1,000 mg = 1 g |
1,000 g - 1 kg | |
1 ml=1cc |
Weights
ALL drug weights are based in Kg.
To convert pounds to kilos, always use your calculator, do not round your answer.
pounds divided by 2.2 gives you kilos.
Ex: 50.6 lb divided by 2.2 = 23 kg
What does this mean??
IV: intravenous
PIV: peripheral intravenous
PO: by moth
SL: sublingual
IM: intramuscular
SC or sometimes SQ: subcutaneous
Gtt: drops
PR: per rectum
Drug Administration
- If the drug is in liquid form, you must always know the dose (mg) and how much to give (mL)
- If the drug is in a pill form, you must know the dose (mg/g) and how many pills to give
- Some orders will say “Metoprolol 50mg give 2 tablets every morning”
- Some orders will say give 100mg metoprolol and you need to figure out how many 50 mg pills you will give.
Order up!
- Give 650 mg PO q12h
- Infuse 50mg drug “x” in 500 ml over 30 minutes (in order we would have to clarify_
- Give 25 mg/k/d in 2 divided doses (/ means per) 25*100=2500/2= answer
- Give 10 mg drug “x” q8h IV
Four Basic Terms
- Drug
- Any chemical that can affect living processes
- Pharmacology
- Study of drugs and their interactions with living systems
- Clinical pharmacology
- Study of drugs in humans
- Therapeutics
- Also known as pharmacotherapeutics
- The use of drugs to diagnose, prevent, or treat disease or to prevent pregnancy
Three Most Important Properties of an Ideal Drug
Effectiveness: Most important property a drug can have
Safety: Drug does not produce harmful effects
Selectivity: Drug elicits only the response for which it is given
….. Name some properties of an ideal drug….
Additional Properties of an Ideal Drug
- Reversible action
- Predictability
- Ease of administration
- Freedom from drug interactions
- Low cost
- Chemical stability
- Simple generic name
- However, no drug is ideal…
There is no perfect drug….
Pharmacology – from the latin root pharmakon
- Literally means remedy and poison
OBJECTIVE: To provide maximum benefit with minimum harm
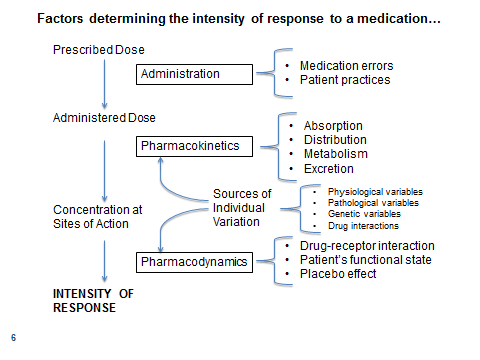
Factors That Determine the Intensity of Drug Responses
- Administration
- Pharmacokinetics- movement of the drugs through the body
- Pharmacodynamics- how the drug affects the body
- Sources of individual variation
Administration
- Important determinants of drug responses: Dosage size, route, and timing
- What does route mean????
- Medication errors
- Patient adherence
Pharmacokinetics
- What the body does to the drug
- Determining how much of the administered dose gets to its sites of action
- Impact of the body on drugs
- Four major pharmacokinetic processes:
- Drug absorption
- Drug distribution
- Drug metabolism
- Drug excretion
Pharmacodynamics
- What the drug does to the body
- Impact of drugs on the body
- Drug-receptor interaction
- Binding of the drug to its receptor
- Patient’s functional state
- Influences pharmacodynamic processes
- Placebo effects
- Also helps to determine the responses a drug elicits
Sources of Individual Variation- impt!
- Physiologic variables
- Age, gender, and weight
- Pathologic variables
- Diminished function of kidneys and liver
- Genetic variables
- Can alter the metabolism of drugs and predispose the patient to unique interactions
- Drug interactions
Question 1
A nurse is caring for a patient who has a bacterial infection. The healthcare provider has ordered an antimicrobial drug for the patient. The nurse understands that which of the following is the most important characteristic of this drug?
That the drug will kill the microorganism
That the drug will be administered orally
That the drug does not have any harmful effects
That the drug does not interact with other drugs
Answer: A
Rationale: The three most important characteristics that any drug can have are effectiveness, safety, and selectivity. Effectiveness is the most important property that a drug can have.
Evolution of Nursing Responsibilities Regarding Drugs- impt!
Right drug
Right patient
Right dose
Right time
Right route
Right assessment
Right documentation
Right evaluation
Right of patient to education
Right of patient to refuse care
The nurse must know:
- What medications are appropriate for the patient
- DRUG ALLERGIES
- What drugs are contraindicated for the patient
- The probable consequences of the interactions between the drug and the patient
- The nurse’s role as advocate
- Last line of defense for the patient
- It is ethically and legally unacceptable to administer a drug that is harmful to the patient, even though the medication has been prescribed by a licensed prescriber and dispensed by a licensed pharmacist
Steps for the Nurse: Preadministration Assessment
- Collecting baseline data
- Needed to evaluate therapeutic responses and adverse effects
- Identifying high-risk patients
- Liver and kidney impairment
- Genetic factors
- Drug allergies
- Pregnancy
- Older adult or pediatric age group
- Certain drugs have more than one indication
- The dosage may differ depending on the indication for which the drug is being used
- Many drugs can be administered by more than one route
- The dosage may differ depending on the route selected
- Certain intravenous agents can cause severe local injury if extravasation occurs
Extravasation
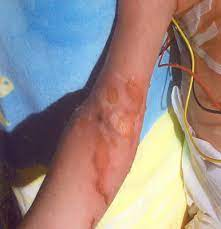
Dosage and Administration (Cont.)
- Read the medication order carefully
- Verify the identity of the patient
- Read the medication label carefully
- Verify dosage calculations
- Implement any special handling that the drug may require
- Do not administer any drug if you do not understand the reason for its use
Steps for the Nurse Evaluating and Promoting Therapeutic Effects
- Evaluating therapeutic responses
- What does this mean?????
- One of the most important aspects of drug therapy
- Must know the rationale for treatment and the nature and time course of the intended response
- Cannot effectively evaluate a drug with multiple applications if the intended use is not known
Steps for the Nurse Evaluating and Promoting Therapeutic Effects (Cont.)
- Promoting patient adherence
- Also known as compliance or concordance
- Extent to which a patient’s behavior coincides with medical advice
- Implementing nondrug measures
- Drug therapy can often be enhanced by nondrug measures
- These include biofeedback, emotional support, smoking cessation, sodium restriction, and so on
Steps for the Nurse Minimizing Adverse Effects
- All drugs have the potential to produce undesired effects
- Always know the following:
- The major adverse effects that the drug can produce
- The times when these reactions are likely to occur
- Early signs that an adverse reaction is developing
- Interventions that can minimize discomfort and harm
- Take a thorough drug history
- Why??? What might this uncover?
- Advise the patient to avoid over-the-counter drugs that can interact with the prescribed medication
- Monitor for adverse interactions that are known to occur
- Be alert for as-yet-unknown interactions
Making PRN Decisions
- PRN: pro re nata; means “as needed”
- The nurse has discretion regarding how much drug to give and when to give it (within order parameters)
- Know the reason for the drug’s use
- Be able to assess the patient’s medication needs
Managing Toxicity
- Early identification makes early intervention possible
- Know the early signs of toxicity
- Know the procedure for toxicity management
Evaluation
- Therapeutic responses
- Adverse drug reactions and interactions
- Adherence to the prescribed regimen
- Satisfaction with treatment
Question
When the nurse reviews a medication order, it is not clear what route should be used for administration. Which action by the nurse is best?
- Use a current drug reference resource to determine the administration route.
- Administer the drug via the oral route.
- Contact the pharmacist for clarification.
- Call the prescriber to verify the route.- impt!
Answer: D
Rationale: If the medication order is unclear, the nurse should verify it with the prescriber.
Question
The nurse administered 2 mg of morphine intravenously to a postoperative patient. In addition to the following Rights of Drug Administration, what responsibility does the nurse have as a patient advocate?
- To administer the drug as often as possible
- To minimize adverse effects by reducing the next dose of morphine
- To know the possible reactions to morphine
- To inform visitors that the patient has received morphine
Answer: C
Rationale: It is important for the nurse to know any possible reactions to the medication in advance.
Which Name to Use: Generic or Trade?
- The little problems with generic names
- More complicated than trade names
- The big problems with trade names
- Single drug can have multiple trade names
- U.S. drugs and drugs outside the United States may have different active ingredients
- Products with the same trade name may have different active ingredients
- For example, Kaopectate
Over-the-Counter Drugs
- Americans spend about $20 billion annually on over-the-counter (OTC) drugs
- OTC drugs account for 60% of all doses administered
- Forty percent of Americans take at least one OTC drug every 2 days
- Four times as many illnesses are treated by a consumer using an OTC drug as by a consumer visiting a physician
- For most illnesses (60% to 95%), initial therapy consists of self-care, including self-medication with an OTC drug
- Always ask if your patient is taking any OTC’s
A nurse is administering a drug that is categorized as Schedule IV. The nurse understands that this means the drug:
Has acceptable medical applications with low potential for abuse.
Is a controlled substance with no accepted medical use.
Is dangerous to administer to pregnant or breast-feeding patients.
Has the potential for serious and life-threatening adverse effects.
Question
Answer: A
Rationale: Categories from Schedules I to V are used for drugs that are considered to have the potential for abuse. The drugs with the highest potential for abuse are Schedule I drugs, and those with the lowest potential for abuse are Schedule V drugs.
You are the night nurse on a busy unit that does hip replacements.
Your client is an 86 year old female post op day 2, and you are due to give her next dose of metoprolol, a pill that lowers blood pressure. This is given every 8 hours. Please highlight the findings below that are significant to this task.
The client is asking for the bedpan as she needs to void
The current blood pressure is 167/98 (high)
Your client at just half of their food tray and is asking you to remove the rest
The client has multiple family members present in the room
The client states “I can’t swallow pills”
The client’s pain is manageable at 3/10
The blood pressure at the last check four hours ago was 169/99
Put it to use!
42
The client is asking for the bedpan as she needs to void Good! But not in your decision process
The current blood pressure is 167/98 (high) shows continued need of the medication, you may want to check what her norms have been in the past.
Your client at just half of their food tray and is asking you to remove the rest Not pertinent to giving this med.
The client has multiple family members present in the room Not part of your decision process, though you will want to maintain the client’s privacy and ask if it is OK to give her pills while they are there.
The client states “I can’t swallow pills” You may need to crush this pill if it is able to be crushed, this requires further research on the drug.
The client’s pain is manageable at 3/10 Good! Should not figure into giving this drug G
The blood pressure at the last check for hours ago was 169/99 Has this drug been effective? This may require further investigation and a note to the provider
Lecture #2
Pharmacokinetics
What the body does to the drug
Objectives - from this unit the student should be able to
Describe absorption, distribution, metabolism and excretion in relation to pharmacotherapeutics
Recall properties of cell membranes
pharmacoKINETICS
The study of drug movement throughout the body - how the body moves the drug
Pharmacokinetic processes:
Absorption
Distribution
Metabolism
Excretion
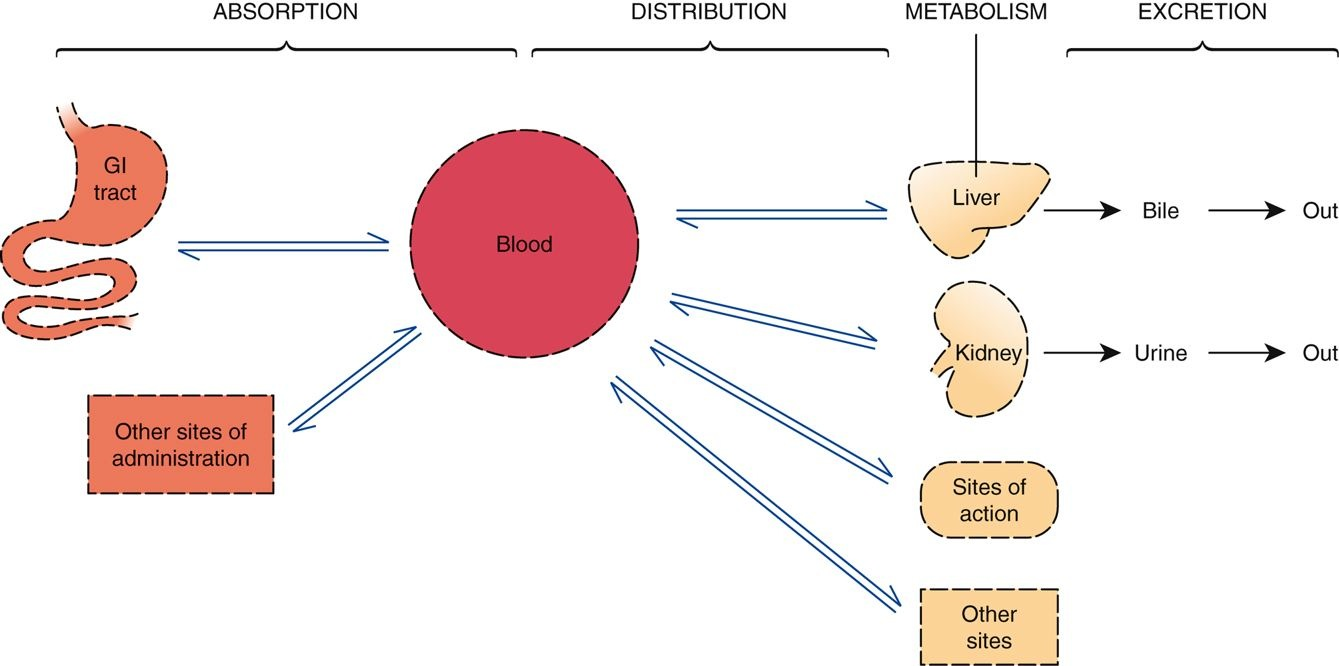
Passage of Drugs Across Membranes
6
Passage of Drugs Across Membranes
- An absolute requirement:
- ‘To move throughout the body, drugs must cross membranes.’
- Physiological Factors nurses should understand:
- Membrane structure
- Channels and pores
- Transport systems
- Direct penetration of the membrane
- For most drugs, movement throughout the body is dependent on the drug’s ability to penetrate membranes directly
- Most drugs are too large to pass through channels or pores
- Most drugs lack transport systems to help them cross all of the membranes that separate them from their sites of action, metabolism, and excretion
- A general rule in chemistry states that “like dissolves like”
- Cell membranes are composed primarily of lipids; therefore, to directly penetrate membranes, a drug must be lipid soluble (lipophilic)
ABSORPTION
- ‘The movement of a drug from its site of administration into the blood.’
- Rate of dissolution (e.g. drug formulation)
- Surface area
- Blood flow
- Lipid solubility
Route | Barriers to Absorption | Absorption Pattern | Advantages | Disadvantages |
|---|---|---|---|---|
PARENTARAL Intravenous (IV) | None | Instantaneous | Rapid onset Precise control over drug levels Permits use of large fluid volumes Permits use of irritant drugs | Irreversible Expensive Inconvenient Difficult Risk of fluid overload, infection, and embolism |
Intramuscular (IM) / Subcutaneous (SubQ) | Capillary wall | Rapid with water- soluble drugs Slow with poorly soluble drugs | Permits use of poorly soluble drugs Permits use of depot preparations | Possible discomfort Inconvenience Potential for Injury |
ENTERAL Oral (PO) | Epithelial lining of GI tract; capillary wall | Slow and variable | Easy Convenient Inexpensive Ideal for self-medication Potentially reversible | Variability Inactivation of some drugs by gastric acid and digestive enzymes Possible nausea and vomiting from local irritation Patient must be conscious and cooperative |
Additional Routes of Administration
- Topical
- Transdermal
- Inhaled
- Rectal
- Vaginal
- Direct injection to a specific site—for example, heart, joints, nerves, central nervous system
A nurse is preparing to administer epinephrine to a patient who is having a severe allergic reaction. Which route of administration should the nurse use to provide the fastest and most complete absorption of epinephrine?
- Intravenous
- Intramuscular
- Subcutaneous
- Oral
Answer: A
Rationale: Intravenous administration results in the fastest and most complete absorption of a drug.
DISTRIBUTION
- ‘The movement of drugs throughout the body’
- Blood flow to tissues
- Exiting the vascular system
- Typical capillary beds
- Blood-brain barrier
- Placental drug transfer
- Protein binding
- Entering Cells
Blood-Brain Barrier
- Tight junctions between the cells that comprise the walls of most capillaries in the central nervous system
- Drugs must be able to pass through the cells of the capillary wall
- Only drugs that are lipid soluble or that have a transport system can cross the blood-brain barrier to a significant degree
Protein Binding
- Drugs can form reversible bonds with various proteins
- Plasma albumin is the most abundant and important protein
- Large molecule that always remains in the bloodstream
- Affects drug distribution
METABOLISM
- ‘Biotransformation: The enzymatic alteration of drug structure’
- Liver: Hepatic Microsomal Enzyme System
- Lungs, Skin, Kidneys, Intestines
- Age affects rate of metabolism
- Pharmacogenetics
- Cytochrome P450
Hepatic Drug-Metabolizing Enzymes
- Most drug metabolism that takes place in the liver is performed by the hepatic microsomal enzyme system, which is also known as the P450 system
- Metabolism does not always result in a smaller molecule
Therapeutic consequences of Biotransformation
- Excretion
- Inactivation
- Increased effectiveness
- Activation of “prodrugs”
- Increased drug toxicity
The nurse identifies which patient as being at highest risk for slow drug metabolism?
A 2-year-old boy who is prescribed an oral antibiotic
A 14-year-old girl who takes four prescription drugs
A 56-year-old man who has chronic hepatic disease
A 76-year-old woman who has an elevated temperature
Answer: C
Rationale: Drug metabolism, which is also known as biotransformation, is the enzymatic alteration of drug structure. Most drug metabolism takes place in the liver.
First Pass Effect
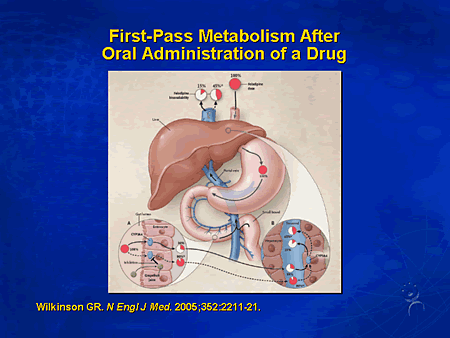
As you take medicine goes to stomach then to liver, so sometimes it can be less effective. You can bypass this by giving via different routes
EXCRETION
- ‘The removal of drugs from the body’
- Renal drug excretion
- Glomerular filtration
- Passive tubular reabsorption
- Active tubular secretion
- Renal drug excretion
- Nonrenal excretion routes
- Breast milk, bile, lungs, skin, GI
When preparing to administer a sustained-release (slowly released) capsule to a patient, the nurse understands that which of the following is true for sustained-release capsules?
