Chapter 4:Particle nature of matter
Atomic nature of matter
Many scientists have performed experiments to study the properties, size and nature of an atom, all the measurements have been indirect and manipulation of large scale to fit the microscopic world.
Einstein’s work on theoretical Brownian motion, additionally confirmed the hypothesis and improved the values of Avogadro’s number in the early 1900s.
The composition of an atom, a heavy positive nucleus and negatively charged particles revolving around it, was proved by multiple discoveries.
The discovery of the law of electrolysis by Michael Faraday, 1833.
The identification of cathode rays as electrons and measurement of e/m by Joseph John, 1897.
Precise measurement of e by Robert Millikan, 1909.
Faraday’s law of electrolysis
- When an electrolyte is in its molten or solution state in the charge passes through the ions, under an electric field these ions move towards the cathode(oxidation) and anode(reduction), respectively lose or gain electrons and is liberated as neutral atoms.
- It provided the proof that matter consists of molecules and molecules consist of atoms.
- It showed that the charge is quantized because only integral number of charges were transferred at the electrodes.
- It also showed that the atom consists or oppositely charges particles, however the mass and size of these charged subatomic particles were unknown at the time.
J.J. Thompsons Experiment
- The Cathode ray experiment, yielded the ratio of e/m in measurable quantities as 1.0 x 10^11 C/kg, although not accurate according to todays 1.758803 x 10^11 C/kg.
- It was clear from these experiment that there exists a particle about 1000 times smaller than the hydrogen, q/m value of which is 10^8 C/kg.
- Photoelectric effect was also observed during the experiment.
- Thompson finally concluded that these particles were the universal constituent of all matter.
Millikan’s Value of the Elementary Charge
By balancing the electric and gravitational force on individual oil drops, Millikan was able to determine the fundamental electric charge and to show that charges always occur in multiples of e.
The quantum charge of an electron is given by
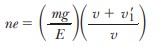 where n is an integer, m is the mass of the drop, E is the magnitude of the electric field, v is the terminal speed of the drop with field off (falling), and v 1 is the terminal speed of the drop with field on (rising).
where n is an integer, m is the mass of the drop, E is the magnitude of the electric field, v is the terminal speed of the drop with field off (falling), and v 1 is the terminal speed of the drop with field on (rising).
Rutherford’s scattering of alpha particles from gold atoms
- This established the nuclear model of the atom. By measuring the rate of scattering of alpha particles into an angle phi .
- Rutherford was able to establish that most of the mass and all of the positive charge of an atom, Ze , are concentrated in a minute volume(nucleus) of the atom with a diameter of about 10^-14 m.
Bohr’s postulates
These postulates explained the motion of electrons within the atom from the diffraction experiment and the rich and elaborate series of spectral lines emitted by the atom.
Electrons move about the nucleus in circular orbits determined by Coulomb’s and Newton’s laws.
Only certain orbits are stable. The electron does not radiate electromagnetic energy in these special orbits, and because the energy is constant with time these are called stationary states.
A spectral line of frequency f is emitted when an electron jumps from an initial orbit of energy Ei to a final orbit of energy Ef, where,
hf=Ei-Ef
The sizes of the stable electron orbits are determined by requiring the electron’s angular momentum to be an integral multiple of h.
These postulates lead to quantized orbits and quantized energies for a single electron orbiting a nucleus with charge Ze, given by 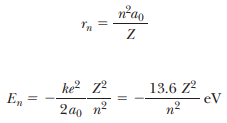 Correspondence principle
Correspondence principle
As a bridge between the familiar domain of classical physics and the more uncertain domain of atomic systems and quantum theory, Bohr provided the correspondence principle.
- This principle states that predictions of quantum theory must correspond to the predictions of classical physics in the region of sizes where classical theory is known to hold.
Direct confirmation of atomics energy levels: the Frank-Hertz experiment
- This experiment shows that mercury atoms can only accept discrete amounts of energy from a bombarding electron beam.
- Therefore, provided clear experimental proof of the existence of quantized energy levels in atoms and showed that the levels deduced from electron bombardment agreed with those deduced from optical line spectra.
- It confirmed the universality of energy quantization in atoms, because the quite different physical processes of photon emission and electron bombardment yielded the same energy levels.