Chapter 20 - Cell Communities and Tissues
Different kinds of cells are organized into tissues, where the cells serve a collective purpose for an organism. Tissues can comprise of different differentiations of cells. Epithelial cell layers divide environments, like the kidney from lumen which contains urine. Connective tissue tends to have lots of extracellular matrix, which is made by fibroblasts.
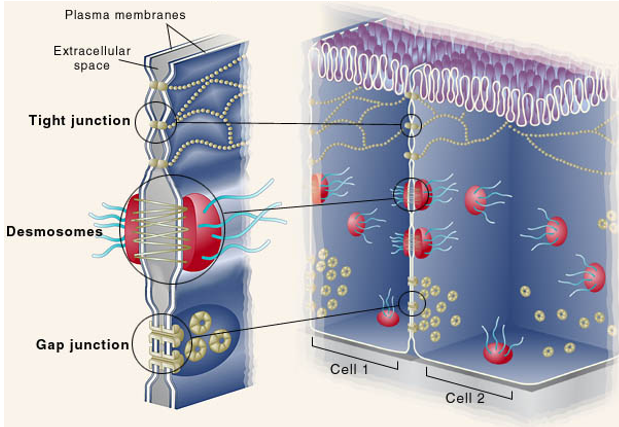
Between cells there is extracellular space. Connecting cells, there may be tight junctions, along with desmosomes and gap junctions. These are comprised of transmembrane proteins from each cell that ‘hold on’ to each other. Cell-cell junctions are between cells, cell-matrix junctions connect the cell to the ECM.
Cartilage is an extracellular matrix that contains many collagen fibrils
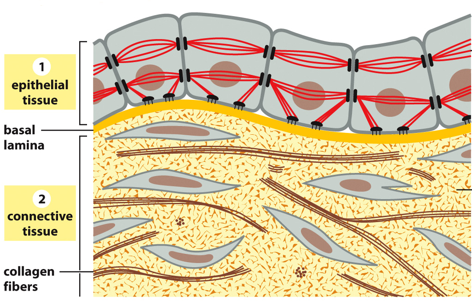
Cells are connected to an environment via epithelial tissue. These cells have cytoskeletal filaments that anchor to the cell matrix and aid in cell-cell adhesion. They absorb mechanical stress transmitted from cell to cell. The basal lamina separates this from various forms of connective tissue, where extracellular matrix directly bears mechanical stresses of tension/compression. The ECM has many carbohydrates to resist tensile forces and bind to water.
There are 3 types of cell junctions, anchoring junctions which anchor cells in a place, occluding junctions which separate cells from each other and prevent things from diffusing from cell to cell (tight junctions like the blood brain barrier), and channel forming junctions which connect neighboring cells and are found only in epithelia/endothelia as they require direct plasma membrane contact between cells. Another 4th type is a signal relaying junction, like those found in the brain that transmit neurotransmitters. Here, there are proteins that help maintain the specific space between cells (synaptic cleft)
Epithelia separates cells from the outside environment, while endothelia separates kinds of internal environments from others (like in blood vessels). Apical refers to the side facing the lumen, and basal is “the outside” or “the bottom”
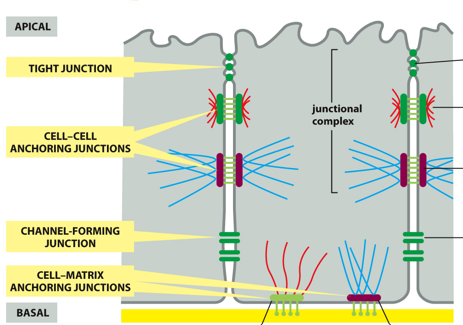
(MEMORIZE THIS IMAGE) A tight junction seals gaps between epithelial cells, and is located near the apical surface of the cell. Cell-cell anchoring junctions below these, connecting actin filament bundles in one cell with actin filament bundles in the next cell. A desmosome connects intermediate filaments between cells with one another. More basal, there are channel-forming junctions like gap junctions, which allow the passage of small molecules from cell to cell
Basally, there are cell-matrix anchoring junctions, like actin-linked cell-matrix junctions that anchor actin to the extracellular matrix and hemidesmosomes which anchor intermediate filaments to the extracellular matrix
A zonula, or bell, includes tight junctions (zonula occludins) and adherins junctions (zonula adherins). They are referred to this way, as they go all around the cell in a circle 3D. Neither of these are connected to the cytoskeleton
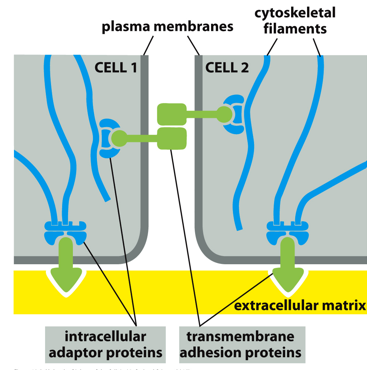
Between two cells, cytoskeletal filaments support intracellular adaptor proteins, which attach to transmembrane adhesion proteins that can attach to one another
Cadherins are calcium dependent adhesin proteins. Cells of similar type have similar cadherins that can bind to one another. (E-cadherin for epithelium, N-cadherin for neural, P-cadherin for placental etc). They are dimers with Ca2+ bridge domains, with a cell adhesion zipper. The presence of these varying cadherins is how different cells are sorted developmentally.
