Chapter 22: Stem Cells and Tissue Renewal
Stem Cells and Renewal in Epithelial Cells
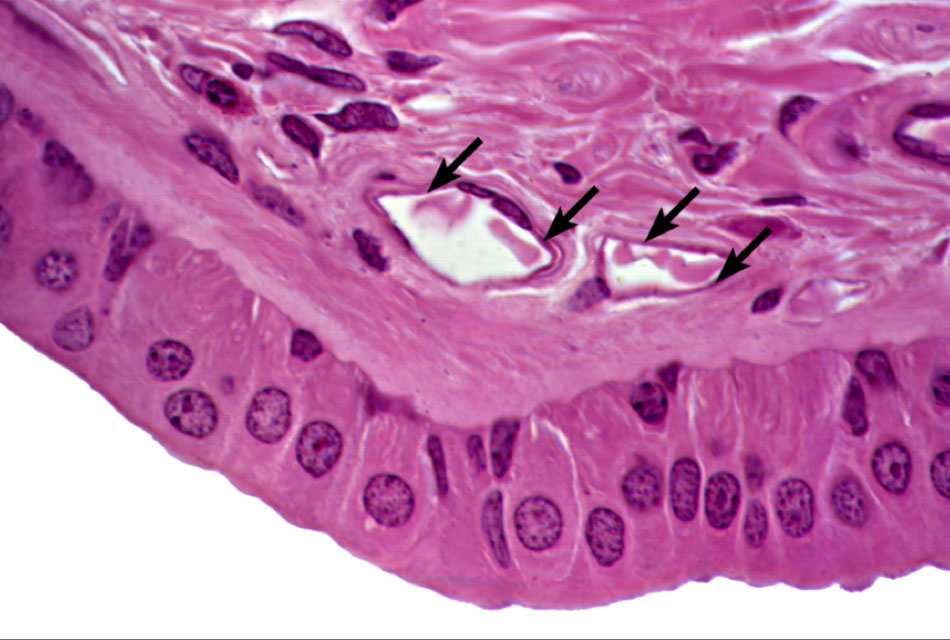
Epithelial tissues are essential for covering body surfaces, lining organs, and forming protective barriers throughout the body.
- The constant renewal and maintenance of epithelial tissues rely on the presence of specialized stem cells.
Stem cells are undifferentiated cells with the capacity for self-renewal and the ability to differentiate into multiple cell types.
Epithelial stem cells reside in specific niches within epithelial tissues and contribute to the continuous replenishment of epithelial cell populations.
- The skin, intestines, respiratory tract, and mammary glands are examples of epithelial tissues that undergo frequent renewal.
Epithelial stem cells can be classified into two types: basal stem cells and transit-amplifying cells.
- Basal stem cells are located in the basal layer of epithelia and have the potential to give rise to differentiating cells.
- Transit-amplifying cells are the immediate progeny of basal stem cells and undergo rapid proliferation before differentiating into specific cell types.
Epithelial stem cells are regulated by various signaling pathways, including Wnt, Notch, and BMP signaling, which play crucial roles in maintaining stemness and controlling differentiation.
The balance between stem cell self-renewal and differentiation is tightly regulated to ensure the maintenance of tissue homeostasis.
Asymmetric cell division is a common mechanism by which epithelial stem cells self-renew while generating differentiating progeny.
- During asymmetric division, the stem cell divides into two daughter cells with different fates: one retains stem cell characteristics, while the other undergoes differentiation.
Epithelial stem cells receive signals from their microenvironment, or niche, which provides the necessary cues for self-renewal and differentiation.
The niche is composed of various cell types, extracellular matrix components, and signaling molecules that regulate stem cell behavior.
Fibroblasts and their transformations: The connective-tissue cell family
Fibroblasts are a type of connective tissue cell that plays a crucial role in the maintenance, repair, and remodeling of various tissues and organs.
- They are the most abundant cells in the connective tissue, which is the most widespread tissue type in the body.
Fibroblasts are responsible for synthesizing and organizing the extracellular matrix (ECM), a complex network of proteins and polysaccharides that provides structural support and biochemical signaling to surrounding cells.
