Organic Lecture 2/21
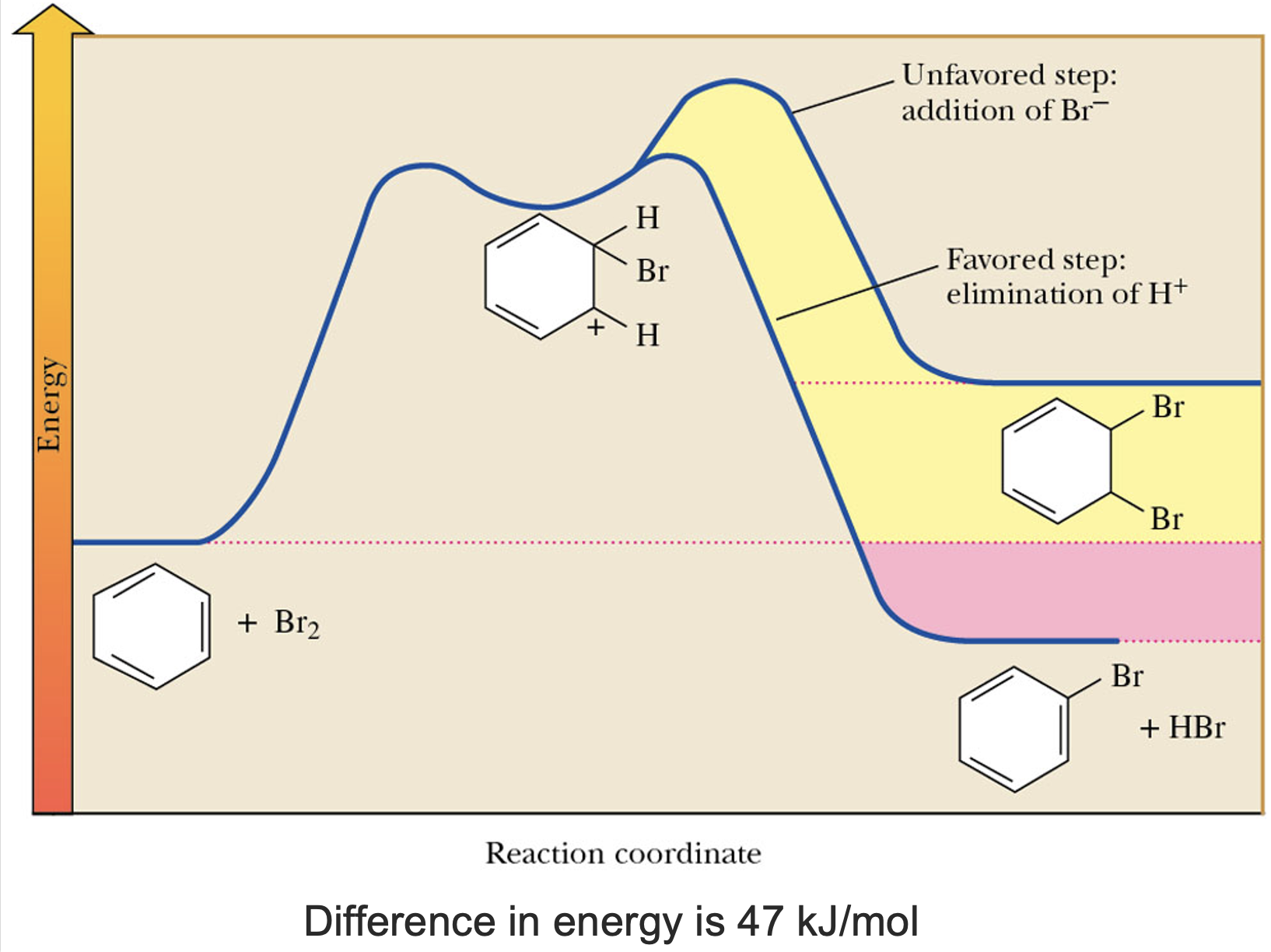
Mechanisms of Electrophilic Aromatic Substitution: Halogen
The process begins with a bromine and a bromine cation (Br+), which can arise from the collapse of an iron pair in a reaction mixture.