There are classical cadherins and nonclassical cadherins. Classical are for adherins junctions, and nonclassical are between desmosomes. Classical connect to actin, nonclassical connect to IFs. Classical only bind to other classical, and nonclassical to other nonclassical.
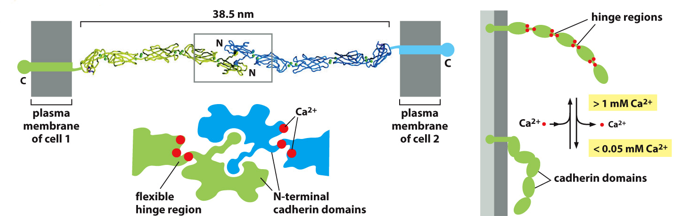
Structurally, a cadherin is many subunits with hinge regions that are flexible. The binding of calcium allows them to “straighten” and reach out to another cadherin, to bind with an N-terminal cadherin domain. They will zipper together, and are held by Ca2+ bridges. These are all noncovalent interactions, but the high number of them is stabilizing (like velcrox)
Due to the importance of calcium fir cadherins, adding a chelator will cause cells to separate, as the calcium will bind to it instead of holding the cadherins together
Homophilic binding will occur with a cadherin if they have the same binding domain, and heterophilic binding if domains are different but they still bind. There is a lot of diversity among the cadherin superfamily. Classical and nonclassical cadherins use homophilic binding. Integrins use heterophilic binding
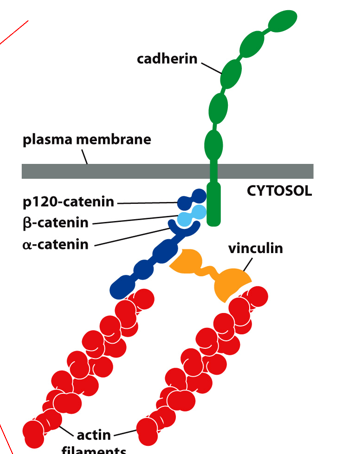
Adherens are classical cadherins connected to actin filaments. They are located centrally, but closer to the apical side of an epithelial cell. This process utilizes adaptor proteins to connect a cadherin to the actin filaments (don’t need to know specifics)
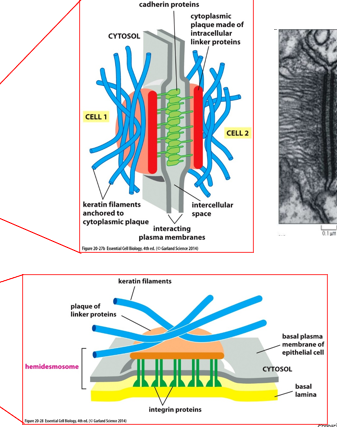
A desmosome joins intermediate filaments between cells, or for the hemidesmosome it joins IFs to the extracellular matrix. Keratin filaments anchor to a cytoplasmic plaque, and cadherins join together between cells. Hemidesmosomes have integrin proteins to attach them to the basal lamina. The rigid structure that IFs come from is a plaque, which form the interface between IFs and cadherins/integrins.