- They are usually more costly than pills.
- They are rapidly absorbed.
- They need to be crushed for appropriate absorption to take place.
- They need to be taken at regular intervals throughout the day.
Answer: A
Rationale: A capsule may cost more than a pill. Sustained-release formulations are capsules filled with tiny spheres that contain the actual drug; the individual spheres have coatings that dissolve at variable rates. Because some spheres dissolve more slowly than others, drug is released steadily throughout the day. These capsules should not be crushed. The primary advantage of sustained-release preparations is that they permit a reduction in the number of daily doses. These formulations have the additional advantage of producing relatively steady drug levels over an extended time (much like giving a drug by infusion). The major disadvantages of sustained-release formulations are their high cost and their potential for variable absorption.
Lecture 3
Pharmacodynamics
- What the drug do to the body
Objectives
- Describe the major pharmacodynamic processes in the body, including
Therapeutic range
Therapeutic index
Half life
Plateau
Drug/Receptor relationships
Understand the importance of drug interactions and ways they occur
pharmacoDYNAMICS
The study of what drugs do to the body and how they do it
Pharmacodynamics
- Study of the biochemical & physiological effects of drugs
- Forms the basis for understanding:
- How we control the effects of medications
- The Dose-response relationship
To control the Effects of Drugs… We control the Dose and Frequency
- Plasma Drug Levels
- Minimum Effective Concentration
- Toxic Concentration
- Therapeutic Range
- Half-life
- Plateau
- Loading dose
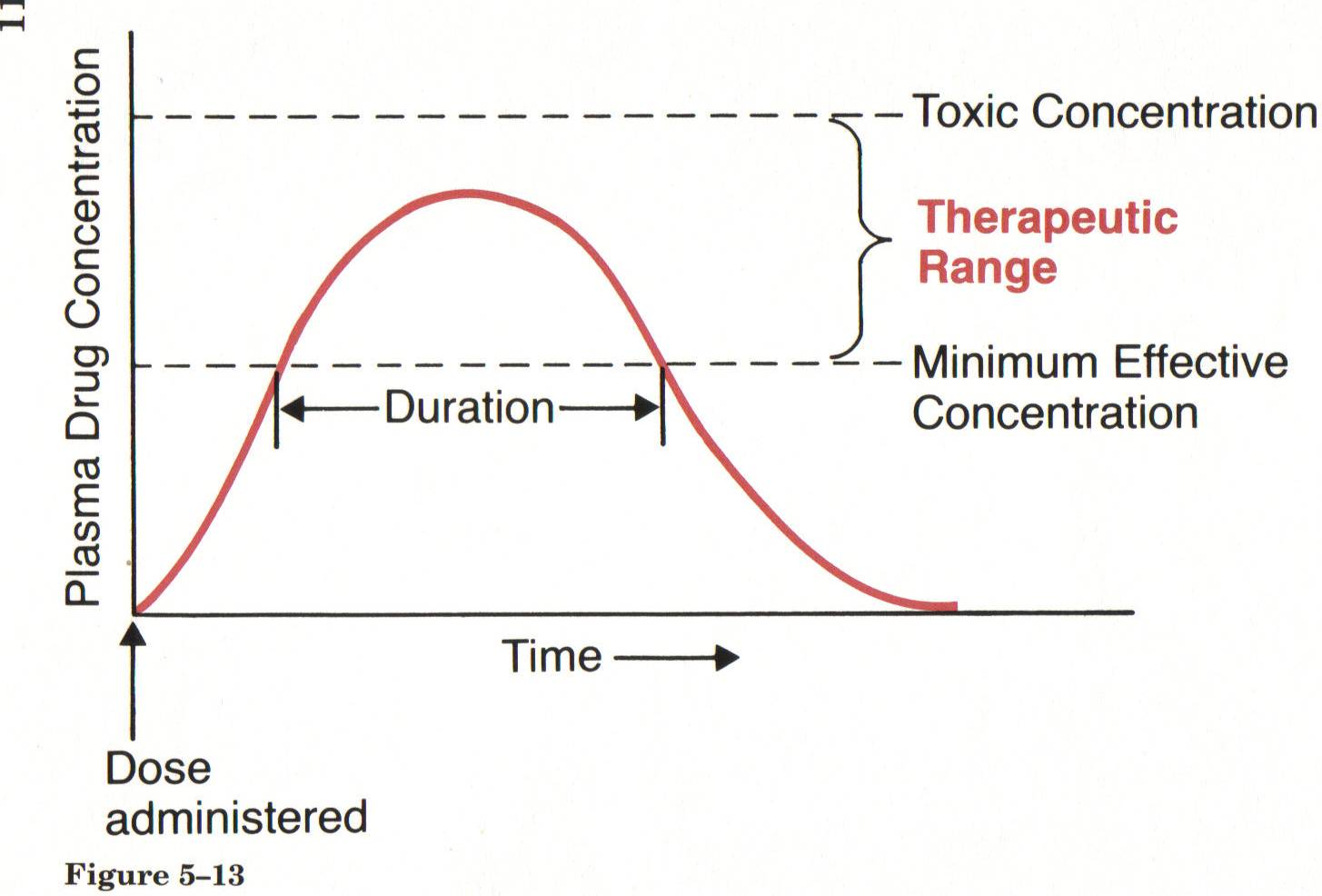
- Plasma Drug Levels:
- These refer to the concentration of a drug in the bloodstream at a particular time. Monitoring plasma drug levels is crucial to ensure that the drug remains within the therapeutic range.
- Minimum Effective Concentration (MEC):
- This is the lowest concentration of a drug in the bloodstream required to produce the desired therapeutic effect. Below this concentration, the drug may not be effective.
- Toxic Concentration:
- The toxic concentration is the level of drug in the bloodstream at which adverse effects or toxicity may occur. It is important to avoid concentrations above this threshold to prevent harm to the patient.
- Therapeutic Range:
- Also known as the therapeutic window, this is the range of drug concentrations in the bloodstream that is effective for treating a particular condition without causing significant adverse effects. It is the range between the minimum effective concentration and the toxic concentration.
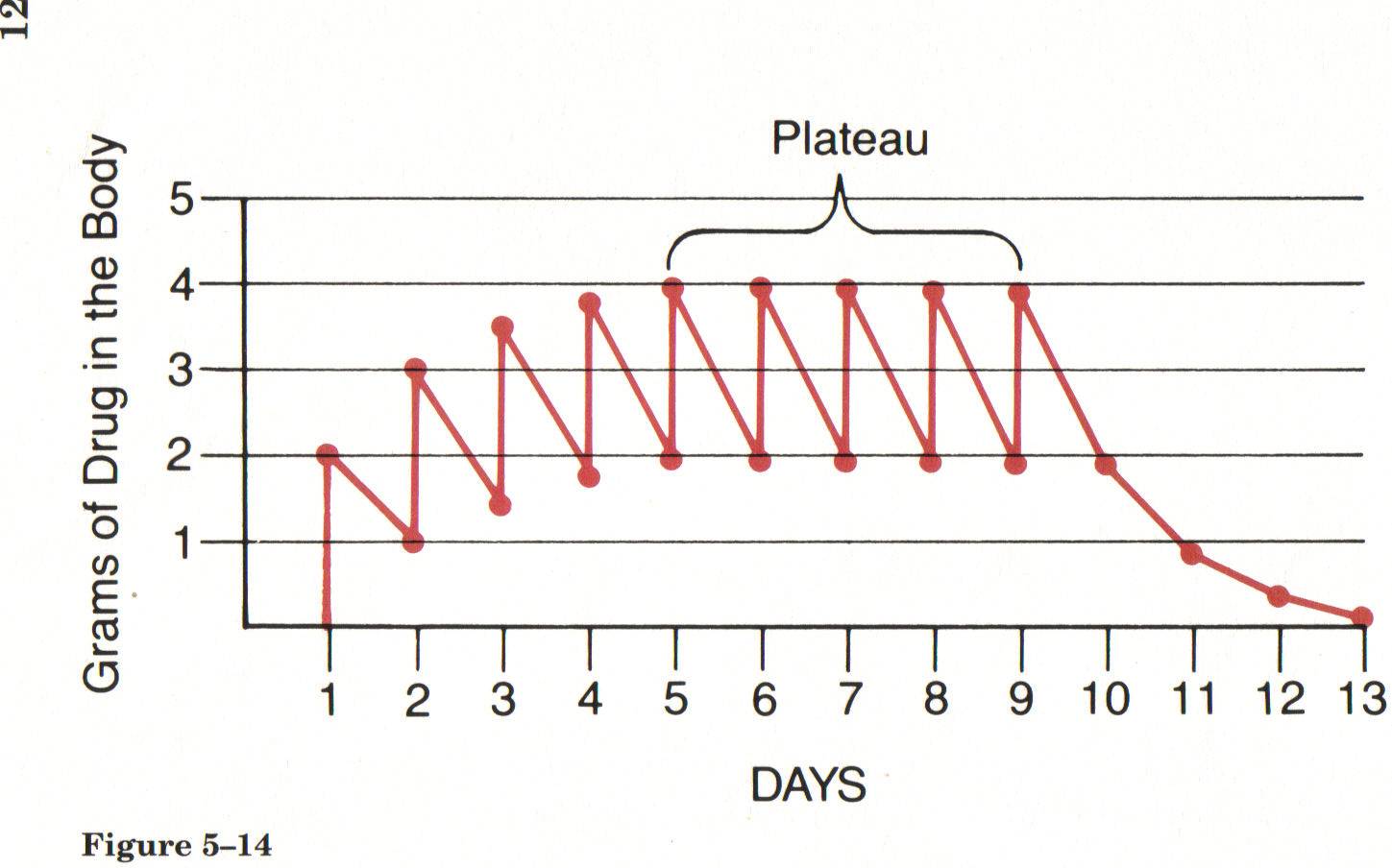
- Half-life:
- The half-life of a drug is the time it takes for the concentration of the drug in the bloodstream to be reduced by half. It helps determine the frequency of drug dosing and how long the drug remains in the body.
- Plateau:
- In the context of drug administration, the plateau refers to the point at which the amount of drug administered is equal to the amount eliminated, resulting in a constant concentration in the bloodstream. This occurs during continuous dosing or when a steady state is reached.
- Loading Dose:
- A loading dose is a higher initial dose of a drug given to achieve a therapeutic concentration quickly. It is often used when a drug has a long half-life, and waiting for the drug to reach a steady state through regular dosing would take too long.
Single-dose time course
Half Life
The half-life of a drug is the time it takes for the plasma concentration of a drug in your body to reduce by half. This depends on how the body processes and gets rid of the drug. It can vary from a few hours to a few days, or sometimes weeks.
Drug accumulation with repeated administration
Note: It takes 4-5 ‘half-lives’ to reach plateau and 4-5 ‘half-lives’ for drug to be completely eliminated - in this example the half life is one day.
(also called Steady State)
The Dose-Response Relationship
- “Graded” not “all-or-nothing”
- Maximal Efficacy
- Relative Potency
Drug-Receptor Interactions (e.g. Mechanism of Action)
- Drug+Receptor = drug-receptor complex 🡪 response
- Binding of drugs to receptors is usually reversible
- Receptors
- Drug actions at the receptor:
- Agonist: goes to the receptor to turn it on to: START a Response
- Antagonist: goes to the receptor and binds with it:
- Partial agonist: binds to the receptor, weak response
- Selectivity of drugs to certain receptors
Which statement about drug agonists does the nurse identify as being true?
- An agonist makes physiologic processes go faster.
- An agonist exerts effects by causing receptor activation.
- An agonist has moderate intrinsic activity.
- An agonist is a dynamic component.
Answer: B
Rationale: It is important to note that agonists do not necessarily make physiologic processes go faster; receptor activation by these compounds can also make a process go slower. Receptors are dynamic components of the cell. A partial agonist is an agonist that has moderate intrinsic activity.
Patient Variability
- Initial dose is an approximation that needs fine tuning
- Nursing Role: ASSESS THE PATIENT TO DECIDE if there is an EFFECTIVE RESPONSE!
- Actual responses should be documented in such a way that prescribers know the response
- Start at the lowest dose and work your way up
Drug-Drug Interactions
- Interactions can occur whenever a patient takes more than one drug
- Some interactions are intended and desired or unintended and undesired
- Patients frequently take more than one drug
- Multiple drugs to treat one disorder
- Multiple disorders requiring different drugs
- Over-the-counter medications, caffeine, nicotine, alcohol, and so on
Consequences of Drug-Drug Interactions
- Intensification of effects
- Increased therapeutic effects
- Increased adverse effects
- Reduction of effects
- Reduced therapeutic effects
- Reduced adverse effects
- Creation of a unique response
Basic Mechanisms of Drug-Drug Interactions
- Drugs can interact through four basic mechanisms:
- Direct chemical or physical interaction
- IV’s can precipitate( drugs did not combine well… on next page)
- Pharmacokinetic interaction- what the body does to the drug
- An elevated gastric pH can change absorption
- Pharmacodynamic interaction- what the drug does to the body
- Drugs may compete for receptor sites
- Combined toxicity
Example of IV precipitate
*Usually caused by drug incompatibility

Drug-Food Interactions
- Drug metabolism
- The grapefruit juice effect (not occurring with other citrus fruits or juices)
- Inhibits the metabolism of certain drugs
- Raises the drugs’ blood levels
- Increase in felodipine
- Others: Lovastatin, cyclosporine, midazolam, and so on
Impact of food on:
- Drug toxicity
- Monoamine oxidase inhibitors (MAOIs) and tyramine-containing foods
- Theophylline and caffeine
- Potassium-sparing diuretics and salt substitutes
- Aluminum-containing antacids and citrus beverages
What is considered an empty stomach?
- 1 hour before meals or 2 hours after eating
Drug-Herb Interactions
- Conventional drugs can interact with herbal preparations
- Interactions with herbal medicines are just as likely as they are with prescription medications
- Reliable information about drug-herb interactions is lacking
- Example of known interaction:
St. John’s wort induces drug-metabolizing enzymes and reduces the blood levels of many drugs, decrease the reliability of bc
A patient is taking two prescription medications that both cause bradycardia. The nurse should monitor the patient for which type of effect?
- An increased functional effect
- An increased adverse effect
- A reduced therapeutic effect
- A reduced adverse effect
Answer: B
Rationale: Both of the drugs have an adverse effect of bradycardia.
A patient is prescribed a medication to be taken on an empty stomach. Which statement should the nurse include when providing patient teaching?
- “Take the medication 1 hour before eating.”
- “Take the medication with a small glass of water.”
- “Take the medication before going to bed at night.”
- “Take the medication 1 hour after a meal.”
Question
23
Answer: A
Rationale: To administer a drug on an empty stomach means to administer it at least 1 hour before or 2 hours after a meal.
Impt to know voab, half life, Drug drug interactions that are common.
Lecture #4
Drug Calculations and their Importance in Medication Administration
Rounding
- Do NOT round unless told to in the problem
- When rounding, only round at the END of the problem
- How do you round up? Down? 4 or less down 5 and up up
CALCULATIONS!!!!!
First some rules:
Use the correct abbreviations
kg | kilogram |
|---|---|
mg | milligram |
mL | milliliter |
mcg | microgram |
Calculations - rules
Always use a leading zero for numbers < 1
YES - 0.6 NOT .6
Never use a trailing zero
YES - 3 NOT 3.0
Use a comma
YES 1,000 NOT 1000
Calculations - Conversions
What do the prefixes mean????
- kg = 1,000
- milli = 0.001 (or 1/1,000th)
- micro = .000001 (or 1/1,000,000th)
Calculations - conversions
Liquids | Solids |
|---|---|
1 ml = 1 cm cubed - also called a “cc” | 1,000 mcg = 1 mg |
1,000 ml = 1 L (liter) | 1,000 mg = 1 g |
1,000 g = 1 kg |
Calculations – more conversions
1 drop | 0.06 ml |
|---|---|
1 tsp | 5 ml |
1 T | 15 ml |
1 oz | 30 ml |
1 cup | 240 ml |
1 lb | 454 g |
2.2 lb | 1 kg |
Do not recommend the use of spoons to patients-lots of variation in size. Use container provided with medication to administer.
** Why do we never use spoons???? HUGE variation in measure – from 3.5 ml to 7 ml
***Which conversion will you use all the time in calculations?? (pounds to kg)
Dimensional Analysis
- Look at labels first!
- What label do you need for the answer – what will you be administering?
- Line up the labels.
- Complete the calculations.
- Answer flows naturally.
Example Order:
Administer 300 milligrams (mg) of Drug A every 4 hours.
The Drug A bottle reads: 600 milligrams (mg) of Drug A per milliliter (ml).
How many ml in one day?
0.5ml
Morphine
- The order is to give Morphine 1mg/10kg
- The patient weighs 63 kg
- Morphine is supplied as 10mg/ml
- What are you solving for? mg? ml? kg?
How much will you give?
1mg
10 kg 63 kg= .63
ml=1ml 1mg 63kg
—- x —----- x —--- =.63 ml
10 mg 10 kg 1 person
Vasotec
The order is to give Vasotec 3mg now
Supplied as 5mg in 1ml
The drug book indicates the need to dilute 5mg to 10ml using NSS
How many ml’s of the diluted Vasotec do you give?
Tylenol
Recommendations for Tylenol are 10mg/kg per dose.
How much should a child who weighs 46 lbs. receive?
10 mg 20.9 kg
—---- x —----- =209 mg
Kg 1
Lasix
A child who weighs 38 lbs is to receive Lasix. Recommendations for Lasix is 3mg/kg. Calculate an appropriate dose for this child.
3 mg 1kg 38lb 114
mg= —---- x —----- x —---= —-------- 51.81
1kg 2.2 lb 1 2.2
Keflin IV
A child who weighs 20 lbs is to receive Keflin IV mixed in 50 ml normal saline. Recommendations for Keflin are 100mg/kg/day in 4 divided doses. How many milligrams of Keflin should this child receive in one dose? (round to the nearest 10th)
100mg 1kg 20lb 2000
mg= ----- x —--- x —---- = —----= 909.09mg/4 =227.27
1kg 2.2 lb 1 2.2
Drip Rates= gtts/min
Calculate the IV drip rate for 1200 mL of NS to be infused in 6 hours. The infusion set is calibrated for a drop factor of 15 gtts/mL.
Gtts 15 gtts 1200ml
—---- = —----- x -------
Min 1 ml 6hr
Calculate the IV flow rate for 1200 mL of NS to be infused in 6 hours. The infusion set is calibrated for a drop factor of 15 gtts/mL.