The benzene ring acts as a nucleophile, exhibiting the ability to attack electrophiles such as Br+.
Formation of the addition product results in the loss of stabilization of the aromatic ring. Formation of substitution product regenerates the resonance-stabilized aromatic ring.
Iodine: Use nitric acid for its reaction
Nitration of Arenes
Benzene reacts with nitric acid in the presence of H2SO4/30-40Celsius, creates a nitrobenzene with water as a side product. Nitration gives very high yields.
Tyrosine: An amino acid that contains a phenolic hydroxyl group. Its structure includes a benzene ring off the side chain, making it susceptible to nitration.
Using nitric acid on tyrosine can lead to nitration, where the hydroxy group can influence reactivity.
During this reaction, the negatively charged oxygen does pick a proton, but this step does not lead to product formation.
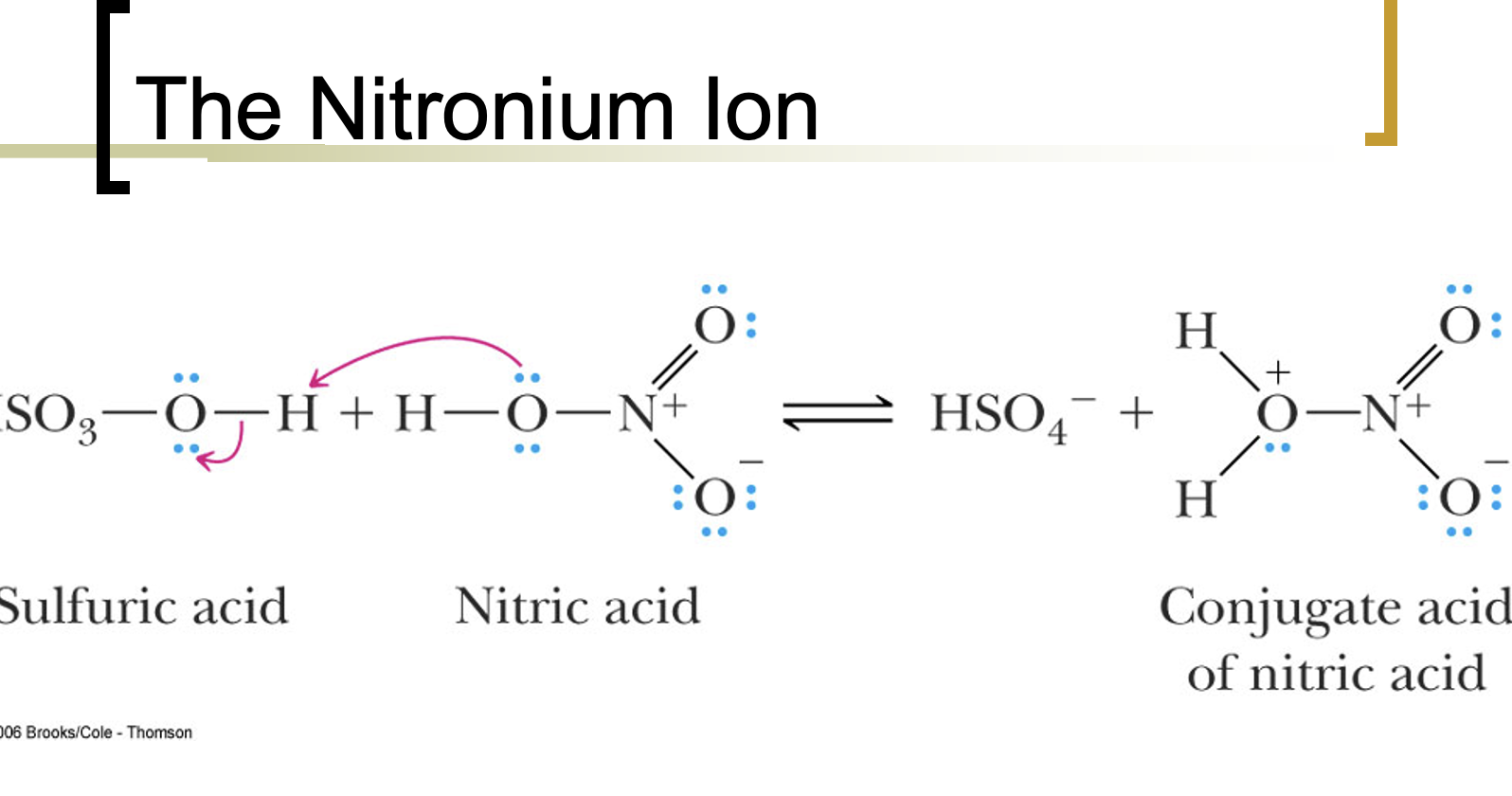
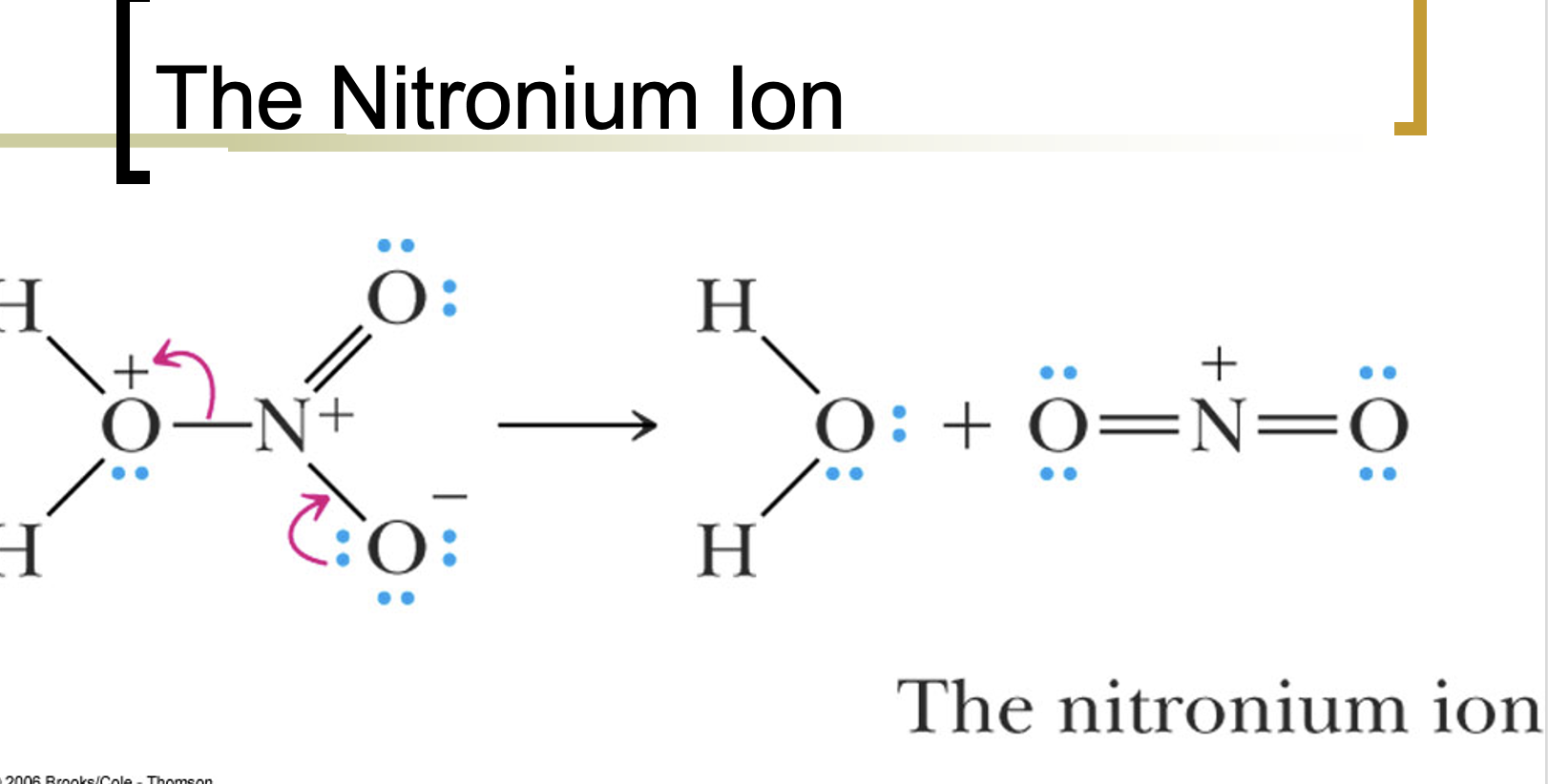
Nitronium ion acts as the electrophile in nitration of arenes.