Fibroblasts secrete ECM components such as collagen, elastin, fibronectin, and proteoglycans, which contribute to the strength, elasticity, and flexibility of tissues.
These cells are characterized by their elongated shape, prominent nucleus, and extensive cytoplasmic processes.
Fibroblasts are found in almost all tissues and organs, including skin, tendons, ligaments, muscles, blood vessels, and organs like the liver, lungs, and kidneys.
Fibroblasts can transform into other cell types under specific conditions or in response to different signals. These transformations are collectively known as fibroblast transformations or myofibroblast differentiation.
Myofibroblasts are a specialized form of fibroblast that exhibit properties of both fibroblasts and smooth muscle cells.
- Myofibroblasts are characterized by the presence of contractile fibers called stress fibers, which allow them to exert mechanical forces on the surrounding ECM.
- Myofibroblasts play a crucial role in wound healing, tissue repair, and the contraction of granulation tissue during the remodeling phase of healing.
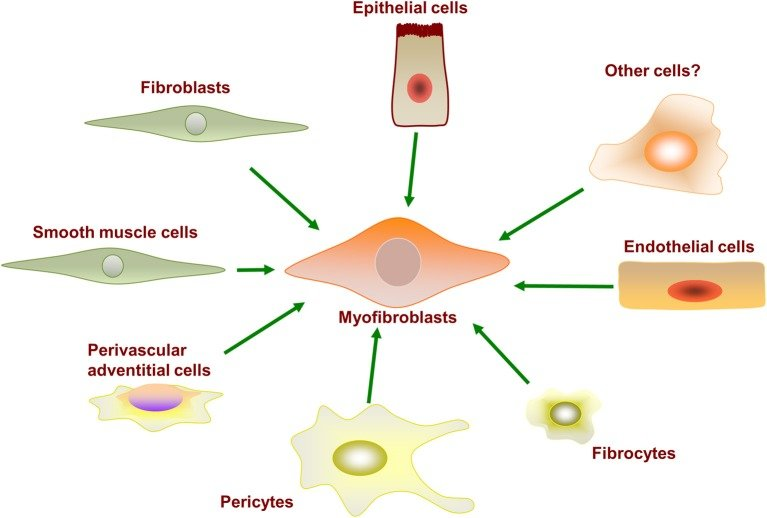
The transformation of fibroblasts into myofibroblasts is regulated by various factors, including growth factors, cytokines, mechanical tension, and inflammatory signals.
Transformations can also occur in p
athological conditions, such as fibrosis, where excessive production and deposition of ECM components lead to tissue scarring and dysfunction.
In addition to myofibroblasts, fibroblasts can undergo other transformations, such as adipogenic transformation (differentiating into fat cells), chondrogenic transformation (forming cartilage-like cells), and osteogenic transformation (forming bone-like cells).
These transformations are influenced by specific differentiation signals and the microenvironment in which the fibroblasts are located.
The plasticity of fibroblasts allows them to adapt and contribute to tissue homeostasis and repair in response to injury or changing physiological conditions.
Fibroblasts also play a role in immune responses, as they can secrete chemokines and cytokines to recruit immune cells and modulate inflammation.
Genesis and Regeneration of skeletal muscle
Genesis of Skeletal Muscle:
Skeletal muscle is derived from a group of cells called myoblasts, which are specialized muscle progenitor cells.
During embryonic development, myoblasts undergo proliferation and migration to form the initial muscle tissue.
As myoblasts differentiate, they fuse together to form multinucleated muscle fibers.
The fusion process is mediated by specific proteins, such as myogenin and myosin, which enable the alignment and merging of myoblasts.