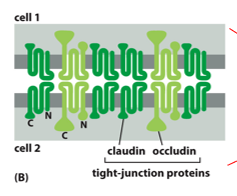
Tight junctions are located apically, and circle the entire cell in a 3D sense. Many of these help form a tight barrier between the extracellular environment and tissues. Each ‘bubble’ or intercellular space is closed off by interactions between claudin and occludin. These don’t cross bind, claudins only bind to claudins and occludins only bind to occludins. Nothing gets in and nothing gets out between these (except white blood cells, but not in the BBB).
The blood brain barrier is one example of where tight junctions are frequently used, the BBB allows for immune cells to pass between cells
Tight junctions also seal the epithelial barrier (midgut) lumen to tissue (interstitium). Tight junctions act as molecular senses, and restrict lateral diffusion of membrane proteins across the plasma membrane. These are vital in maintaining concentration gradients across a membrane, like that of glucose. Molecular fences are tight junctions
Gap junctions are for intercellular communication, cytosols connect via hydrophilic channels and allow for the diffusion of molecules. These can be opened or closed. This process is relatively non-selective, a functional unit is all cells that make cytoplasmic contact
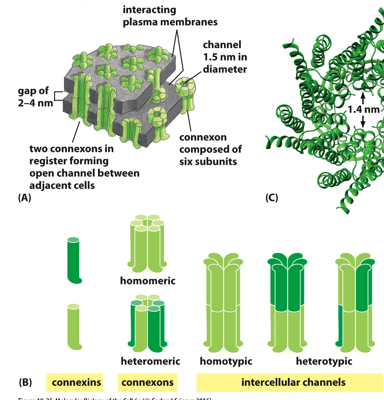
Structurally, a gap junction is made of multiple connexons, each of which have 6 subunits, called connexins. Two of these from 2 different connected cells form an open channel, allowing things to diffuse across. 6 connexins form a connexon (1/2 the total protein), and 2 connexons form one gap junction. Many gap junctions are grouped together. They can only allow in smaller particles, larger ones have a hard time getting through.
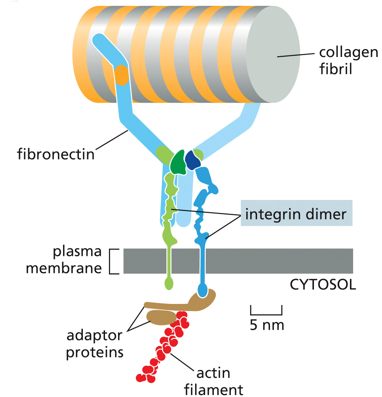
Integrins are proteins that anchor cells to the extracellular matrix, having an active and an inactive form.
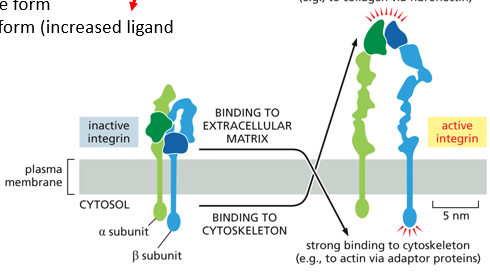
Actively, they have increased ligand affinity, and strongly bind to the extracellular matrix. There are alpha and beta subunits. When the inactive form senses a signal, it will unfold into the active position
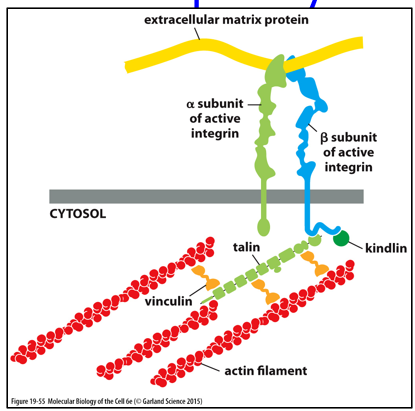
Focal adhesion is between integrins and actin, and is more temporary. This is seen in white blood cells, that can move between tissue types. An integrin will bind to different components on a medium (like a petri dish or kind of issue), but the temporary nature allows for the cell to move faster.