Dimensional Analysis
We want gtts/min so:
gtts 15 gtts 1200 ml 1 hr 18,000
_________ = ______________ x _________________ x ____________ = ________________ =
min 1 ml 6 hr 60 min 360
50 gtt/min
Now you try……
Calculate the IV drip rate for 200 mL of 0.9% NaCl IV over 120 minutes. Infusion set has drop factor of 20 gtts/mL.
33 gtts/min
Gtts 20gtts 200ml 4000
—--- —----- x —------- = —------ = 33.333= 33 gtts/min
Min 1ml 120 min 120
Common Calculation Errors!!
Confusing dosage with volume
Conversion errors
Decimal points
Lack of common sense
Distractors (0.9% saline, HR 90, Rm 250)
Don’t make these errors!!!!!
Lecture #5
Drug Safety/Neuropharm Intro
-Drug Safety
-Intro to Neuropharm
Drug Calculation Practice
Order: KCL 25 meq PO daily
Available: KCL 40 meq/15 ml
How many ml will the nurse administer per dose?
Order: D5W 500 ml to infuse over 6 hrs
Available: D5W 500 ml bag
15 gtt/ml administration set
How many gtts/min will the nurse regulate the IV?
Answers:
Order: KCL 25 meq PO daily
Available: KCL 40 meq/15 ml
How many ml will the nurse administer per dose?
ml = 15 ml x 25 meq = 375 = 9.375 =9.4ml/dose
dose 40meq dose 40
Order: D5W 500 ml to infuse over 6 hrs
Available: D5W 500 ml bag
15 gtt/ml administration set
How many gtts/min will the nurse regulate the IV?
gtts = 500ml x 15 gtt x 1 hour = 7500 = 20.8333 =21 gtt/min
min 6 hours 1ml 60 min 360
3
Objectives - following this class students should be able to:
- Perform nursing drug calculations safely and accurately
- Describe common sources of medication errors
- Document safely and correctly - avoiding common documentation errors
Objectives - following this lecture students should be able to:
- Describe principles of individual variation in drug responses
- Describe adverse and side effects and note the difference between the two
- Recall the functions of the peripheral nervous system
- Sympathetic response
- Parasympathetic response
- Identify cholinergic and adrenergic receptors in the body
Two Issues Related to Drug Safety
- Adverse drug reactions (ADRs)
- Also known as adverse drug events (ADEs)
- Medication errors
Medication Errors
- Major cause of morbidity and mortality
- Documented in two landmark reports from the Institute of Medicine:
- To Err Is Human, 1999
- Preventing Medication Errors, 2006
- It is estimated that medication errors:
- Injure 1.5 million people per year
- Kill 7,000 people per year
What Is a Medication Error and Who Makes Them?
- The risk for error in hospitals is high because each medication order is processed by several people
- The nurse is the last person in this sequence
- Thus the nurse is the last line of defense against mistakes
- This places a heavy responsibility on the nurse to ensure patient safety
What kinds of errors can be made? (Think rights…..)
What Is a Medication Error
and Who Makes Them?
Causes of Medication Errors
- Of the human factors that can cause errors, performance deficits are the most common, followed by knowledge deficits and the miscalculation of dosage
- 90% of all errors are due to:
- Human factors
- Communication mistakes
- Drug name confusion
Ways to Reduce Medication Errors
- Help and encourage patients and their families to be active and informed members of the healthcare team
- Create an institutional culture that is dedicated to safety
- Give healthcare providers the tools and information they need to prescribe, dispense, and administer drugs as safely as possible
- Institute safety checklists for high-alert drugs Institute for Safe Medication Practices High-Alert Medications.
- About 20 drugs cause 80% of medication error–related deaths
- Replace handwritten medication orders with a computerized order entry system
- Have a senior clinical pharmacist accompany physicians on rounds
- Use a barcode system
- Do not use error-prone abbreviations
- Institute for Safe Medication Practices
- Perform medication reconciliation
Individual Variation in Drug Responses
- Key factors that cause one patient to respond to drugs differently than another patient
- Important for nurses to know these factors to be better prepared to reduce individual variation in drug responses
- Body weight and composition
- Body surface area versus weight
- Age
- Significant variability with age
- Infants and older patients especially sensitive to drugs
- Infants: Organ immaturity
- Older patients: Organ degeneration
- Due to increased severity of illness, multiple pathologies, treatment with multiple drugs
- Pathophysiology
- Kidney disease
- Reduced excretion and increased toxicity
- Liver disease
- Reduced metabolism and increased toxicity
- Acid-base imbalance
- pH changes that alter absorption, distribution, metabolism, and excretion of drugs
- Altered electrolyte status
- Rare for electrolyte changes to have a significant impact on drug responses
- Tolerance
- Decreased responsiveness to a drug as a result of repeated drug administration
- Higher doses required
Three Types of Drug Tolerance
- Pharmacodynamic tolerance
- Associated with long-term administration of drugs such as morphine and heroin
- Metabolic tolerance
- Results from accelerated drug metabolism
- Tachyphylaxis
- Reduction in drug responsiveness brought on by repeated dosing over a short time - a rapid response, ex. Afrin if you keep using it loses its effect and makes it worse
Placebo Effect
- Any response that a patient has to a placebo is based solely on his or her psychological reaction to the idea of taking a medication and not to any direct physiologic or biochemical action of the placebo itself
- Nurses need to present a positive but realistic assessment of the effects of therapy
- Placebos are primarily used for the control groups in clinical trials
Terms Related to Adverse Drug Reactions
- Side effect
- Toxicity
- Allergic reaction
- Idiosyncratic effect- we do not know what caused it
- Paradoxical effect - benodryal makes kids sleepy but sometimes it can make u hype
- Iatrogenic disease - thing in medical field causes a problem
- Physical dependence
- Carcinogenic effect
- Teratogenic effect - fetus related
Identifying Adverse Drug Reactions
- Can be very difficult to determine whether a specific drug is responsible for an observed adverse event
- Other factors to consider:
- Underlying illness
- Other drugs
Boxed Warnings- for the test
- Also known as black box warnings
- Strongest safety warning a drug can carry and still remain on the market
- Purpose of this warning is to alert prescribers to:
- Potentially severe side effects/adverse effects (eg, life-threatening dysrhythmias, suicidality, major fetal harm)
- Ways to prevent or reduce harm (eg, avoiding a teratogenic drug during pregnancy)
- The most dangerous that you can still give to pt, like sertraline has black box warning for suicide
- Impt things to know: what's going to cause pronblems
- What can you do as a nurse to prevent those from happen
Neuropharmacologic Agents
- Peripheral Nervous System drugs (our focus)
- Central Nervous System drugs
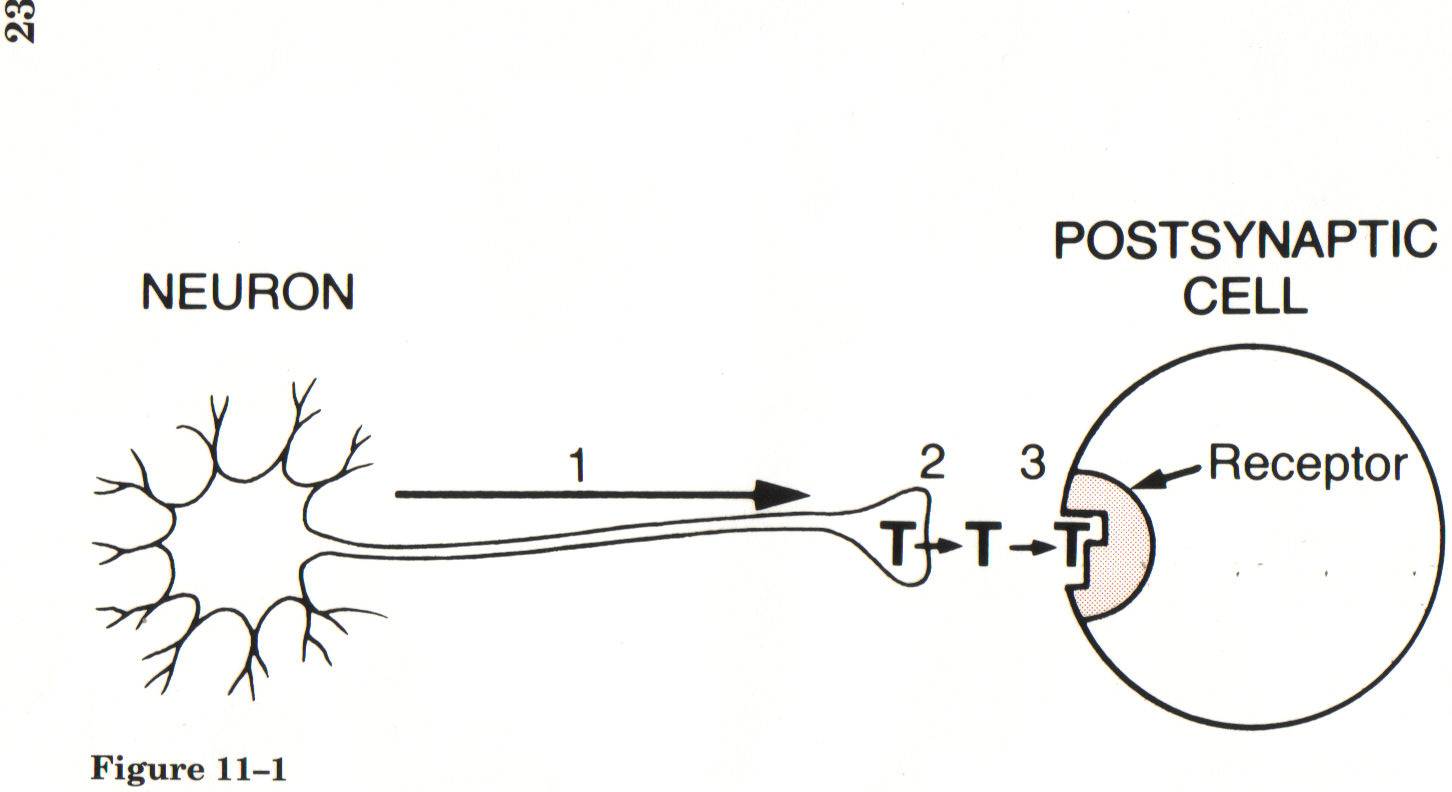
How neurons regulate physiological processes…
- Conduction of an action potential along the axon of the neuron
- Release of neurotransmitter from the axon terminal and
- Binding of transmitter molecules to receptors on the postsynaptic cell
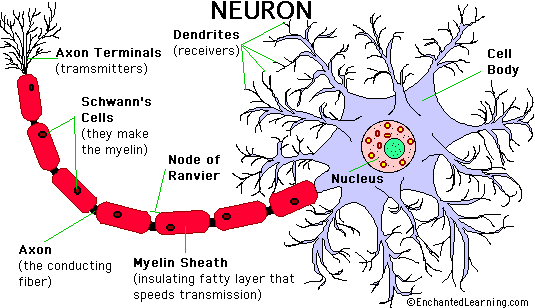
Sites of Action
- Axonal conduction
- Synaptic transmission (Primary site of drug action)
- Transmitter synthesis
- Transmitter storage
- Transmitter release
- Receptor binding
- Termination of transmission
- Receptors
Synaptic Transmission
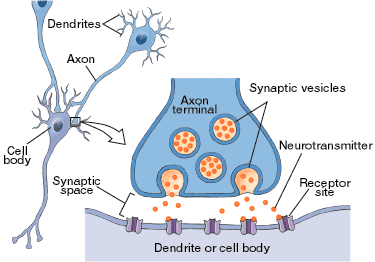
An approach to learning about PNS drugs
- First, know the types of receptors through which the PNS works to influence the function of a specific organ
- Second, know what the normal response to activation of those receptors is
- Third, know what receptor the drug acts upon and its effect upon receptor function (agonist vs. antagonist) ( binds with receptor and second does not allow a )
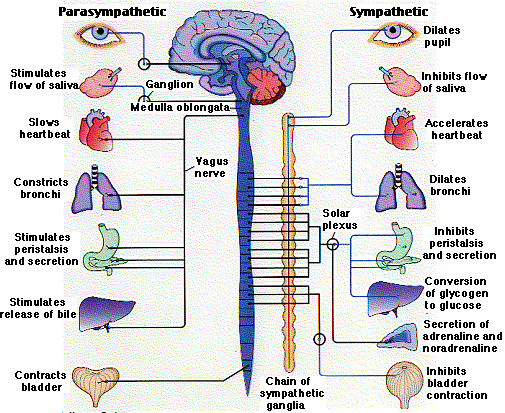
Divisions of the Peripheral NS
- Somatic motor system – controls movement of voluntary muscles
- Autonomic nervous system – regulates heart, secretory glands, smooth muscles (involuntary activities)
- Parasympathetic nervous system
- Sympathetic nervous system
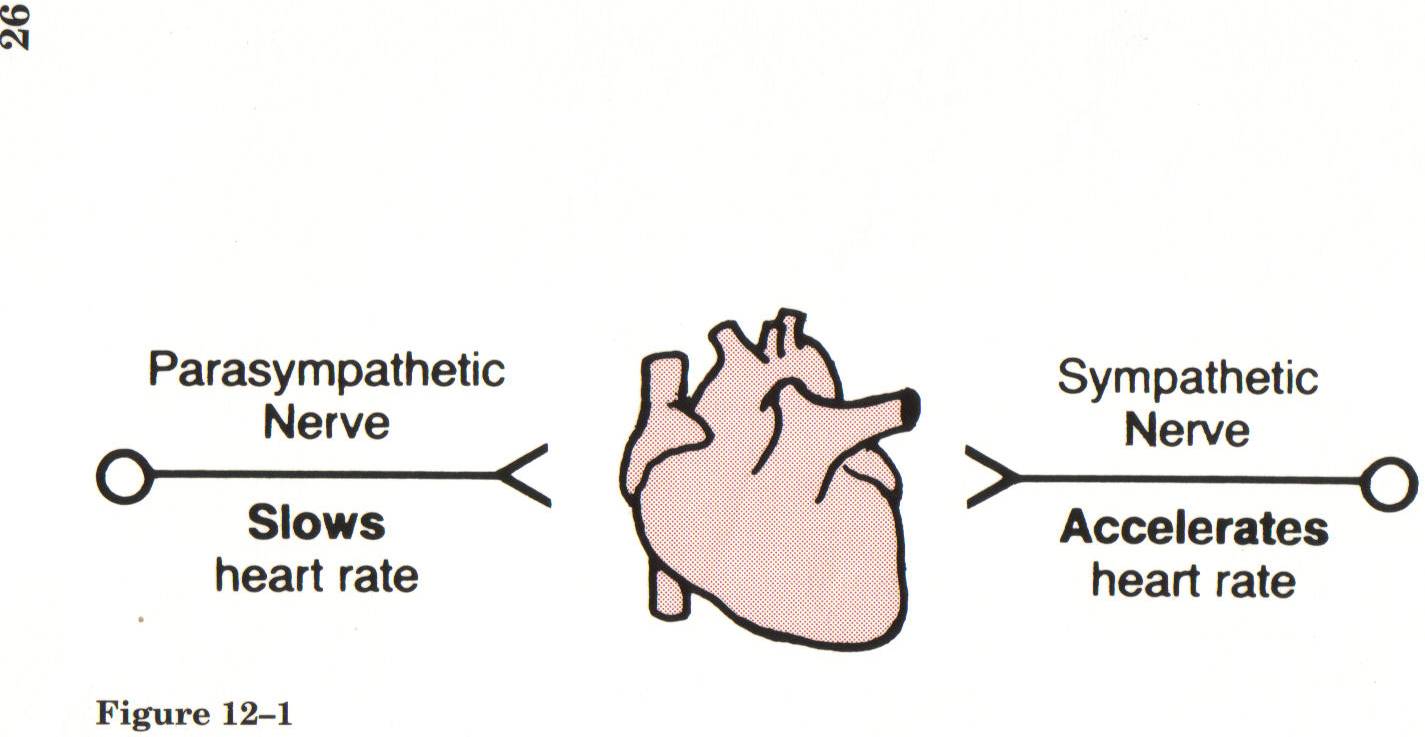
Principal Functions of the Autonomic NS
- Parasympathetic-”rest and digest”
- Slowing of heart rate
- Increased gastric secretions
- Emptying of bladder
- Emptying of bowel
- Focusing of eye for near vision
- Constriction of pupil
- Constriction of bronchial smooth muscle
- Sympathetic “fight or flight”
- Regulation of cardiovascular system
- Regulation of body temperature
- Implementation of “fight-or-flight” reaction:
- Increased heart rate & BP
- Bronchodilation
- Vasodilation in skeletal muscle and heart muscle
- Vasoconstriction in superficial capillaries
- Decreased gastric secretions and motility
- Pupil Dilation
Opposing effects of parasympathetic and sympathetic nerves
Neurotransmitters of the Peripheral NS
- Acetylcholine
- Norepinephrine
- Epinephrine
- Dopamine
Know that the peripheral nervous system needs neurotransmitters and the receptors help control the netureogenic
Receptors of the Peripheral NS -know test, understand which are which… cholernergic have three and Adrenergic have 4
- Cholinergic receptors
- Nicotinic n
- Nicotinic m
- Muscarinic - most cholinergic drugs work here, and activate parasympathetic system
- Adrenergic receptors - activate sympathetic system
- Alpha 1
- Alpha 2
- Beta 1
- Beta 2
Understand difference between between parasympathetic and sympathetic why that's important and how affects medicine
Hematology Lecture 1 and 2
Objectives
- Know the function and normal lab values for the various blood cells
- Understand how anemia happens, and the manifestations
- Differentiate the major types of anemia
- List treatment and patient teaching for anemia
- Understand treatment of medications used to treat various low blood counts
- Describe hemophilia - particularly the most common form.