Water is the base of the reaction.
Sulfonation of Arenes
Mixing red powder (likely sulfur trioxide) with sulfuric acid yields fuming sulfuric acid, which releases toxic SO2 gas.
Reaction: Benzene + SO3 in the presence of H2SO4 adds SO3 to benzene (benzenesulfonic acid)
Generate electrophile (HSO3+), benzene attacks electrophile to form carbocation, then regenerate aromaticity. The base is either HSO4- or another molecule of the HSO3.
This reaction is the only electrophilic addition to the aromatic ring that is reversible: heating with water can reverse these reactions. Low heat adds ortho; high heat adds para.
Influence of Substituents on the Aromatic Ring
The benzene ring’s role as a nucleophile means substituents can influence its reactivity.
Electron-Withdrawing Groups (EWGs): These reduce nucleophilicity by pulling electron density away from the ring, making it less likely to undergo electrophilic substitution.
Electron-Donating Groups (EDGs): Conversely, groups like alkyl chains can donate electrons via hyperconjugation, increasing the reactivity of the ring.
Example: A methyl group (–CH3) added to benzene increases its electron density, hence it reacts quicker with additional electrophiles compared to benzene alone.
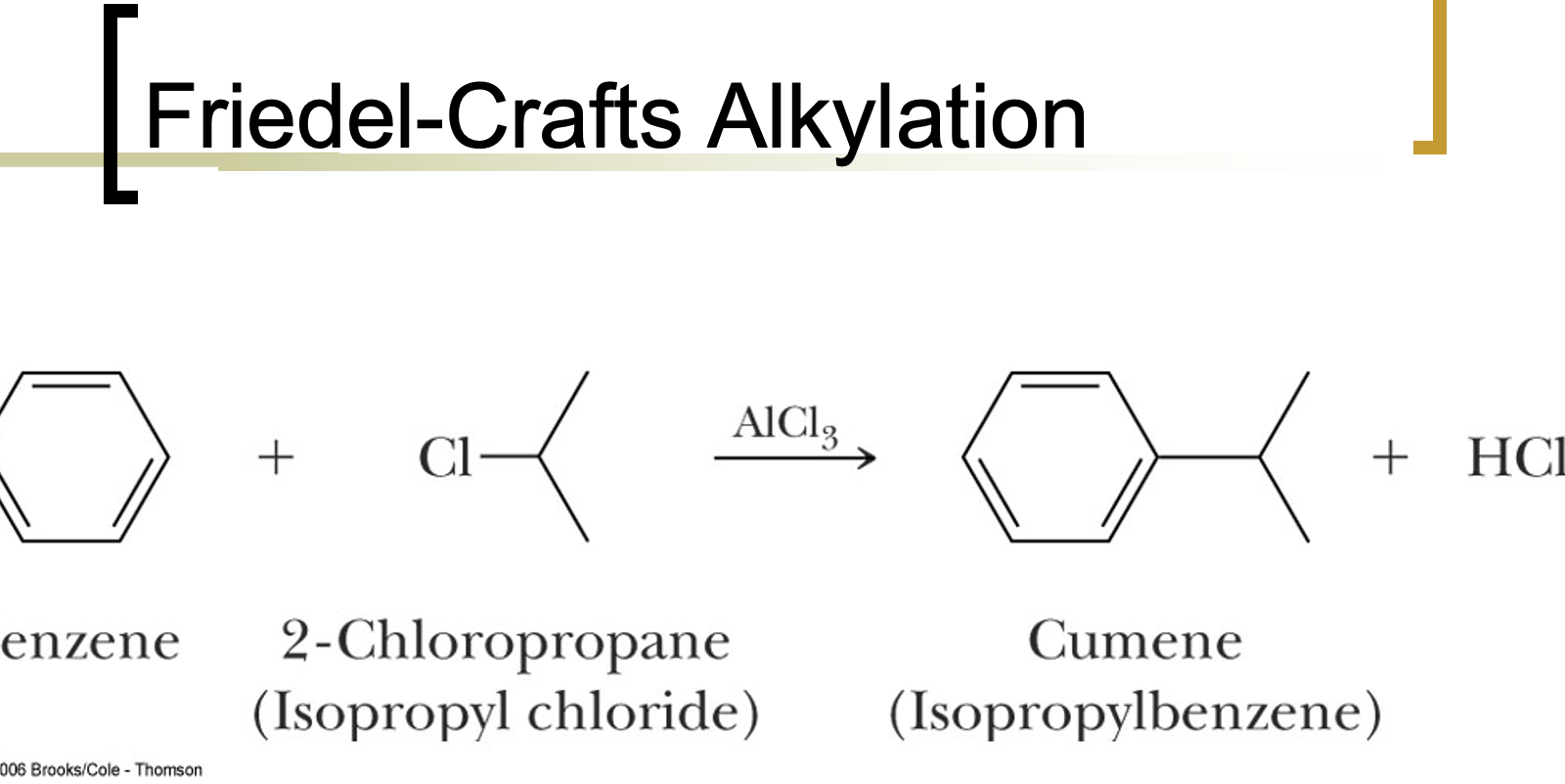
Friedel-Crafts Alkylation Reactions
Friedel-Crafts alkylation and acylation can add alkyl groups to the aromatic ring. Alkylation introduces a methyl group, which then facilitates further reactions.
If one benzene has a methyl group, it reacts quicker than another benzene without a methyl group due to already increased electron density.