White blood cells roll along the endothelium of blood vessels, scanning the cell surfaces for changes in the glycocalyx, which may indicate infection. They have proteins called selectins, which scans carbohydrates. If a difference is detected, instead of a selectin, and integrin is used to bind to the cell, breaking tight junctions and targeting the cell. This process is homing, selectins bind to oligosaccharides
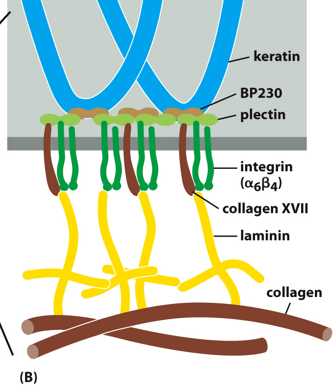
With hemidesmosomes, integrin interacts with IFs in a long-term stable manner. The binding is heterophilic
The extracellular matrix (ECM) is an organized network of extracellular materials, that is dynamic and determines shape and activity of a cell. This comprises anything not inside the cell. Components include fibrous proteins and glycoproteins. There are also proteoglycans and GAGs. Glycoproteins are functionally proteins that happen to carry a glycosyl group, and proteoglycans are mainly sugars that may contain a protein to aid in binding function.
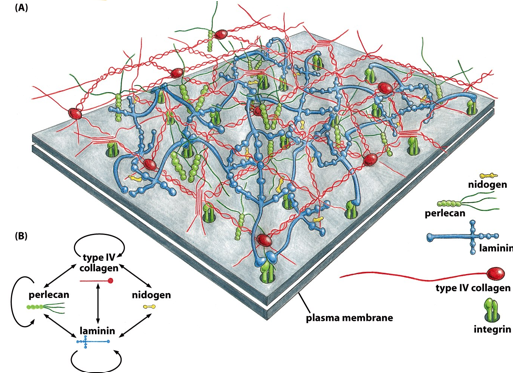
In the basal lamina, the ECM is well developed. The grey square is the cell’s plasma membrane. In the basal lamina, there are nidogens, integrins, perlecans, laminins, and collagens. They only connect to specific other molecules, as outlined in the bottom left. This is a well organized matrix.
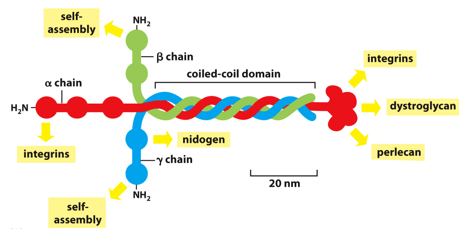
Laminin self assembles (binds to other laminins), being a coiled coil of different proteins, alpha beta and gamma. This is why it is called basal LAMINA. Nidogen connects laminins and collagens to each other, with 3 binding domains, it acts as an adaptor.
Proteoglycans are protein and polysaccharide complexes, consisting of a core protein and many sugar chains. They look like centipedes, many of these subunits (aggrecans) will bind around hyaluronic acid, forming a sort of pine tree structure.
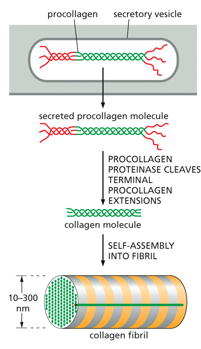
Collagen is a supportive structure, a fibril is made up of a triple stranded collagen molecule. This kind of helix is much more shallow than an alpha helix, as it contains many glycines and prolines. Hydrogen bonds and disulfide bridges stabilize the triple threaded structure, many group up to form a collagen fibril, which will then associate with many others to form a collagen fiber.
A single strand of collagen will have many repeats of glycine and proline, sometimes containing a hydroxylproline (hyp), which allows for typically non-polar proline to form hydrogen bonds to stabilize collagen. Vitamin C is a cofactor for the protein that hydrolyzes proline
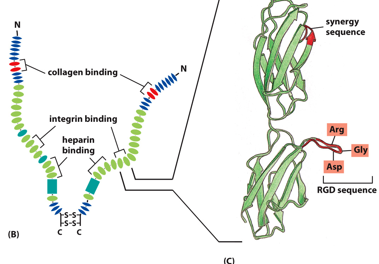
Fibronectins also help attach the cell to the ECM. Soluble ones help initiate blood clots. There are soluble forms in blood, and insoluble forms in the basal lamina. It has disulfide bridges, and is a dimer. It has multiple integrin binding sites, allowing things with integrins to bind to one other, with it as an intermediate (like a glue).