Blood Types - know for the test

a
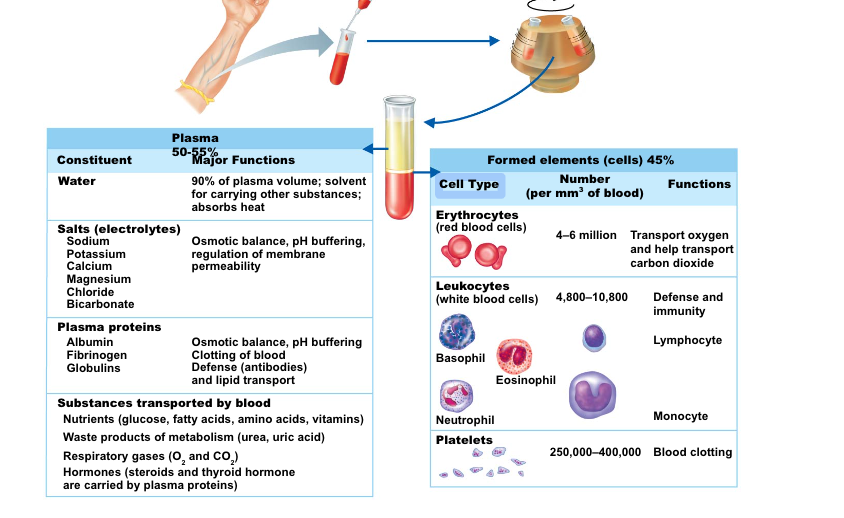
Blood Plasma
- Composed of approximately 90 percent water—liquid portion of blood
- Includes many dissolved substances:
- Nutrients
- Salts (electrolytes)
- Respiratory gases
- Hormones
- Plasma proteins: albumin, antibodies, clotting proteins
- Waste products
Formed Elements
- Erythrocytes
- Red blood cells (RBCs)
- Leukocytes
- White blood cells (WBCs)
- Platelets
- Cell fragments
Formed Elements
- Erythrocytes (red blood cells, or RBCs)
- Main function
- Transport oxygen (O2) from lungs to systemic tissues
- Carry carbon dioxide from the tissues to the lungs
- Main function
- Anatomy of circulating erythrocytes
- Biconcave disks
- Essentially bags of hemoglobin
- No nucleus
- 4-6 million RBCs per cubic millimeter of blood is the normal count
RBC formed elements: labs to look at health of RBC
- Hemoglobin (Hg, Hgb)
- Contained in erythrocytes
- Iron-containing protein
- Binds strongly, but reversibly, to oxygen
- Normal blood contains 12–18 g of hemoglobin per 100 mL of blood
- Men are a bit higher than women
- Hematocrit: (Hct) ratio of RBCs to plasma ~ 39 to 50% is normal (males on the higher end)
Formed Elements (Cont.)
- Leukocytes (white blood cells, WBCs)
- Defend the body against infection, and remove debris
- Normal level: 5,000 to 10,000 cells/mm3
- Granulocytes: Phagocytes
- Contain many granules in cytoplasm
- Neutrophils, basophils, and eosinophils
- Agranulocytes
- Relatively few granules are contained in cytoplasm
- Monocytes and macrophages: Phagocytes
- Lymphocytes: Immunocytes
Formed Elements (Cont.)
Platelets
- Small cell fragments
- Needed for the clotting process
- Platelet count ranges from 150,000 to 400,000 per cubic mm of blood
- 300,000 is considered a normal number of platelets per cubic millimeter of blood
Formation of Red Blood Cells
- Since RBCs are anucleate (have no nucleus), they are unable to multiply or grow
- RBCs wear out in 100 to 120 days
- When worn out, RBCs are eliminated by phagocytes in the spleen or liver
- Hematopoiesis is the process of blood cell formation
- Occurs in red bone marrow
Control of Erythrocyte Production
- Rate of RBC production is controlled by a hormone called erythropoietin
- Kidneys produce most erythropoietin as a response to reduced oxygen levels in the blood
- RBC production is in equilibrium with RBC destruction and loss
- If someone has kidney disease they have problems with erythropoetin makin more problems with RBC
Anemia
- A deficiency in the quantity or quality
- Number of erythrocytes (RBCs)
- Quantity of hemoglobin
- Volume of packed RBCs (hematocrit)
Classification of Anemia- what causes it?
- Decreased RBC production
- Decreased hemoglobin synthesis
- Defective DNA synthesis
- Decreased number of RBC precursors
- Blood loss
- Acute
- Chronic
- Increased RBC destruction
- Hereditary
- Acquired
Pt is anemic, short of breath, faint feeling, fatigued, bc RBC carry oxygen and if they have lack of oxygen.
Anemia
- Not a specific disease
- Manifestation of a pathologic process
- Classified by laboratory review of
- Complete blood count (CBC)
- Reticulocyte count
- Peripheral blood smear
Once anemia is identified, further investigation is done to determine its specific cause.
Anemia can result from primary hematologic problems or can develop as a secondary consequence of diseases/disorders of other body systems.
Causes of Anemia
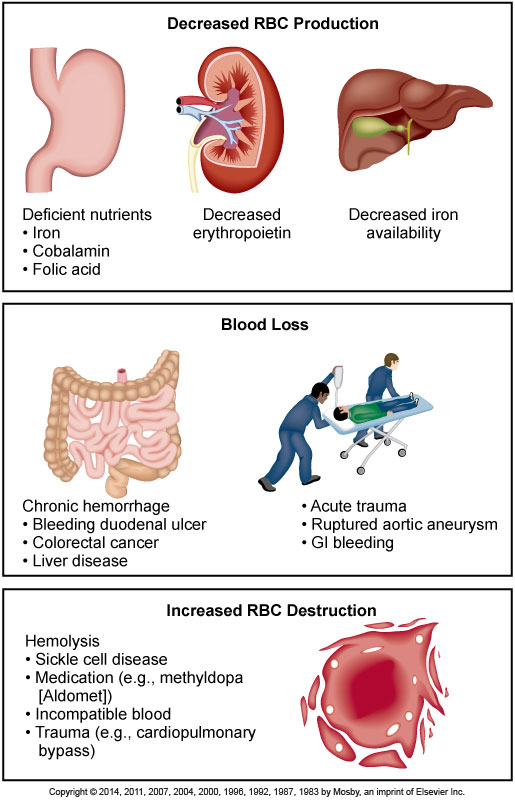
It is a prevalent condition with many diverse causes such as blood loss, impaired production of erythrocytes, or increased destruction of erythrocytes.
Anemia - Clinical Manifestations
- Caused by the body’s response to tissue hypoxia
- Manifestations vary based on rate of development, severity of anemia, presence of co-existing disease.
- Hemoglobin (Hgb) levels are used to determine the severity of anemia.
- Manifestations: signs and symptoms
- Based on body system
Mild states of anemia (Hgb 10 to 12 g/dL [100 to 120 g/L]) may exist without causing symptoms. If symptoms develop, it is because the patient has an underlying disease or is experiencing a compensatory response to heavy exercise. Symptoms include palpitations, dyspnea, and mild fatigue.
In moderate anemia (Hgb 6 to 10 g/dL [60 to 100 g/L]), cardiopulmonary symptoms are increased. The patient may experience them while resting, as well as with activity.
In severe anemia (Hgb less than 6 g/dL [60 g/L]), the patient has many clinical manifestations involving multiple body systems (See Table 31-3).
Anemia
Integumentary Manifestations
- Pallor
- ↓ Hemoglobin
- ↓ Blood flow to the skin
- Jaundice
- ↑ Concentration of serum bilirubin
- Pruritus
- ↑ Serum and skin bile salt concentrations
In addition to the skin, the sclerae of the eyes and mucous membranes should be evaluated for jaundice because they reflect the integumentary changes more accurately, especially in a dark-skinned individual.
Anemia
Cardiopulmonary Manifestations
- Result from additional attempts by heart and lungs to provide adequate O2 to the tissues
- Cardiac output maintained by increasing the heart rate and stroke volume
Low viscosity of the blood contributes to development of systolic murmurs and bruits.
In extreme cases, or when concomitant heart disease is present, angina pectoris and myocardial infarction (MI) may occur if myocardial O2 needs cannot be met.
Heart failure (HF), cardiomegaly, pulmonary and systemic congestion, ascites, and peripheral edema may develop if the heart is overworked for an extended period of time.
Anemia
Gerontologic Considerations
- Common in older adults
- Chronic disease
- Nutritional deficiencies
- Signs and symptoms may go unrecognized or may be mistaken for normal aging changes.
In healthy older men, a modest decline in hemoglobin of about 1 g/dL occurs between ages 70 and 88 years, in part because of the decreased production of testosterone. Only a minimal decrease in hemoglobin occurs between these ages in healthy women (about 0.2 g/dL).
Among older adults with anemia:
About one third have a nutritional type of anemia (e.g., iron, folate, cobalamin).
About another third have renal insufficiency and/or chronic inflammation.
The other third havconfusion, ataxia, fatigue, worsening angina, and heart failure.
Anemia Caused by Decreased RBC Production
- Iron-deficiency
- Anemia of chronic illness
- Aplastic anemia
Decreased hemoglobin synthesis may lead to iron-deficiency anemia, thalassemia, and sideroblastic anemia.
Defective DNA synthesis in RBCs (e.g., cobalamin [vitamin deficiency, folic acid deficiency) may lead to megaloblastic anemias.
Diminished availability of erythrocyte precursors may result in aplastic anemia and anemia of chronic disease.
Iron-Deficiency Anemia
- Iron-deficiency anemia
- Most common type of anemia worldwide
- At-risk groups
- Premenopausal women
- Pregnant women
- Persons from low socioeconomic backgrounds
- Older adults
- Individuals experiencing blood loss
- Associated with cognitive impairment in children
Is found in 2-5% of adult men and postmenopausal women in developed countries.
Those most susceptible to iron-deficiency anemia are the very young, those on poor diets, and women in their reproductive years.
Normally, 1 mg of iron is lost daily through feces, sweat, and urine.
Iron-Deficiency Anemia
Etiology
- Inadequate dietary intake
- 5% to 10% of ingested iron is absorbed.
- Malabsorption
- Iron absorption occurs in the duodenum.
Diseases or surgery that alter, destroy, or remove the absorption surface of this area of the intestine cause anemia.
Table 31-5 lists nutrients needed for erythropoiesis.
Dietary iron is adequate to meet the needs of men and older women, but it may be inadequate for those individuals who have higher iron needs such as menstruating or pregnant women.
As iron absorption occurs in the duodenum, malabsorption syndromes may involve diseases of the duodenum in which the absorption surface is altered or destroyed, or after certain types of GI surgery where the removal or bypass of the duodenum occurs.
APLASTIC ANEMIA IS WHEN YOU HAVE RED AND WHITE BLOOD CELLS AND PLATELETS!!!- on test
Iron-Deficiency Anemia
Etiology
- Blood loss
- 2 mL whole blood contain 1 mg iron.
- Major cause of iron deficiency in adults
- Chronic blood loss most commonly through GI and GU systems
- Hemolysis
- Pregnancy contributes to this condition.
50-75 mL of blood loss from the upper GI tract is required for stools to appear black (melena; the black color is from the iron in the RBCs).
Common causes of GI blood loss are peptic ulcer, gastritis, esophagitis, diverticuli, hemorrhoids, and neoplasia.
GU blood loss occurs primarily through menstrual bleeding.
The average monthly menstrual blood loss is about 45 mL and causes the loss of about 22 mg of iron.
Postmenopausal bleeding can contribute to anemia in a susceptible older woman.
In addition to anemia of chronic kidney disease, dialysis treatment may induce iron-deficiency anemia as the result of blood lost in the dialysis equipment and frequent blood sampling.
Pregnancy contributes to iron deficiency because of the diversion of iron to the fetus for erythropoiesis, blood loss at delivery, and lactation.
Iron-Deficiency Anemia
Clinical Manifestations
- General manifestations of anemia
- Pallor is the most common finding.
- Glossitis is the second most common.
- Inflammation of the tongue
- Cheilitis
- Inflammation of the lips
In the early course of iron-deficiency anemia, the patient may not have any symptoms, but as the disease becomes chronic, any of the general manifestations of anemia may develop.
In addition, the patient may report headache, paresthesias, and a burning sensation of the tongue, all of which are caused by lack of iron in the tissues.
See Table 31-3 for a list of general manifestations.
Glossitis and Cheilitis
Glossitis- strawberry red tongue

Cheilitis 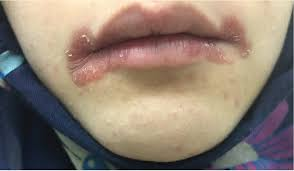
28
Iron-Deficiency Anemia
Collaborative Care
- Goal is to treat the underlying disease causing reduced intake or absorption of iron.
- Efforts are aimed at replacing iron.
- Nutritional therapy
- Oral or occasional parenteral iron supplements
- Transfusion of packed RBCs
The main goal of collaborative care of iron-deficiency anemia is to treat the underlying disease that is causing reduced intake (e.g., malnutrition, alcoholism) or absorption of iron.
Teach the patient which foods are good sources of iron.
If nutrition is already adequate, iron supplements are used.
If the iron deficiency is from acute blood loss, the patient may require a transfusion of packed RBCs.
Iron
- Daily requirements
- Determined by rate of erythrocyte production
- Increased requirement during pregnancy
- Dietary sources
- Available in foods of plant and animal origin
- Liver, egg yolk, brewer’s yeast, wheat germ, muscle meats, fish, fowl, cereal grains, beans, and green leafy vegetables
Oral Iron Preparations
- Ferrous sulfate
- Indications: Drug of choice
- Prophylactic therapy
- Adverse effects
- Gastrointestinal (GI) disturbances
- Staining of teeth
- Toxicity: Nausea, vomiting, diarrhea, and shock, followed by acidosis, gastric necrosis, hepatic failure, pulmonary edema, and vasomotor collapse
Oral Iron Preparations (Cont.)
- Drug interactions
- Antacids: reduce absorption
- Tetracycline: reduce absorption of both drugs
- Ascorbic acid: promotes absorption
- Other oral iron preparations
- Ferrous gluconate, ferrous fumarate, and ferrous aspartate
Iron-Deficiency Anemia
Drug Therapy
- Oral iron
- Inexpensive
- Convenient
- Factors to consider
- Enteric-coated or sustained-release capsules are counterproductive.
- Daily dose is 150 to 200 mg.
Iron is absorbed best from the duodenum and proximal jejunum. Therefore enteric-coated or sustained-release capsules, which release iron farther down the GI tract, are counterproductive and expensive.
The daily dosage should provide 150 to 200 mg of elemental iron. This can be ingested in three or four daily doses, with each tablet or capsule of the iron preparation containing between 50 and 100 mg of iron.
Iron-Deficiency Anemia
Drug Therapy
- Oral iron
- Factors to consider
- Best absorbed as ferrous sulfate in an acidic environment
- Liquid iron should be diluted and ingested through a straw.
- Side effects
- Heartburn, constipation, diarrhea
- black stools
- Factors to consider
Iron is best absorbed as ferrous sulfate (Fe2+) in an acidic environment. For this reason and to avoid binding the iron with food, iron should be taken about an hour before meals, when the duodenal mucosa is most acidic. Taking iron with vitamin C (ascorbic acid) or orange juice, which contains ascorbic acid enhances iron absorption.
Undiluted liquid iron may stain teeth, thus the reason for ingesting it through a straw.
Side effect example: Many individuals who need supplemental iron cannot tolerate ferrous sulfate because of the effects of the sulfate base. However, ferrous gluconate may be an acceptable substitute.
All patients need to be told that iron will cause their stools to become black because excess iron is excreted in the GI tract.
Because iron causes constipation, patients should be started on stool softeners and laxatives, if needed, when started on iron.
Anemia of Chronic Disease
The cytokines released in these conditions, particularly interleukin 6 (IL-6), cause an increased uptake and retention of iron within macrophages (see Fig. 30-3).
This leads to a diversion of iron from circulation into storage sites with subsequent limited iron available for erythropoiesis.
There is also reduced RBC life span, suppressed production of erythropoietin, and an ineffective bone marrow response to erythropoietin.
For any chronic disease, there may also be additional factors contributing to the anemia.
For example, with renal disease, the primary factor causing anemia is decreased erythropoietin, a hormone made in the kidneys that stimulates erythropoiesis. With impaired renal function, erythropoietin production is decreased.
Anemia of Chronic Disease
Anemia of Inflammation
- Caused by
- Chronic inflammation
- Autoimmune and infectious disorders
- HIV, hepatitis
- Heart failure
- Malignant diseases
- Bleeding episodes
Anemia of Chronic Disease
Anemia of Inflammation
- Associated with
- Underproduction of RBCs
- Mild shortening of RBC survival
- Normocytic, normochromic, and hypoproliferative RBCs
- Usually a mild anemia but can become severe if the underlying disorder is untreated
This type of anemia, which usually develops after 1 to 2 months of disease activity, has an immune basis.
Anemia of Chronic Disease
- Anemia of chronic disease findings
- ↑ Serum ferritin
- ↑ Iron stores
- Normal folate and cobalamin levels
- Treating underlying cause is best.
- Rarely blood transfusions
- Conservative use of erythropoietin therapy
Anemia of chronic disease must first be recognized and differentiated from anemia of other etiologies.
Elevated serum ferritin and increased iron stores distinguish it from iron-deficiency anemia.
Normal folate and cobalamin blood levels distinguish it from those types of anemias.
The best treatment of anemia of chronic disease is correction of the underlying disorder.
If the anemia is severe, blood transfusions may be indicated, but they are not recommended for long-term treatment.
Erythropoietin therapy (Epogen, darbepoetin) is used for anemia related to renal disease and may be used for anemia related to cancer and its therapies.
However, it needs to be used conservatively, as there is an increased risk of thromboembolism and mortality in some patients.
Megaloblastic Anemias
- Group of disorders
- Caused by impaired DNA synthesis
- Presence of megaloblasts
- result in large, fragile, defective RBC’s
- Majority result from deficiency in
- Cobalamin (vitamin B12)
- Folic acid
Megaloblastic anemias are a group of disorders caused by impaired deoxyribonucleic acid (DNA) synthesis and characterized by the presence of large RBCs.
When DNA synthesis is impaired, defective RBC maturation results.
The RBCs are large (macrocytic) and abnormal and are referred to as megaloblasts.
Macrocytic RBCs are easily destroyed because they have fragile cell membranes.
Although the overwhelming majority of megaloblastic anemias result from cobalamin (vitamin B12) and folic acid deficiencies, this type of RBC deformity can also occur from suppression of DNA synthesis by drugs, inborn errors of cobalamin and folic acid metabolism, and erythroleukemia (malignant blood disorder characterized by a proliferation of erythropoietic cells in bone marrow).
Megaloblastic Anemia
Cobalamin Deficiency (B12)
- Intrinsic factor (IF)
- Protein secreted by the parietal cells of the gastric mucosa
-====
- IF is required for cobalamin absorption in the distal ileum.
- If IF is not secreted, cobalamin will not be absorbed.
Cobalamin deficiency is vitamin B12 deficiency.
Cobalamin Deficiency
Etiology
- Most commonly caused by pernicious anemia
- Which is caused by an absence of IF
- Insidious onset
- Begins in middle age or later
- Predominant in Scandinavians and African Americans
Cobalamin deficiency is most commonly caused by pernicious anemia, which results in poor cobalamin absorption through the GI tract.
In pernicious anemia the gastric mucosa is not secreting IF because of either gastric mucosal atrophy or autoimmune destruction of parietal cells.
In the autoimmune process antibodies are directed against the gastric parietal cells and/or IF itself.
Because parietal cells also secrete hydrochloric (HCl) acid, in pernicious anemia there is a decrease in HCl in the stomach.