no vinyl halides
can go through carbocation rearrangements
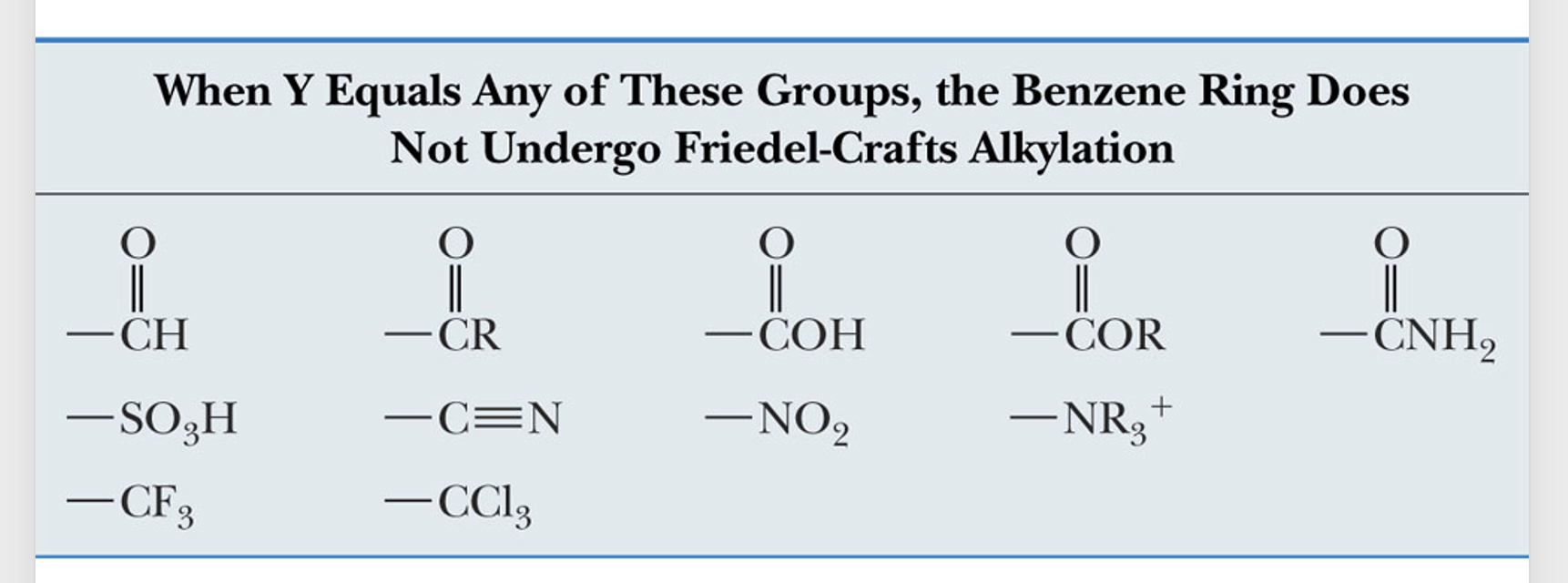
reaction fails if the electron-withdrawing group is attached to the ring because aromatic ring acts as the nucleophile
Hard to stop at just one alkylation
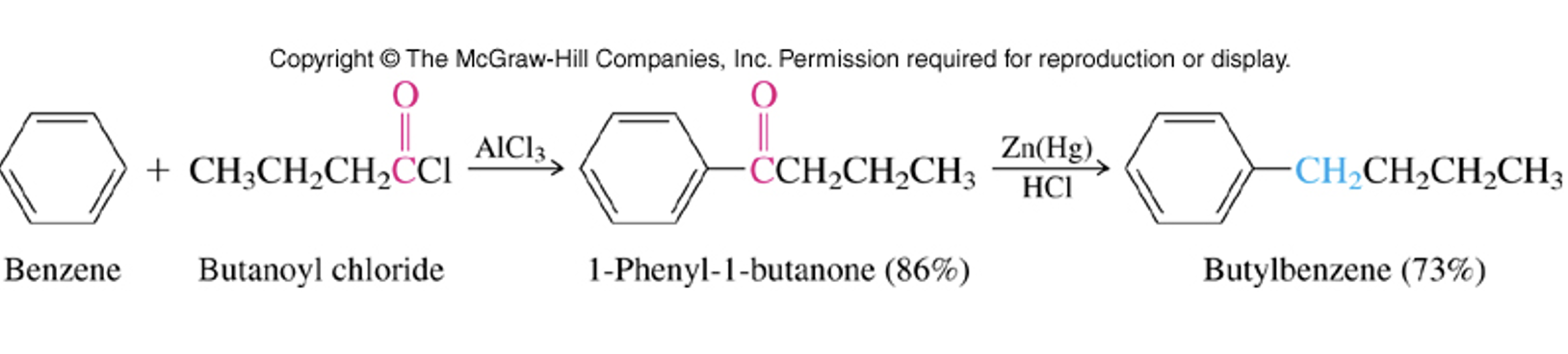
Friedel-Crafts Acylation
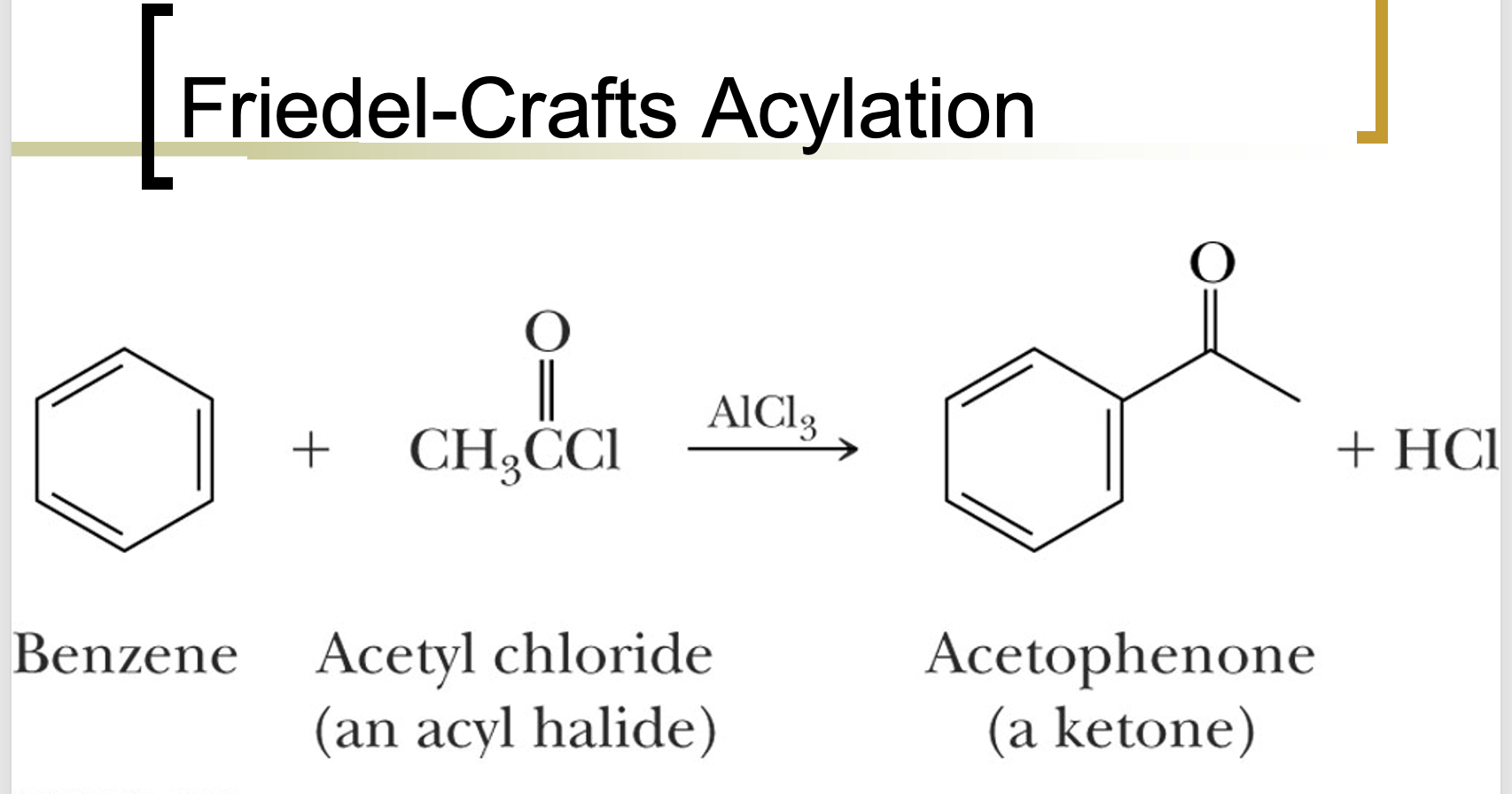
Chlorine attacks aluminum. This leads to the formation of an acylium ion, which then reacts with the aromatic compound to introduce an acyl group.