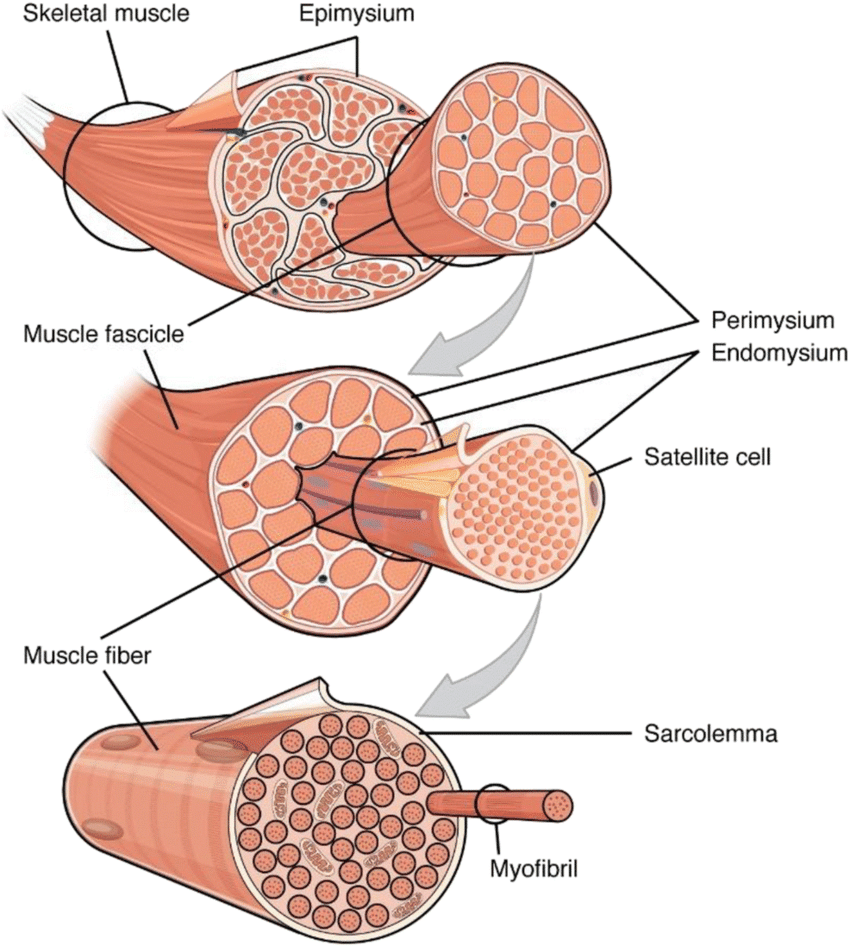
Regeneration of Skeletal Muscle:
- Skeletal muscle has a remarkable ability to regenerate and repair itself after injury or damage.
- The regeneration process is initiated by satellite cells, which are a type of quiescent (dormant) muscle stem cell located between the muscle fiber and its surrounding extracellular matrix.
- Upon injury, satellite cells are activated and undergo proliferation to generate myoblasts.
- The myoblasts then differentiate and fuse together to form new muscle fibers, leading to the repair and regeneration of the damaged muscle tissue.
- The regeneration process is regulated by various growth factors and signaling pathways, such as insulin-like growth factor (IGF) and Notch signaling, which orchestrate the proliferation, differentiation, and fusion of myoblasts.
- Additionally, immune cells, such as macrophages, play a crucial role in muscle regeneration by clearing cellular debris and secreting factors that promote tissue repair.
- The regenerating muscle undergoes a process called myofiber hypertrophy, where the new muscle fibers increase in size and lengthen to restore the functional integrity of the muscle.
Factors Affecting Muscle Regeneration:
- Several factors can influence the efficiency of skeletal muscle regeneration, including age, nutrition, and the extent of the injury.
- Aging can impair the regenerative capacity of skeletal muscle due to a decline in the number and function of satellite cells.
- Proper nutrition, particularly an adequate intake of protein, is crucial for providing the building blocks necessary for muscle regeneration.
- In cases of severe muscle damage, such as large injuries or chronic diseases, the regenerative capacity may be compromised, leading to the formation of fibrotic tissue instead of functional muscle.
Blood Vessels, Lymphatics, and Endothelial Cells
Blood Vessels:
Blood vessels are part of the circulatory system and play a crucial role in transporting blood throughout the body.