An acid environment in the stomach is required for the secretion of IF.
Pernicious anemia is a disease of insidious onset that begins in middle age or later (usually after age 40) with 60 years being the most common age at diagnosis.
Pernicious anemia occurs frequently in persons of Northern European ancestry (particularly Scandinavians) and African Americans.
In African Americans, the disease tends to begin early, occurs with higher frequency in women, and is often severe.
Parenteral or intranasal administration of cobalamin is the treatment of choice.
Cobalamin Deficiency
Etiology
- Can also occur in the following situations:
- GI surgery
- Chronic diseases of the GI tract
- Chronic alcoholics
- Long-term users of H2-histamine receptor blockers and proton pump inhibitors
- Strict vegetarians - vegans
Cobalamin deficiency can also occur in patients who have had GI surgery such as gastrectomy, gastric bypass, small bowel resection involving the ileum, and chronic diseases of GI tract such as Crohn’s disease, ileitis, celiac disease, diverticuli of the small intestine, chronic atrophic gastritis.
In these cases, cobalamin deficiency results from the loss of IF-secreting gastric mucosal cells or impaired absorption of cobalamin in the distal ileum.
Cobalamin Deficiency
Clinical Manifestations
- General manifestations of anemia develop slowly due to tissue hypoxia.
- Gastrointestinal manifestations:
- Sore tongue, anorexia, nausea, vomiting, & abdominal pain
- Gastrointestinal manifestations:
- Neuromuscular manifestations:
- Weakness, paresthesias of feet & hands, ataxia, muscle weakness, and impaired thought processes
Because cobalamin deficiency–related anemia has an insidious onset, it may take several months for these manifestations to develop.
Cobalamin Deficiency
Diagnostic Studies
- Macrocytic RBCs have abnormal shapes and fragile cell membranes.
- Serum cobalamin levels are decreased.
- Normal serum folate levels and low cobalamin levels suggest megaloblastic anemia is due to cobalamin deficiency.
- Upper GI endoscopy with biopsy of gastric mucosa
Laboratory data reflective of cobalamin–deficiency anemia are presented in Table 31-6.
Abnormal RBCs are susceptible to erythrocyte destruction.
A serum test for anti-IF antibodies may be done that is specific for pernicious anemia.
The potential for gastric cancer is increased in patients with pernicious anemia.
Testing of serum methylmalonic acid (MMA) (elevated mainly in cobalamin deficiency) and serum homocysteine (elevated in both cobalamin and folic acid deficiencies) can also be done.
Cobalamin Deficiency
Collaborative Care
- Parenteral or intranasal administration of cobalamin is the treatment of choice. Recently noted that higher doses of PO cobalamin can increase levels, as even without IF there is some absorption.
- Patients will die in 1-3 years without treatment.
- This anemia can be reversed with ongoing treatment but long-standing neuromuscular complications may not be reversible.
Increasing dietary cobalamin does not correct this anemia if intrinsic factor is lacking or if there is impaired absorption in the ileum. However, good nutrition should still be taught.
The dosage and frequency of cobalamin administration may vary. A typical treatment schedule consists of 1000 mg of cobalamin IM daily for 2 weeks and then weekly until the Hgb is normal, then monthly for life.
High-dose oral cobalamin and sublingual cobalamin are also available for those in whom GI absorption is intact.
Vitamin B12 (Cobalamin)
- Essential for synthesis of DNA
- Absorption requires intrinsic factor
- Elimination occurs very slowly
- Daily requirement
- Dietary sources
- Animal products (liver, dairy products)
- Fortified foods
Vitamin B12 Deficiencies: Causes, Consequences, and Diagnosis
- Causes
- Usually result of impaired absorption
- Regional enteritis
- Celiac disease
- Absence of intrinsic factor: Pernicious anemia
Vitamin B12 Preparations: Cyanocobalamin
- Cyanocobalamin is medication used to treat
- Administration
- Oral, parenteral, intranasal
- Adverse effect
- Hypokalemia
- Long-term treatment
- With lack of intrinsic factor, vitamin B12 therapy is lifelong
Megaloblastic Anemia
Folic Acid Deficiency
- Also a cause of megaloblastic anemia
- Folic acid is required for DNA synthesis.
- RBC formation and maturation
- Clinical manifestations are similar to those of cobalamin deficiency, but absence of neurologic problems differentiates them.
Folic acid deficiency develops insidiously, and the patient’s symptoms may be attributed to other coexisting problems (e.g.,9 cirrhosis, esophageal varices).
GI disturbances include dyspepsia and a smooth, beefy red tongue.
Folic Acid Deficiency
Common causes
- Dietary deficiency
- Malabsorption syndromes
- Drugs
- Increased requirement
- Alcohol abuse and anorexia
- Loss during hemodialysis
Folic Acid Deficiency
- Serum folate level is low.
- Normal is 3 to 25 mg/mL (7 to 57 mol/L).
- Serum cobalamin level is normal.
- Treated by replacement therapy
- Usual dose is 1 mg per day by mouth.
- Encourage patient to eat foods with large amounts of folic acid.
The diagnostic findings for folic acid deficiency are presented in Table 31-6.
Also during diagnostic studies, the gastric analysis is positive for hydrochloric acid.
Replacement therapy is the treatment of choice: In malabsorption states or with chronic alcoholism, up to 5 mg per day may be required. Duration of treatment depends on the reason for the deficiency.
Folic Acid Anemia:
Causes and Consequences
- Causes
- Poor diet (malnutrition and alcoholism)
- Malabsorption syndrome
- Consequences for developing fetus
- Neural tube defects (eg, spina bifida, anencephaly)
- Adequate intake before conception is critical
- Consequences for all individuals
- Megaloblastic anemia
- Leukopenia, thrombocytopenia, injury to the oral and GI mucosa
- May increase risk of colorectal cancer and atherosclerosis
Folic Acid Preparations
Nomenclature
- Inactive form: Folacin, folate, pteroylglutamic acid, or folic acid
- Active form: Leucovorin calcium, folinic acid, or citrovorum factor
- Inactive form is by far the most commonly used preparation
\
Aplastic Anemia/ Pancytopenia
- Decrease in all blood cell types
- Red blood cells (RBCs)
- White blood cells (WBCs)
- Platelets
- Hypocellular bone marrow
- Ranges from chronic to critical
Aplastic anemia is a disease in which the patient has peripheral blood pancytopenia.
The spectrum of the anemia can range from a chronic condition managed with erythropoietin or blood transfusions to a critical condition with hemorrhage and sepsis.
Etiology
- Low incidence, not happen a lot
- Affecting 2 of every 1 million persons
2 Major Types
- Congenital: Chromosomal alterations
- Acquired: Results from exposure to ionizing radiation, chemical agents, viral and bacterial infections
Approximately 75% of the acquired aplastic anemias are idiopathic and are thought to have an autoimmune basis.
Clinical Manifestations
- Abrupt or gradual development
- Symptoms caused by suppression of any or all bone marrow elements
- General manifestations of anemia
- Fatigue, dyspnea
- Cardiovascular and cerebral responses
- Neutropenia: low neutrophils
The patient with neutropenia (low neutrophil count) is susceptible to infection and is at risk for septic shock and death. Even a low-grade temperature (>100.4o F) should be considered a medical emergency.
Thrombocytopenia is manifested by a predisposition to bleeding evidenced by petechiae, ecchymosis, and epistaxis.
Diagnostic Studies
- Diagnosis confirmed by laboratory studies
- Low Hgb, WBC, and platelet values
- Low reticulocyte count
- Prolonged bleeding time
- Elevated serum iron
- Hypocellular bone marrow with increased fat content (yellow marrow)
All marrow elements are affected in this disorder.
The condition is classified as a normocytic, normochromic anemia because although Hgb, WBC, and platelet values are decreased, other RBC indices are generally normal.
Aplastic anemia can be further evaluated by assessing various iron studies.
The serum iron and total iron-binding capacity (TIBC) may be elevated as initial signs of erythropoiesis suppression.
Bone marrow biopsy, aspiration, and pathologic examination may be done for any anemic state. However, the findings are especially important in aplastic anemia because the marrow is hypocellular with increased yellow marrow (fat content).
Nursing & Collaborative Management
- Identify and remove causative agent (when possible).
- Provide supportive care until pancytopenia reverses.
- Prevent complications from infection.
- Prevent hemorrhage.
- Prognosis of severe untreated aplastic anemia is poor.
- Median survival is 3 to 6 months.
- 20% survive longer than 1 year.
- Treatment options
- Immune therapies and bone marrow transplantation can be curative.
Advances in medical management, including hematopoietic stem cell transplant (HSCT) and immunosuppressive therapy with antithymocyte globulin (ATG) and cyclosporine or high-dose cyclophosphamide (Cytoxan), have improved outcomes significantly.
ATG is a horse serum that contains polyclonal antibodies against human T cells. It can cause anaphylaxis and a serum sickness. The rationale for this therapy is that idiopathic aplastic anemia is considered an autoimmune disorder resulting from activated cytotoxic T cells that target and destroy the patient’s own hematopoietic stem cells.
The treatment of choice for adults less than 55 years of age who do not respond to the immunosuppressive therapy and who have a human leukocyte antigen (HLA)–matched donor is an HSCT.
The best results occur in younger patients who have not had previous blood transfusions.
Prior transfusions increase the risk of graft rejection.
For older adults without an HLA-matched donor, the treatment of choice is immunosuppression with ATG or cyclosporine or high-dose cyclophosphamide.
High-dose corticosteroids may also be used. However, this therapy may be only partially beneficial.
Patients who need ongoing supportive blood transfusion should be on an iron-binding agent to prevent iron overload.
Hematopoietic Agents
- Hematopoiesis: The process by which red blood cells, white blood cells, and platelets are produced
- Therapeutic applications
- Acceleration of neutrophil and platelet repopulation after cancer chemotherapy
- Acceleration of bone marrow recovery after autologous bone marrow transplantation (BMT)
- Stimulation of erythrocyte production in patients with chronic renal failure
Epoetin Alfa (Erythropoietin)
- Brand names: Epogen, Procrit
- Hematopoietic growth factor
- Significant effects outside hematopoietic system
- Recombinant DNA technology
Uses
- Anemia associated with chronic renal failure
- Chemotherapy-induced anemia
- For HIV-infected patients taking zidovudine
- Anemia in patients facing surgery
- HIV, Human immunodeficiency virus.
Adverse effects
- Hypertension
- Autoimmune pure red-cell aplasia
- Very rare
- Cardiovascular events
- Cardiac arrest
- Hypertension
- Heart failure
- Thrombotic events (stroke and MI)
MI, Myocardial infarction.
63
Epoetin Alfa (Erythropoietin) (Cont.)
- Warnings
- Excessive dosage
- Cancer patients: ESAs can accelerate tumor progression and shorten life in certain cancer patients
- Renal failure patients: ESAs can increase the risk of serious cardiovascular events and death if hemoglobin levels are driven too high
- Preoperative patients
IMPT on TEST!: If a patient has cancer do not give them erythopoetin!
- Risk Evaluation and Mitigation Strategy
Epoetin Alfa (Erythropoietin) (Cont.)
ESAs, Erythropoiesis stimulating agents.
64
Dosage
- To minimize risks
- Use minimum needed to gradually raise hemoglobin content to eliminate the need for RBC transfusions
- Hemoglobin level should not exceed 12 g/dL
Longer-Acting Erythropoietins
- Darbepoetin alfa (long acting)
- Similar risks and need for monitoring as for epoetin alfa
- Patients with hypertension may need increased hypertension medications
- Monitoring
- Dosing guidelines
Thrombocytopenia- low platelets
- Platelets are below 150,000/uL
- Can have multiple causes
- inherited
- immune mediated
- Drug related
- Infection
- Malignancy
- Bleeding Precautions- on test
- Can transfuse platelets
Oprelvekin
- Thrombopoietic
- similar to interleukin-11
- Acts on platelet progenitor cells to increase platelet production
- Administered SQ
- Can cause severe allergies, fluid retention, cardiac dysrhythmias and conjunctival irritation.
Neutropenia
- Neutropenia - decrease in neutrophils- specific type of WBC
- Leukopenia - a decrease in total WBC counts (granulocytes, monocytes and lymphocytes)- all of the wbc’s
- Can happen with chemo, immunosuppression, some disease states
What are these people at risk for?
What does a fever mean in conjunction with neutropenia?
Filgrastim [Neupogen]
Granulocyte colony-stimulating factor
Leukopoietic growth factor
Recombinant DNA technology
Reduces the incidence of severe neutropenia
Produces dose-dependent increase in circulating neutrophils
Reduces the incidence of infection, need for hospitalization, and need for intravenous antibiotics
Copyright © 2019 by Elsevier, Inc. All rights reserved.
70
70
Uses
Cancer
Severe chronic neutropenia
Investigational uses
Adverse effects
Bone pain
Leukocytosis
Other adverse effects
Filgrastim [Neupogen] (Cont.)
Copyright © 2019 by Elsevier, Inc. All rights reserved.
71
71
Pegfilgrastim (Granulocyte Colony-Stimulating Factor)
- Long acting
- Derivative of filgrastim [Neupogen]
- Stimulates myeloid cells to increase production of neutrophils
Adverse effects
- Similar to those of filgrastim
- May require opioids for pain relief
Inherited Hemorrhagic Disease
Hemophilia
- A group of hereditary bleeding disorders that result from deficiencies of specific clotting factors
- Typically, an X-linked recessive pattern
- Meaning primarily men will have it!
Inherited Hemorrhagic Disease
Hemophilias
- Serious bleeding disorders
- Hemophilia A (classic hemophilia): Factor VIII deficiency, x-linked (test)
- Hemophilia B (Christmas disease): Factor IX deficiency
- Hemophilia C: Factor XI deficiency
- von Willebrand disease: Autosomal dominant trait of factor VIII deficiency
- Two primary defects: Point mutation and gene deletion
Know the difference what factor causes which anemia
Inherited HemorrhagicDisease (cont’d)
Hemophilias (cont’d)
- Clinical manifestations
- Hematoma formations
- Persistent bleeding from relatively minor traumatic lacerations
Tests
- Phase III: Thrombin time
- Phase II: Prothrombin time
- Phase I: Activated partial thromboplastin time, prothrombin consumption time, thromboplastin generation test (most sensitive)
Inherited HemorrhagicDisease (cont’d)
Hemophilias (cont’d)
- Treatment
- Recombinant factor VIII
- Recombinant antihemolytic factor plasma; albumin-free method: For hemophilia A
Types of Hemophilia
Hemophilia A
- Classic hemophilia (deficiency of factor VIII)
- Accounts for 80% of cases of hemophilia
Hemophilia B
- Christmas disease (deficiency of factor IX)
- von Willebrand disease (vWD)
Deficiency, abnormality, or absence of vWF and factor VIII
- Affects both males and females
IMPT!
Etiology of Hemophilia A
- X-linked recessive trait
- Males are affected
- Females may be carriers
- Degree of bleeding depends on the amount of clotting factor present and the severity of a given injury
- Up to one third of cases have no known family history
- In these cases, disease is caused by a NEW mutation
Manifestations of Hemophilia (1 of 2)
- Bleeding tendencies range from mild to severe
- Symptoms may not occur until 6 months of age
Hemarthrosis
- Bleeding into joint spaces of the knee, ankle, or elbow leads to impaired mobility and, eventually, bony changes and disability
- Symptoms include warmth, pain, bruising, and decreased movement
- Epistaxis(nose bleed)
- Bleeding in the gastrointestinal tract
- Bleeding after procedures
- Minor trauma, tooth extraction, minor surgeries
- Large subcutaneous and intramuscular hemorrhages may occur
- Bleeding into the neck, chest, or mouth may compromise the airway
- Bleeding in the spinal cord may cause paralysis
Diagnostic Evaluation
- Can be diagnosed through amniocentesis
- Genetic testing of family members is needed to identify carriers
- Diagnosis is made on the basis of the history, laboratory studies, and the examination
- Laboratory tests will show low levels of factor VIII or IX and a prolonged partial thromboplastin time (PTT)
- Platelet count, prothrombin time (PT), and fibrinogen levels are normal
Medical Management of Hemophilia
- Replacement of missing clotting factors
- Desmopressin (DDAVP)
- IV
- Increases factor VIII activity by two to four times
- Used for mild hemophilia
- Transfusions
- Prompt intervention to reduce complications
- Medications
- Exercise and physical therapy
Prognosis for Hemophilia
- Historically, most patients died by 5 years of age
- Currently, patients with mild to moderate hemophilia live nearly normal lives
- Gene therapy for the future
- Infused carrier organisms act on target cells to promote manufacture of the deficient clotting factor
Nursing Care Management
- Prevent bleeding episodes
- Recognize and control bleeding
- Support the patient
- Pain
- Mobility
- Promote growth and development
- Support the family
- Education
- Prepare for home care
Interventions for Hemophilia
- Close supervision and safe environment
- Dental procedures in a controlled situation
- Shave only with an electric razor
- For superficial bleeding, apply pressure for at least 15 minutes and ice to promote vasoconstriction
- If significant bleeding occurs, transfusion for factor replacement
Managing Hemarthrosis
- During bleeding episodes, elevate and immobilize the joint
- Ice
- Analgesics
- Range-of-motion exercises after the bleeding stops will help to prevent contractures
- Physical therapy
- Avoid obesity to minimize joint stress
More for you…..
Patient Profile
R.D is a 51-year-old male who comes to the emergency department with complaints of weakness and abdominal pain. He reports he is homeless. His medical history includes Methicillin-Resistant Staphylococcus Aureus (MRSA) bacteria of the nares.
Subjective Data
Reports he does not drink or eat food consistently
Says he has no family or any social support
Had “a drink or two a little while ago”
Objective Data
Physical Examination
Blood pressure 144/86, pulse 92, temperature 98.2°F, respirations 24
Height 6’1”, weight 170 lbs
Heart rhythm irregular
Oxygen saturation 89% on room air
Alert, oriented to person and place
Capillary refill sluggish in lower extremities, normal in upper extremities
· 2+ edema in lower extremities
(see next slide)
88
…more for you
Discussion Questions
What health history information would you obtain from R.D.?
Describe what R.D.’s physical assessment should include.
Based on the clinical manifestations that brought R.D. to the emergency department, what diagnostic tests you would expect to be ordered and what each would contribute?
What factors in R.D.’s history place him at risk for anemia?
What are the priority nursing diagnoses for R.D.?
What resources might be available to this R.D. after he is discharged from the hospital?
Try to answer these on your own!!! Next slide has answers.
89
Answers!!! (don’t look til you try!!!)
Discussion Questions
What health history information would you obtain from R.D.?