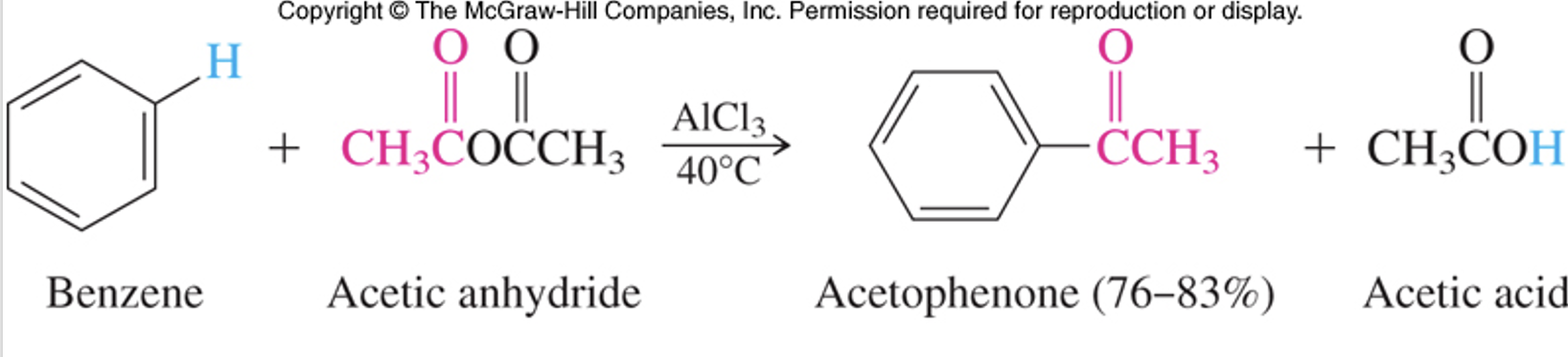
Multiple additions not probable because electron withdrawing group is attached to the ring. Acyl ions are easily formed and can be made from acid anhydrides as well.
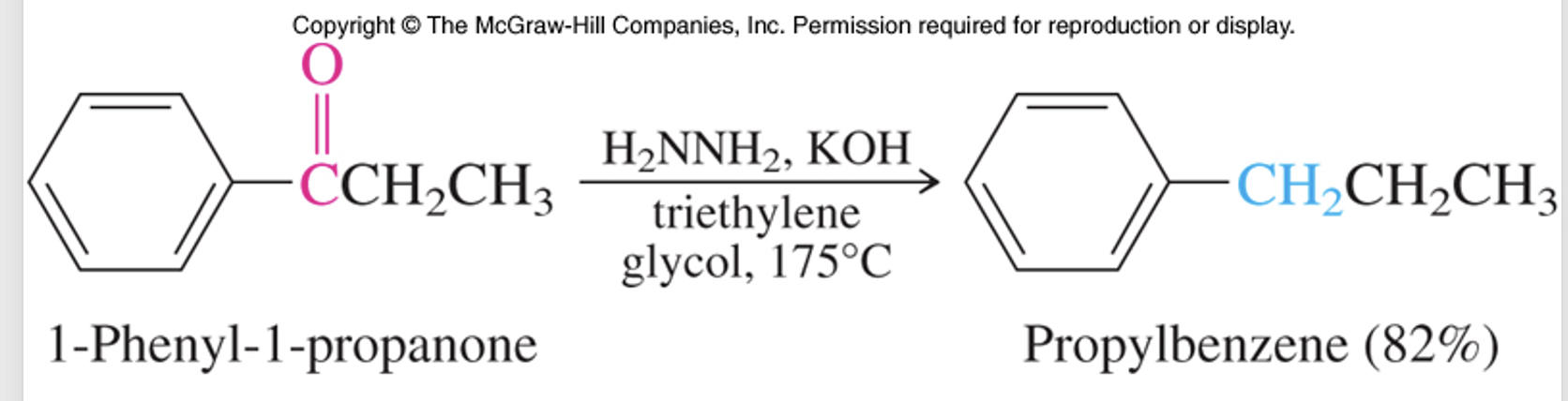
An alternative for introducing alkyl groups involves converting bromine into a Grignard reagent to perform an SN2 attack on a primary halide.
This is often followed by reduction using hydrazine (N2H4) solvated in basic triethylene glycol (representing a solvent with particular boiling point conditions).
Clemmensen Reduction
Reduction of carbonyls. Does not reduce carboxylic acids or interfere with alkenes/alkynes
Wolff-Kishner Reduction
This reduction process is essential for turning acylated products into alkylated products, crucial for aromatic compound modifications.
Does not reduce carboxylic acids or interfere with alkenes/alkynes
The selection of triethylene glycol, with a higher boiling point (around 75 °C), is key for energy-demanding reactions to ensure proper conditions.
method of choice most of the time