Answer: Begin by assessing R.D.’s presenting problem using the functional health patterns as a guide. Fully explore the symptoms he is experiencing, including the length of time he has had symptoms and the presence of any accompanying symptoms, including chest pain, bleeding or bruising, orthopnea, dyspnea, nausea, paresthesia, palpitations and dark, tarry stools. Inquire the relationship between his symptoms and activity. Ask about any changes in his weight, typical nutritional intake, tobacco, alcohol, and medication use. Ask about a family history of cardiovascular disease and review his past medical history.
Describe what R.D.’s physical assessment should include.
Answer: Since hematologic disorders affect a variety of systems, perform a complete physical examination. Areas particularly relevant include obtaining vital signs, auscultating heart and lung sounds, assessing capillary refill time, inspecting the skin, palpating lymph nodes, assessing oral cavity, including dentition, mucus membranes and tongue, palpating abdomen for liver and spleen enlargement, and inspecting appearance of conjunctiva and sclera.
90
more answers
Based on the clinical manifestations that brought R.D. to the emergency department, what diagnostic tests you would expect to be ordered and what each would contribute?
Answer: Tests that may be ordered for R.D. include:
Since his heart rhythm is irregular, a 12-lead ECG would be used to determine heart rhythm and presence of any potential ischemia.
Complete blood count would provide a rapid screening of his RBC, hemoglobin, hematocrit, red blood cell indices (MCV, MCH), and platelets that would be used in making a potential diagnosis.
A metabolic panel will provide information regarding R.D.’s liver and kidney function, electrolyte status, acid-base balance, and nutritional status.
A blood alcohol level may be used to determine current alcohol use and to evaluate if an elevated blood alcohol concentration is responsible for any of R.D.’s symptoms.
Estimated glomerular filtration rate (eGFR) would be calculated to determine if R.D. has chronic kidney disease.
Urinalysis would be useful in evaluating liver and kidney function and determining if any coexisting problems, such as infection or diabetes, are present.
A urine drug screen would provide information regarding R.D.’s alcohol and substance use.
A culture swab of nares would be used to screen for the presence of a current MRSA infection.
What factors in R.D.’s history place him at risk for anemia?
Answer: R.D.’s risk factors include low socioeconomic background, being homeless without adequate nutrition, and a potential history of alcoholism.
What are the priority nursing diagnoses for R.D.?
Answer: Priority nursing diagnoses include impaired gas exchange, decreased cardiac output, noncompliance, ineffective peripheral tissue perfusion, fatigue, excess fluid volume, and imbalanced nutrition of less than body requirements.
What resources might be available to this R.D. after he is discharged from the hospital?
Answer: Involve social services in R.D.’s care to evaluate if he is eligible for any government services. It is possible that R.D. is eligible for placement in a special facility or in subsidized housing. If R.D. will continue to remain homeless, investigate the possibility of having R.D. register with an interim care shelter, such as the Salvation Army, to provide meals and to store medications. Another option is to advise R.D. to attend alcohol support group meetings.
Test: Hemophilia: the types what causes it how they present, what you as a nurse need to educate them
DRUG | CLASS and MOA | THERAPEUTIC ROLE | ROUTE | SIDE EFFECTS | NURSING IMPLICATIONS/PATIENT EDUCATION/NOTES | |
|---|---|---|---|---|---|---|
Iron | GI disturbances, staining of teeth, toxicity: nausea, vomiting, diarrhea, and shock, followed by acidosis, necrosis, hepatic failure, pulmonary edema, and vasomotor collapse | Drug interactions Antacids: reduce absorption Tetracycline: reduce absorption of both drugs Ascorbic acid: promotes absorption Other oral iron preparations Ferrous gluconate, ferrous fumarate, and ferrous aspartate | ||||
Vitamin B12 | ||||||
Folic Acid | ||||||
Erythropoietin | ||||||
Filgrastim | ||||||
Pegfilgrastim | ||||||
Oprelvekin |
Fluid and Electrolytes Lecture 1
1
Calculation Practice
The nurse practitioner writes and order for you to administer 15mg of Robitussin every four hours. Supplied: Robitussin Liquid 30mg/3ml. How many ml will you give at each dose? How many ml in 24 hours?
The physician writes an order to start an IV and infuse fluids at 1pm. The patient weighs 185 lb. Infuse 1500ml of 0.9% NS over 12 hours, tubing drip factor 15gtt/ml. What is the drip rate (gtts/min)?
Answers
The nurse practitioner writes and order for you to administer 15mg of Robitussin every four hours. Supplied: Robitussin Liquid 30mg/3ml. How many ml will you give at each dose? How many ml in 24 hours?
ml = 3ml x 15mg = 45 = 1.5 ml per dose
30mg 1 dose 30 9 ml per 24 hours (1.5 x 6 doses)
The physician writes an order to start an IV and infuse fluids at 1pm. The patient weighs 185lb. Infuse 1500ml of 0.9% NS over 12 hours, tubing drip factor 15gtt/ml. What is the drip rate (gtts/min)?
gtt = 15gtt x 1500 ml x 1 hr = 22,500 = 31.25 =31 gtt/min
min 1 ml 12 hr 60 min 720
Objectives
Describe the composition of major body fluid compartments
Describe the etiology and clinical manifestations of:
-fluid imbalances
- electrolyte imbalances
Homeostasis
- The tendency of the biological system to maintain a relatively constant condition (equilibrium) in the internal environment while continuously interacting with and adjusting to changes originating within or outside the system.
Who Contains the Highest Percentage of Water?
- Newborn -70-80%
- Normal adult woman- 50-60%
- Normal adult man50-60%
- Elderly adult man: 45-55%
Percentage of Water
- Average water content is 50-60% body weight.
- Body water % inversely proportional to body fat %.
- Women generally have more body fat.- maybe have less than man
- Older adults generally have more body fat.
- % water decreases with age.
Men > women
Babies > adults
adults >elderly
Therefore…. water content comparisons (< or >)
“Rule of Thirds”
Intracellular Fluid is 2/3 of fluid (40% TBW)
Extracellular Fluid is 1/3 of fluid (20% TBW)
Interstitial fluid and lymph – 2/3 (15% TBW)
Intravascular - 1/3 (5% %TBW)
ICF vs. ECF vs. Transcellular
ICF = inside the cell
- 2/3 of total body fluid (60%)
- 30 liters = 42% of body weight
- Potassium major cation (+ ion)
- Phosphate major anion (- ion)
ECF = outside of cells
- 1/3 of body fluid (40%)
- liters = 17% of body weight
- includes interstitial and intravascular/plasma
- Sodium major cation (+ ion)
- Chloride major anion (- ion)
Transcellular
- = fluid secreted by cells in specialized cavities = 1 liter, e.g. CSF, synovial
Fluid Spacing
- First spacing: Normal distribution of fluid in the ECF and ICF
- Second spacing: Abnormal accumulation of interstitial fluid (edema)
- Third spacing: Fluid accumulation in areas that normally have little or no fluid & can not be easily exchanged (ascites, pleural effusion)
- First spacing: Completely normal and how the body should be
- Second spacing: Edema
- Third spacing: fluid is trapped and unavailable for functional use. Examples: ascites, peritonitis, pleural effusion 🡪 requires aspiration to remove the fluid (paracentesis or thoracentesis)
Mechanisms Controlling F & E Movement
- Body fluid is composed of water (the solvent) and particles that are dissolved or suspended in water (the solutes).
- To maintain a stable internal environment the body uses 4 processes
- Diffusion
- Facilitated Diffusion
- Osmosis
- Active Transport
F & E Movement Across Membranes
- Cell membrane is semi-permeable: some materials can get through while others can't.
- Small molecules (water, CO2, O2, ammonia, lipids, cholesterol) can cross the membrane easily.
k
- Larger molecules (glucose, amino acids, proteins) have a harder time and may need assistance.
F&E Movement…
- Passive Transport (no outside energy required)
- Simple diffusion: movement of solutes/particles from higher to lower concentration until particles are “mixed” and equilibrium is achieved.
- Facilitated diffusion: requires a protein carrier to assist with passage through the members (too large).
- Moves from high to low concentrations until equilibrium is achieved
- Osmosis: movement of only water between compartments to equalize concentration.
- Water moves from areas of lower to higher concentration
F&E Movement…
- Active Transport (external energy is required for movement of particles)
- Particles move against the concentration gradient, from areas of low to high concentration by combining with a carrier on the outside of the cell.
https://docs.google.com/presentation/d/101gAvggIZVJIXY_sa_fIjwrmqN1Ltp0s/edit#slide=id.p21
Slides 17-21 review
Osmotic Pressure
- Osmotic Pressure: pulling strength of solutes that pull water into a space- pulls water into cells
- Determined by the concentration of solutes in solution.
- Oncotic Pressure (colloidal osmotic pressure) is a type of osmotic pressure exerted by colloids/ proteins in blood.
- Protein/ colloid is too big to be pushed by hydrostatic force through the capillary to the interstitial space. Therefore it remains in the vascular system and pulls fluid into the vascular system.
- Albumin is the most common protein in blood.
On test
Fluid Movement
The amount and direction of fluid movement is determined by 4 factors:
Arterial side (high intravascular pressure system):
(1) Capillary hydrostatic pressure and
(2)Interstitial oncotic pressure causes movement of fluid out of the capillaries.
Venous side (low intravascular pressure system):
(3)Plasma oncotic pressure and
(4)Interstitial hydrostatic pressure cause the movement of fluid into the capillary.
24
Fluid movement in disease
From plasma to interstitial
- Results in Edema - can be from:
- Heart failure
- Fluid overload
- Liver failure
- Renal disease
- Trauma
- Burns
From interstitial to plasma
- Results in movement of fluid from the interstitial space back to vascular
- Done by:
- Medications (mannitol, colloids, hypertonic solutions)
- Compression hose (TEDS or ACE wrap)
Certain diseases will cause fluids to shift…
Movement of fluid from Plasma (blood vessels) to the interstitial results in edema 🡪 fluid overload, CHF, liver failure, obstruction of venous return to the heart (Constrictive clothing, tourniquet, venous thrombus, varicose veins)
Back up of fluid results in – heart failure
Deficient protein synthesis- liver disease
Damaged capillary walls- trauma and burns
Draws fluid into the plasma/blood vessels from the interstitial space 🡪 may be done by medications, colloids, compression hose
Mannitol- medication given to decrease ICP due to edema
Compression hose- decrease peripheral edema
Water Balance Regulation
- Hypothalamus : osmoreceptors control thirst & produces ADH to be stored by posterior pituitary
- Pituitary : Stores and secretes ADH to increase reabsorption of fluid in kidneys
- Adrenals : regulate H20 & electrolyte levels via aldosterone & cortisol
- Kidneys : regulate renin & aldosterone thru urine, Na+, & K+ secretion
Insensible water loss= 900 ml/daily- Fluid lost through vaporization from respiration and skin.
- Term to know!
Ideally
Input = Output
HOMEOSTASIS
Intake | Output |
|---|---|
Fluids: 1200 ml | Insensible loss (skin & lungs): 900 ml |
Solid Food: 1000 ml | Through Feces: 100 ml |
Water from Oxidation: 300 ml | Urine: 1500 ml |
TOTAL: 2500 ml | TOTAL: 2500 ml |
27
*Fluid Volume Deficit - impt
- Hypovolemia: less than the body requires
Causes: abnormal loss of body fluids
- Diarrhea
- fistula drainage
- Hemorrhage
- Polyuria
- Burns
- inadequate fluid intake
- Excessive perspiration (heat stroke, high fever)
Signs & Symptoms:
-restless
-drowsy
-thirst
-dry membranes
-tachypnea
-tachycardia
-weakness
-weight loss
-seizure
-decreased urine output
Treatment: correct the underlying cause
- Replace fluid & electrolytes
- Blood, Lactated Ringers, or Isotonic normal saline (rapid replacement)
*Fluid Volume Excess - impt
- Hypervolemia: more than the body requires
Causes:
- Excess intake of fluids
- Abnormal fluid retention (heart or renal failure)
- Fluid shifts
- Long term steroid use
Signs & Symptoms:
- Weight gain
- Edema
- Headache
- JVD
- bounding pulse
- increased BP
- Dyspnea
- crackles
- muscle spasms
- Seizure
- lethargy
Treatments: Remove fluid without causing other imbalances & identify cause
- Diuretics
- fluid restriction
- Sodium restriction
- Paracentesis, thoracentesis
Subtypes of Fluid Volume Imbalance
Hypovolemia
- Isotonic
- Equal loss of Na & H2O
- Need Isotonic fluids
- Hypertonic
- Greater H2O loss than Na
- Need hypotonic solution
- Hypotonic
- Greater loss of Na than H2O
- Need hypertonic solution
Hypervolemia
- Increased extracellular fluid
- Edema
- Treat with diuretics
- Fluid restriction
How should we assess fluid status?
- Daily weights most accurate- considerations???
- I&O
- Vital signs
- Neuro signs
- Skin turgor/ edema
Electrolytes & Acid Base Balance
Objectives
- Describe the composition and indications for common IV fluid solutions
- Discuss the etiology, diagnostic findings, clinical manifestations, and nursing management of acid base imbalances
Little Maggie may help you remember electrolytes…..
Little Maggie is 1.5 to 2.5 years old (magnesium). She ate 3.5 to 5 bananas (potassium) and drank 8.5 to 10.5 oz of milk (calcium). Then she took a 135- 145 minute nap after swimming in the ocean (sodium).
*think about foods associateied with these
- Sodium
Regulates:
- Neurological function
- Neuromuscular function
- Control osmotic pressure/ water regulation
- Acid- base balance
Regulated by:
- Kidneys & ADH
- Primarily gained from diet
- Primarily Extracellular
- Normal serum levels 135-145 mEq/L
- Normal Saline solution = 0.9% ≈ body concentration
* Remember the levels and symptoms
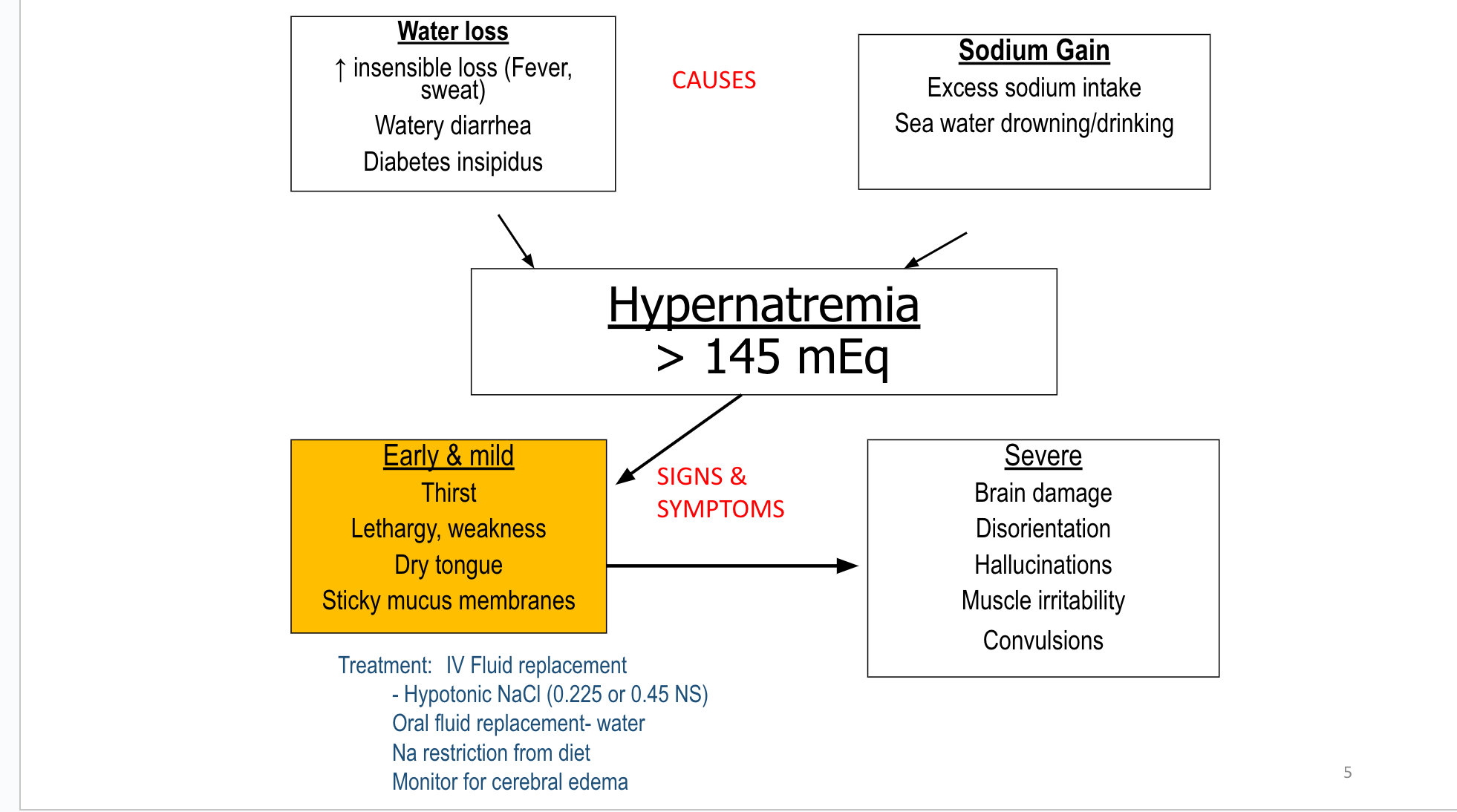
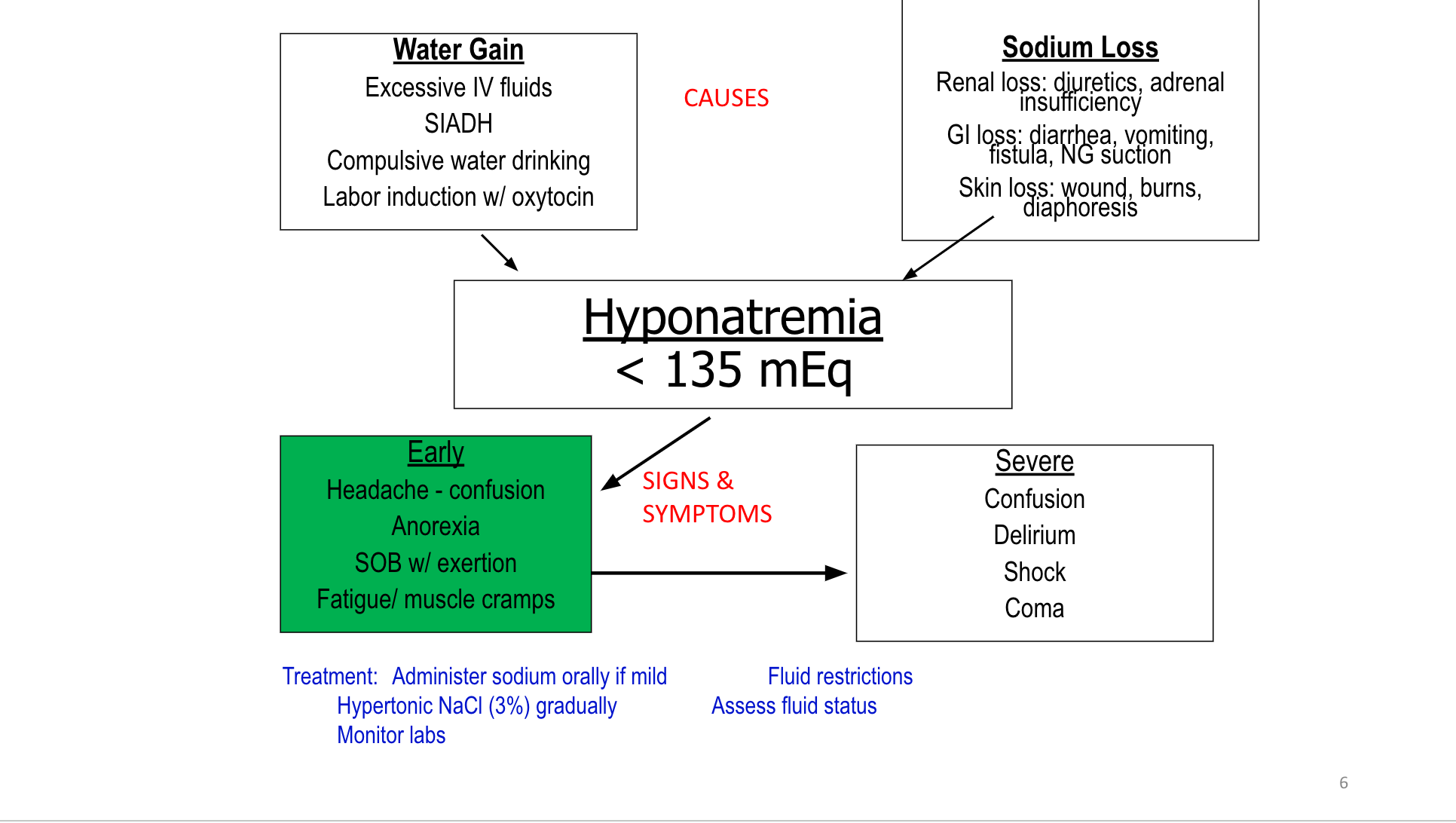
- Potassium
Regulates:
- Primary for neuromuscular & cardiac function
- ICF Osmolarity
- Regulate acid-base balance
Regulated by:
- Kidneys
- Primarily gained from diet
- Abundant in the ICF (98%)
- Normal serum levels are 3.5 to 5 mEq/L
Potassium is the most abundant electrolyte in the intracellular fluid and is essential for the generation of electrical impulses that allow the muscles (including heart) and brain to function.
The Sodium Potassium pump maintains concentration by pumping K into the cell and Na out of the cell
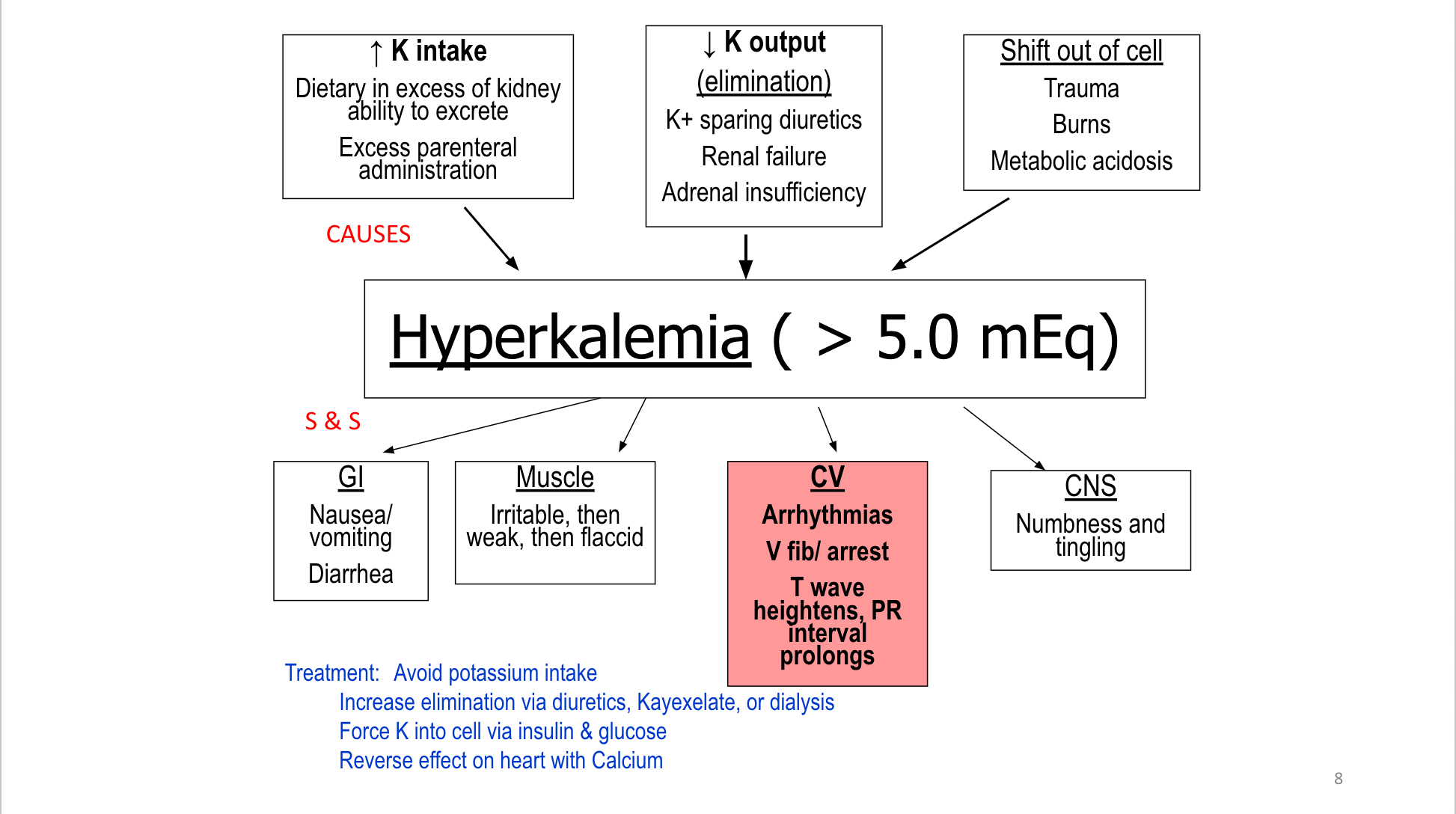
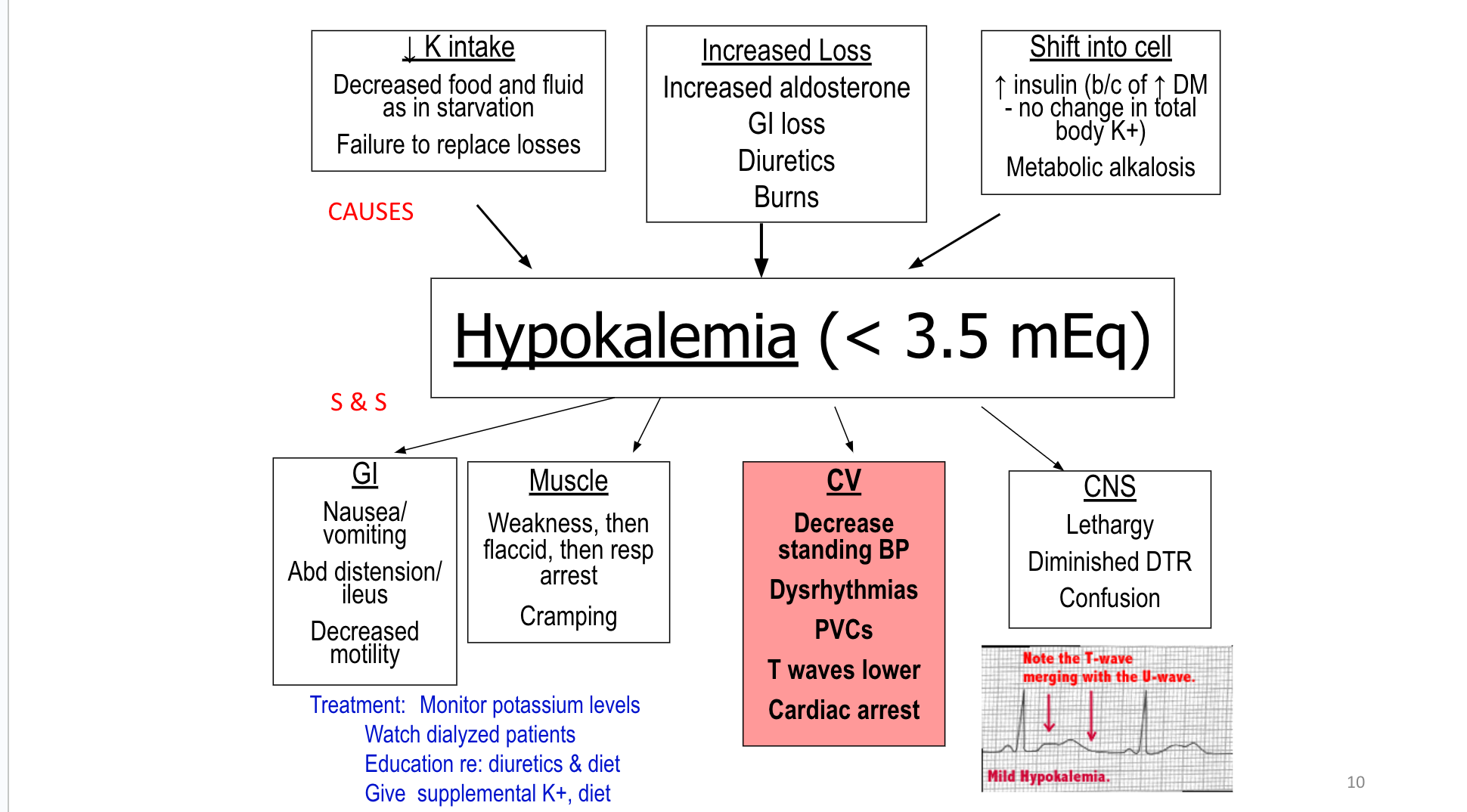
HypoKalemia Treatment
- Oral form of K supplements the safest
- IV never to exceed 10-20 mEq/hr
- Avoid IV concentration > 40mEq/liter
- IV may cause pain at IV site
- Orally can cause GI irritation 🡪 give with food
- Monitor K if taking digoxin or diuretics
- Foods high in K (raisins/ bananas/potatoes/ OJ/citrus/ green leafy)
- ALERT: patients with renal failure
- Calcium
- Regulates:
- Forming bones & teeth
- Transmit nerve impulses
- Muscle contraction
- Blood clotting
- Most abundant electrolyte in the body
- Normal serum levels 8.5-10.5 mg/dL
HyperCalcemia (>10.5 mg/dl)
- Causes:
- Hyperparathyroidism, malignancy, & prolonged immobility
- S & S:
- Weak neuromuscular system, kidney stone formation, abdominal pain, depression, cardiac dysrhythmias
- Treatment:
- Hydrate with force fluids & IV NS with diuretics to excrete extra Ca through urine
- Weight bearing activities
- Low Calcium diet
- Assess for fluid overload
Hypocalcemia ( < 8.5 mg/dL)
Causes:
- Malabsorption, ESRD, post- thyroidectomy, decreased production of PTH
S & S:
- Weakness, muscle spasms, cardiac dysrhythmia (V-tach, Prolonged QT interval), hypotension, bradycardia
Acute hypocalcemia = med emergency
*Tetany (sustained muscle contraction), Hyper-excitable neuromuscular system
Treatments:
- Oral Calcium
- IV calcium diluted b/c irritating to veins (give slowly to prevent bradycardia or arrest)
- Educate in those at risk for osteoporosis
Always place on seizure precautions
Encourage Ca rich foods- Dairy and dark green leafy veg
Practice Question
The nurse is caring for a patient with metastatic bone cancer. Which of the following clinical manifestations would alert the nurse to the possibility of hypercalcemia in this patient?
A. Paresthesia
B. Weakness
C Facial Spasms
D. Muscle Tremor
Magnesium
Regulates:
- Intracellular metabolism
- Relaxing muscle contractions
- Transmit nerve impulses
- Cardiac function
- 2nd most abundant intracellular electrolyte
- Mostly located in bone
- Serum Mg level 1.5 -2.5 mg/dL
Hypermagnesemia > 2.5 mg/dL
Cause:
- Increased intake, renal failure
S & S:
- Muscle weakness, cardiac dysrhythmias, NV, dyspnea
Treatments:
- Withhold Mg, hemodialysis, emergent = administer IV Calcium chloride or Calcium gluconate
Hypomagnesemia < 1.5 mg/ dL
Cause:
- Limited Mg intake, increased renal losses, malnutrition, alcohol ingestion
S & S:
- Neuromuscular hyperactivity, CNS irritability, cardiac dysrhythmias
Treatments:
- Mild: diet, PO or IV Mg replacement
- assess reflexes when giving
- slow infusion to avoid cardiac/resp arrest (1.5 mL/min or less of a 10% concentration)TEST
*Think about these past electrolytes for test
pH and Hydrogen Ion (H+)
- Acidity and Alkalinity of a solution depends on H+ concentration
- Increase in H+ concentration = acidity
- Decrease in H+ concentration = alkalinity
- pH ranges from 1 – 14
- Less than 7 = Acidic
- Greater than 7 = Alkaline
Hydrogen ions shift btw the extracellular and intracellular compartments to compensate for acid-base imbalances
pH of Blood
- Blood is slightly alkaline
- 7.35 – 7.45
- pH < 6.8 = death
- pH > 7.8 = death
Arterial blood is the most accurate blood source!
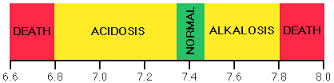
Acid Base Regulation
- Acid: H+ ion donor, release H+
- Increasing _acidity____/ decreasing the __pH____.
- Base: H+ ion receiver, decreasing H+
- Decreasing _acidity____/ increasing the __pH______.
- Buffer: A substance that prevents rapid pH change.
AcidBase regulation is determined by 3 pieces Acid, Base, and buffers
Increasing acidity/ decreasing pH
Decreasing Acidity/ increasing pH
Examples of Acid: Carbonic acid (CO2), sulfuric acid, lactic acid
Examples of bases: Bicarbonate (HCO3), Ammonia
Buffers are substances that bring body fluids back to normal pH range of 7.35-7.45, either by releasing H+ ions to make fluids more acidic or by binding with hydrogen ions to make fluids more alkaline
Buffers can be acidic or alkaline
If a fluid is basic then buffers release H+ ions to decrease the pH
If a fluid is acidic then buffers acts as binders, to bind with the H+ and increase the pH
Examples of Buffers: Bicarbonate (hCO3), phosphate, protein buffers
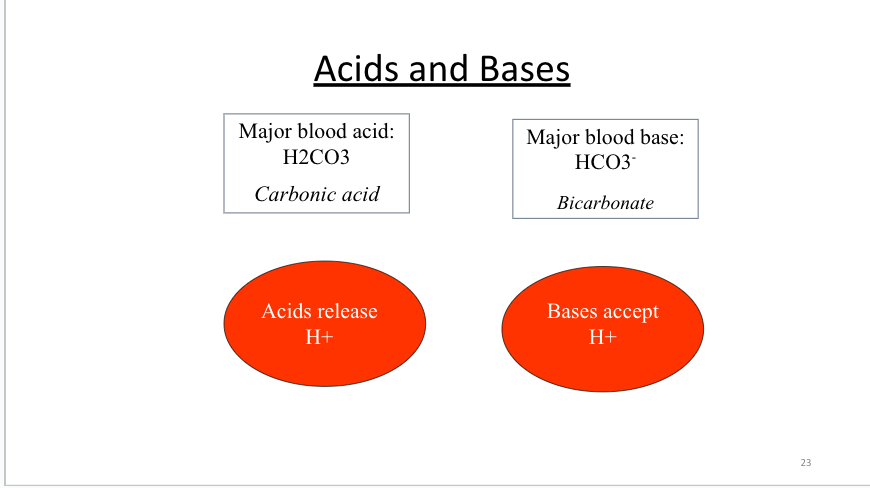
Notice that H2CO3 (carbonic acid) donates a H ion and becomes a base HCO3 ion.
MAJOR ACID is CARBONIC ACID
MAJOR BASE is BICARBONATE
Acidosis vs Alkalosis
- Acidosis
- Too much acid or too little base
- Alkalosis
- Too little __acid_______ or too much__base_______.
Buffers are used to maintain acid-base homeostasis
Acidosis: too much acid or too little base
Alkalosis: too little acid or too much base
Buffers are substances that bring body fluids back to normal pH range of 7.35-7.45, either by releasing H+ ions to make fluids more acidic or by binding with hydrogen ions to make fluids more alkaline
Buffers can be acidic or alkaline
If a fluid is basic then buffers release H+ ions to decrease the pH(make for acidic)
If a fluid is acidic then buffers acts as binders, to bind with the H+ and increase the pH (make more basic)
Examples of Buffers: Bicarbonate (hCO3), phosphate, protein buffers
Chemical, Respiratory, & Renal Buffers
- Chemical Buffers: react instantaneously to changes in pH to maintain normal pH of blood
- Respiratory Buffers: Lungs control/excrete carbon dioxide (CO2) to maintain normal pH of blood
* pH changes occur in minutes
- Renal Buffers: Kidneys control/excrete Bicarbonate (HCO3- ) to maintain normal pH of blood
* pH changes occur in hours or days
Chemical buffers are the first line of defense- either bind or release H+ ions as needed, respond quickly to pH changes
Respiratory Buffers are the second line of defense- control level of H+ ions in the blood through the CO2 levels (carbon dioxide), responds in minutes to hours
Hyperventilation = decrease hydrogen ions
Hypoventilation = increase hydrogen ions
Renal Buffers are the third line of defense , much slower to respond, but is the most effective buffering system with the longest duration, responds in hours to days
Looking at HCO3 bicarbonate levels
Respiratory vs Metabolic Imbalances
- Respiratory Imbalances
- An alteration in CO2, carbonic acid concentrations
- Respiratory acidosis: increase in CO2
- Respiratory alkalosis: decrease in CO2
- Metabolic Imbalances
- An alteration in HCO3, bicarbonate concentrations
- Metabolic acidosis: decrease in HCO3
- Metabolic alkalosis: increase in HCO3
Acid-base imbalance are classified as either Respiratory or metabolic, depending on the underlying cause of the alteration.
During a respiratory problem the kidneys compensate and if it is a metabolic problem the lungs compensate for the imbalance
27
*Respiratory Acidosis : Test purposes- review
- Increased CO2
- Increased H+ concentration = acidity
- Blood to acidic
REMEMEMEBER **** acidosis would be hypoventilation, if you do acid you will be hypoventilation also if you shit/diarhea its gonna be acidic
S & S:
- Confusion, drowsy, HA, tachypnea, tachycardia (mild), bradycardia (severe), hypotension, dysrhythmia, seizure, hypoventilation
Treatment
Oxygen therapy, patent airway, enhance gas exchange, pursed lip breathing, bronchodilators
- Caused by the retention of CO2 due to decreased respiratory rate or depth.
- Hypoventilation
- Inadequate chest expansion, muscle weakness, pneumo/hemothorax, flail chest, obesity, etc.
- Airway obstruction
- Inadequate mechanical ventilation
- Respiratory depression from poisons, anesthetics, trauma, or neurological diseases
WHAT ARE SOME EXAMPLES OF DISEASES or ILLNESSES THAT MIGHT RESULT IN RESPIRATORY ACIDOSIS??
🡪 COPD, CHF, Pneumonia, atelectasis, undervenilated, Narcotic/opioid overdose, pulmonary edema, pneumothorax
*Respiratory Alkalosis
- Decreased CO2
- Decreased H + concentration = alkalosis
REMEMBER Alkalosis, AL is super anxious! And vomitting is alkalotic
S & S:
- Dizzy, confusion, HA, tachypnea, tachycardia, dysrhythmia, N/V, epigastric pain, tetany, numbness, tingling, seizure
Treatment:
- Slow breathing to retain co2, bag breathing, anxiety reduction, o2 therapy
- Caused by an excessive loss of CO2 through spontaneous or mechanical hyperventilation
- Hyperventilation caused by
- Fear
- Anxiety
- Exercise
- Pain
- Head injury
- Sepsis
- Stroke
- Asthma
- Pneumonia
- Etc….
- Hyperventilation caused by
*Metabolic Acidosis
- Decreased HCO3
- Increased H+ concentration = acidosis
S & S:
- Tachycardia (mild), Bradycardia (severe), Kussmaul respirations, lethargy, confusion, weakness, anorexia, N/V/D, dysrhythmia, hypotension
Treatments:
- Depends on underlying cause.
- Administer bicarbonate
- DKA = insulin
- GI loss = antidiarrheal
- Occurs when the body produces too much acid or when the kidneys do not remove enough acid from the body
- Diabetic Keto-Acidosis (DKA)
- Shock
- Renal Failure
- Diarrhea, ileostomy
- Starvation
- Lactic Acidosis
- Heavy Exercise
- Pancreatitis
- Liver Failure
*Metabolic Alkalosis (continued)
Increased HCO3
Decreased H+ concentration = alkalosis
S & S:
- Dizzy, confusion, HA, Tachycardia, dysrhythmia, N/V, anorexia, tremor, tetany, tingling, cramps, seizure, hypoventilation
Treatment:
- Depends on underlying cause
- Antiemetic, fluids, electrolyte replacement
- Diamox (excretes HCO3)
- Caused by a loss of hydrogen ions from the body
- Shift of H+ into cells
- Gain of Bicarbonate
- Diuretic use (K+ depletion)
- Loss of gastric secretions (vomiting, NG suction)
- Oral ingestion of antacids
Diamox SEQUELS (Acetazolamide Extended-Release Capsules) are an inhibitor of the enzyme carbonic anhydrase.
Acts as a diuretic to excrete bicarbonate
A Helpful Hint
- Food going up (vomiting), pH going up (alkalotic), food going down (diarrhea) pH going down (acidotic).
- Look at the pH first and then compare.
ABG (Arterial Blood Gas)
- ABGs usually done by respiratory therapists (RT)
- Reason to test:
- lung problems, check how lungs are functioning, acid-base balance, kidney failure
Normal Arterial Blood Gas Values
(Table 17.15, p. 323 Lewis’s Med/Surg Text , 12th Ed)
*remember these top three
- pH = 7.35 to 7.45
- PaCO2 = 35 to 45 mmHg
- Bicarbonate (HCO3-) = 22-26mEq/L - tells us if it is metabolic
- PaO2 = 80-100 mmHg
- O2 Saturation = 96% - 100%
- Base Excess = ± 2.0 mEq/L
Base Excess: (relates only to metabolic): metabolic alkalosis if too high (more than +2 mEq/L) or metabolic acidosis if too low (less than −2 mEq/L).
BE is better indicator of metabolic acidosis or alkalosis because it removes the respiratory influences.
If BE and HCO3 conflict, use BE.
Indicator of metabolic
If increased ( 2 or greater) = metabolic alkalosis
If decreased (-2 or less) = metabolic acidosis
Steps to evaluating ABGs
1. Evaluate pH – normal, acidotic, or alkalotic.
2. Evaluate respiratory process(ventilation/breathing pattern or pCO2)? – normal or abnormal?
3. Evaluate the metabolic process (HCO3)?–nl or abnl?
4. Compare pH with pCO2 or HCO3
5. Make diagnosis
6. Evaluate perfusion (gas ex/oxygenation/pO2), if low then hypoxemia (decreased O2 in arterial blood)? (this is a skill we will get to later in the program)
First 5 steps is what we will focus on in class.
Compensated: if pH is back to “normal” (remember 7.35 - 7.4 is either normal OR compensated acidosis, 7.4 to 7.45 is either normal OR compensated alkalosis).
Base excess (relates only to metabolic): metabolic alkalosis if too high (more than +2 mEq/L) or metabolic acidosis if too low (less than −2 mEq/L). BE is better indicator of metabolic acidosis or alkalosis because it removes the respiratory influences. If BE and HCO3 conflict, use BE.
Mnemonics: “ROME”
- Respiratory
- Opposite
- Alkalosis ↑ pH ↓ PaCO2
- Acidosis ↓ pH ↑ PaCO2
- Metabolic
- Equal
- Acidosis ↓ pH ↓ HCO3
- Alkalosis ↑ pH ↑ HCO3
Food going up (vomitting), pH going up (alkalotic), food going down (diarrhea) pH going down (acidotic).
Look at the pH first and then compare.
If pH and CO2 are going in the same direction then it is metabolic
If pH and Co2 are going in opposite directions then it is respiratory
36
Acidotic, Alkalotic, Normal? * Remember the normal values
pH 7.36 - normal
pH 7.32- acidic
pH 7.49- alkaltoic
pH 7.55- alkalotic
pH 7.44 - normal
Interpret the pH
37
37
CO2-
PaCO2 36-
PaCO2 47
- hihgh
PaCO2 70
PaCO2 19
PaCO2 37
Interpret the PaCO2
38
38
HCO3- 22-26
HCO3 25
HCO3 35
HCO3 16
HCO3 22
HCO3 26
Interpret the bicarbonate values
39
39
ABG Interpretation Chart
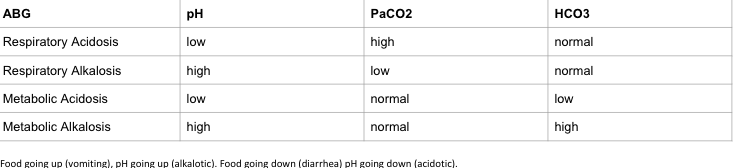
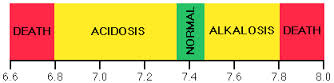
ABG Case Studies
metabolic acidosis
respiratory acidosis
metabolic alkalosis
41
Question …
A patient is anxious and hyperventilating. The blood gas pH result is 7.51. The nurse determines that this most likely represents
A. Respiratory acidosis
B. Respiratory alkalosis
C. Metabolic acidosis
D. Metabolic alkalosis
42
B. What is the initial tx for a person hyperventilating?
42
Question
The nurse is admitting a patient with complaints of abdominal pain and
vomiting. A bowel obstruction is suspected. The nurse assesses this
patient for which of the following anticipated primary acid-base
imbalances if the obstruction is high in the intestine.
A. Metabolic Alkalosis
B. Metabolic Acidosis
C. Respiratory Alkalosis
D. Respiratory Acidosis
Review & Diuretics
Objectives
- Identify clients in need of diuretic treatment
- Determine appropriate diuretic use for specific clients
Case Study 1
A 35 year-old male client is seen in the ED with reports of nausea, vomiting, dizziness, and weakness. History reveals that he had just completed a 10-mile run in preparation for upcoming marathon when the onset of symptoms occurred. Physical assessment reveals dry oral mucous membranes, temp. 38.5 (101.3), pulse 92 beats/min and thready, respirations 20/min, skin cool and diaphoretic, BP 102/64 mm Hg. His urine is concentrated with high specific gravity.
Is he experiencing fluid volume excess or deficit?
What client findings are consistent with the presence of this volume imbalance?
What history is supportive of the diagnosis?
Deficit
Dry oral mucous membranes, elevated body temp, hypotension, tachycardia, thread pulse, concentrated urine with high SPG, diaphoretic
N/V, dizzy, strenuous exercise
Serum electrolytes (Na especially), BUN/Creatinine
Identification and treatment of underlying cause, encouraging oral fluids, and administering isotonic IV fluids
Deficient fluid volume r/t abnormal fluid loss- diaphoresis
Impaired oral mucous membrane r/t dehydration
Hyperthermia r/t dehydration
Risk for injury
3
Case Study 2
An 83 year-old female client is admitted to an acute care facility with dyspnea, weakness, weight gain of 2 lb, 1+ pitting edema of both lower extremities to mid calf, and bilateral crackles in lung bases.. Assessment finding include: temp 37.2 (99), pulse 96 and bounding, RR 26/min, pulse ox 94 % on 3 L O2, and BP 152/96. She is diagnosed with congestive heart failure (CHF).
1. Is she experiencing fluid volume excess or deficit?
2. What data supports the presences of this volume imbalance?
Excess
Dyspnea, weakness, wt gain, pitting edema, crackles, temp, pulse bounding, RR 26, 94% on 3L, BP elevted
CHF occurs when the cardiac muscle is to weak to maintain adequate cardiac output. This results in decreased renal blood flow and renal perfusion. This prompts the retention of Na and water. Poor myocardial contraction also results in insufficient ventricle emptying resulting in pulmonary and or peripheral edema
There is an increased risk for fluid volume excess related to changes and decreased function in cardiac and or renal function
Diuretic (Furosemide, Lasix)
K+ because furosemide is a K+ wasting loop diuretic which may result in hypokalemia Get K on board
Position client in high fowlers
reduce IV fluid rate, fluid restriction, monitor I & O, elevate extremities to reduce edema, o2 as needed, monitor wt, monitor ABG
4
Question …
A patient is anxious and hyperventilating. The blood gas pH result is 7.51. The nurse determines that this most likely represents
A. Respiratory acidosis
B. Respiratory alkalosis
C. Metabolic acidosis
D. Metabolic alkalosis
B. What is the initial tx for a person hyperventilating?
5
Scenario: A 62-year old male client who is a widower, retired, and living alone, enters the emergency department reporting shortness of breath and tingling in his fingers. His breathing is shallow and rapid (30/min). He denies diabetes and his blood glucose is normal. There are no ECG changes. He has no significant respiratory or cardiac history. He takes several anti-anxiety medications. He says he has had anxiety attacks before. While the client is being worked up for chest pain, an ABG is done. Results are:
pH 7.48, PaCO2 28mm Hg, PaO2 85 mm Hg, HCO3 22 mEq/L.
1. What, if any, acid-base imbalance is the client experiencing?
2. Which client findings support this conclusion?
3. What nursing interventions are appropriate for this client?
6
Diuretics
Anatomy
- Basic functional unit of the kidney: Nephron
- Four functionally distinct regions
- Glomerulus
- Proximal convoluted tubule
- Loop of Henle
- Distal convoluted tubule
Introduction to Diuretics
- How diuretics work: Mechanism of action
- Blockade of sodium and chloride reabsorption
- Site of action
- Proximal tubule produces greatest diuresis
- Adverse effects
- Hypovolemia
- Acid-base imbalance
- Electrolyte imbalances
Classification of diuretics
- Four major categories
- Loop: Furosemide, bumetanide, torsemide
- Thiazide: Hydrochlorothiazide, chlorthalidone, indapamide
- Osmotic: Mannitol
- Potassium-sparing: Two subcategories
- Aldosterone antagonists (spironolactone)
- Nonaldosterone antagonists (triamterene)
Loop Diuretics
- Furosemide (Lasix): Most frequently prescribed loop diuretic
- Mechanism of action
- Acts on ascending loop of Henle
- Mechanism of action
- Pharmacokinetics
- Rapid onset (PO 60 minutes; IV 5 minutes)
- Therapeutic uses
- Pulmonary edema
- Edematous states
- Hypertension
Furosemide [Lasix] -
All are hypo– lowww
- Adverse effects
- Hyponatremia, hypochloremia, and dehydration
- Hypotension
- Loss of volume
- Relaxation of venous smooth muscle
- Hypokalemia- impacting CV system
- Ototoxicity
Drug interactions (for Lasix)
- Digoxin
- Ototoxic drugs-aminoglycoside antibiotics, antimalarials
- 0 Potassium-sparing diuretics
- Lithium
- Antihypertensive agents
- Nonsteroidal anti-inflammatory drugs
Preparations, dosage, and administration
- Oral
- Parenteral (IV)
Thiazides and Related Diuretics
- Also known as benzothiadiazides
- Effects similar to those of loop diuretics
- Increase renal excretion of sodium, chloride, potassium, and water
- Elevate levels of uric acid and glucose
- Maximum diuresis is considerably lower than with loop diuretics
- Not effective when urine flow is scant (unlike with loop diuretics)
Hydrochlorothiazide [HydroDIURIL]- HCTZ
- Most widely used
- Action: Early segment distal convoluted tubule
- Peaks in 4 to 6 hours
Therapeutic uses
- Essential hypertension
- Edema
- Diabetes insipidus-body makes large amt of urine
Adverse effects
- Hyponatremia, hypochloremia, and dehydration
- Hypokalemia- CV system
- Use in pregnancy and lactation
- Hyperglycemia
- Hyperuricemia
- Impact on lipids, calcium, and magnesium
Potassium-Sparing Diuretics
- Useful responses
- Modest increase in urine production
- Substantial decrease in potassium excretion
- Rarely used alone for therapy
- Aldosterone antagonist
- Spironolactone
- Nonaldosterone antagonists
- Triamterene
- Amiloride
Spironolactone [Aldactone]
- Mechanism of action
- Blocks aldosterone in the distal nephron
- Retention of potassium
- Increased excretion of sodium
- Therapeutic uses
- Hypertension
- Edematous states
- Heart failure (decreases mortality in severe failure)
- Primary hyperaldosteronism
- Premenstrual syndrome
- Polycystic ovary syndrome
- Acne in young women
- Hypertension
- Adverse effects
- Hyperkalemia- CV systme HYPER HYPER HYPER!!!!! TEST
- Endocrine effects
- Drug interactions
- Thiazide and loop diuretics
- Agents that raise potassium levels
- Food interactions
- avoid salt substitutes- some are potassium salts make with KCl which can have additive effect when used with spironolactone (hyperkalemia)
Osmotic Diuretic
- Mannitol [Osmitrol]
- Promotes diuresis by creating osmotic force within lumen of the nephron-draws free water out of brain to be eliminated
- Pharmacokinetics
- Drug must be given parenterally
- Therapeutic uses
- Prophylaxis of renal failure
- Reduction of intracranial pressure-(tumor, bleeding, swelling of brain)
- Reduction of intraocular pressure
Mannitol [Osmitrol]
- Adverse effects
- Edema
- Headache
- Nausea
- Vomiting
- Fluid and electrolyte imbalance
- Nursing Considerations
- check for crystals, ? if present
- filter needle and in-line IV filter
A patient who sustained a head injury is admitted to the critical care unit with increased intracranial pressure (ICP). The healthcare provider says that a diuretic will be used to lower the patient’s ICP. The nurse anticipates that which diuretic will be ordered, and why?
Case Study
Copyright © 2019 by Elsevier, Inc. All rights reserved.
27
The nurse anticipates that mannitol, an osmotic diuretic, will be ordered. Intracranial pressure (ICP) that has been elevated by cerebral edema can be reduced with mannitol. The drug lowers ICP because its presence in the blood vessels of the brain creates an osmotic force that draws edematous fluid from the brain into the blood. There is no risk of increasing cerebral edema because mannitol cannot exit the capillary beds of the brain.
27
Mannitol IV has been ordered for the patient. When the IV solution of mannitol arrives from the pharmacy, the nurse notes crystals in the fluid. What is the most appropriate action by the nurse?
Case Study (Cont.)
Copyright © 2019 by Elsevier, Inc. All rights reserved.
28
Mannitol may crystallize out of solution if exposed to a low temperature. Accordingly, preparations should be observed for crystals before use. Preparations that contain crystals should be warmed (to redissolve the mannitol) and then cooled to body temperature for administration.
28
Are there any other steps the nurse should take regarding IV administration of mannitol?
Case Study (Cont.)
Copyright © 2019 by Elsevier, Inc. All rights reserved.
29
Along with following all the appropriate safety precautions for medication administration, the nurse should use a filter needle to withdraw mannitol from the vial, as well as an in-line filter for IV infusion, to prevent crystals from entering the circulation.
29
When providing discharge teaching for a patient who has been prescribed furosemide [Lasix], it is most important for the nurse to include which dietary items to prevent adverse effects of furosemide [Lasix] therapy?
Oranges, spinach, and potatoes
Baked fish, chicken, and cauliflower
Tomato juice, skim milk, and cottage cheese
Oatmeal, cabbage, and bran flakes
Question
Copyright © 2019 by Elsevier, Inc. All rights reserved.
30
30
Answer: A
Rationale: Furosemide may have the adverse effect of hypokalemia. Hypokalemia can be reduced by consuming foods that are high in potassium, such as nuts, dried fruits, spinach, citrus fruits, potatoes, and bananas.
A patient is prescribed spironolactone [Aldactone] for treatment of hypertension. Which foods should the nurse teach the patient to avoid?
Baked fish
Low-fat milk
Salt substitutes
Green beans
Question
Copyright © 2019 by Elsevier, Inc. All rights reserved.
31
31
Answer: C
Rationale: Spironolactone is a potassium-sparing diuretic. Medications that are potassium sparing, potassium supplements, and salt substitutes should be avoided. High-potassium foods should also be avoided.
DRUG TABLE