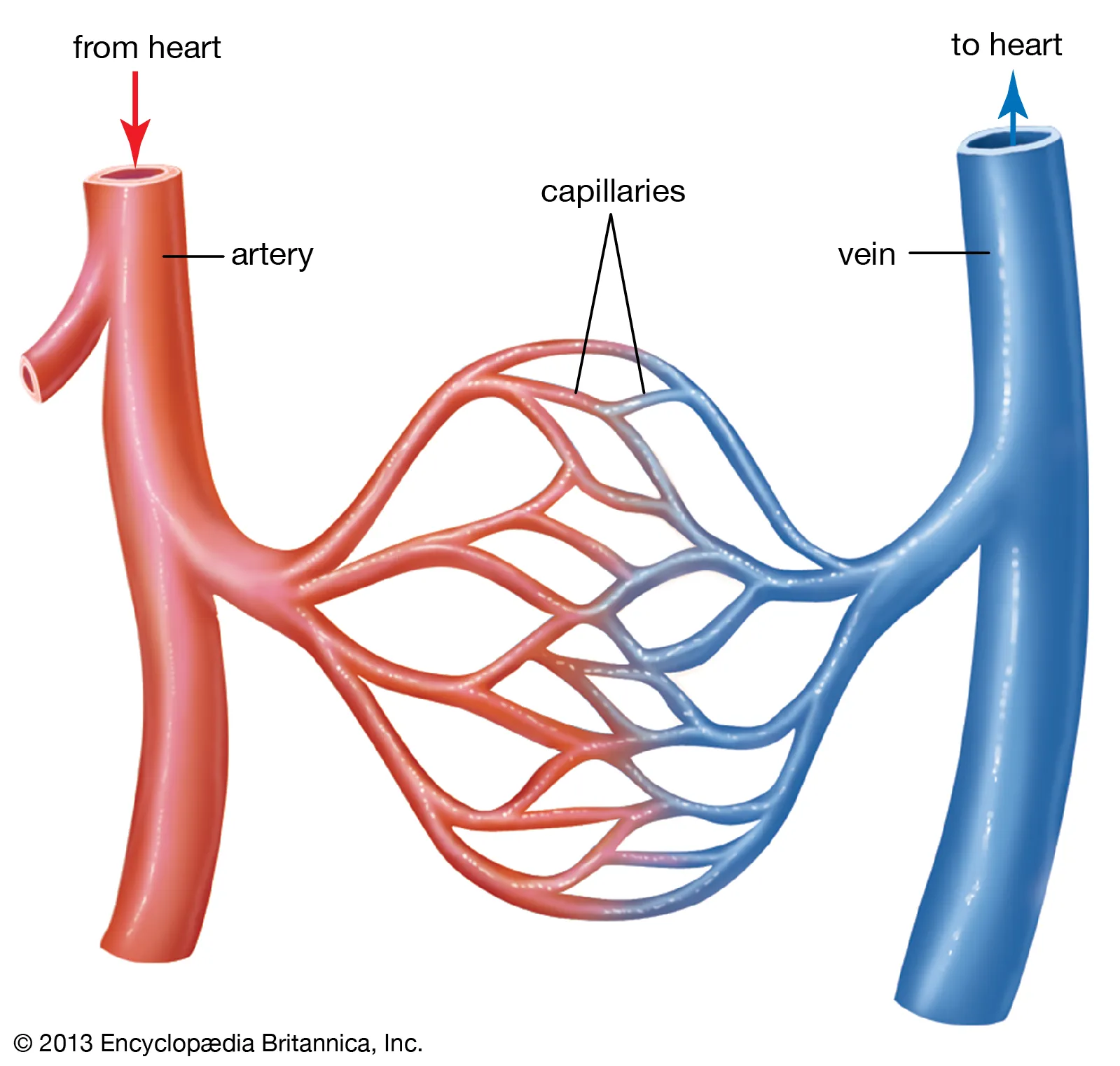
There are three main types of blood vessels: arteries, veins, and capillaries.
- Arteries carry oxygenated blood away from the heart to various tissues and organs.
- Veins return deoxygenated blood back to the heart.
- Capillaries are tiny, thin-walled vessels that connect arteries and veins. They enable the exchange of nutrients, oxygen, waste products, and hormones between the blood and surrounding tissues.
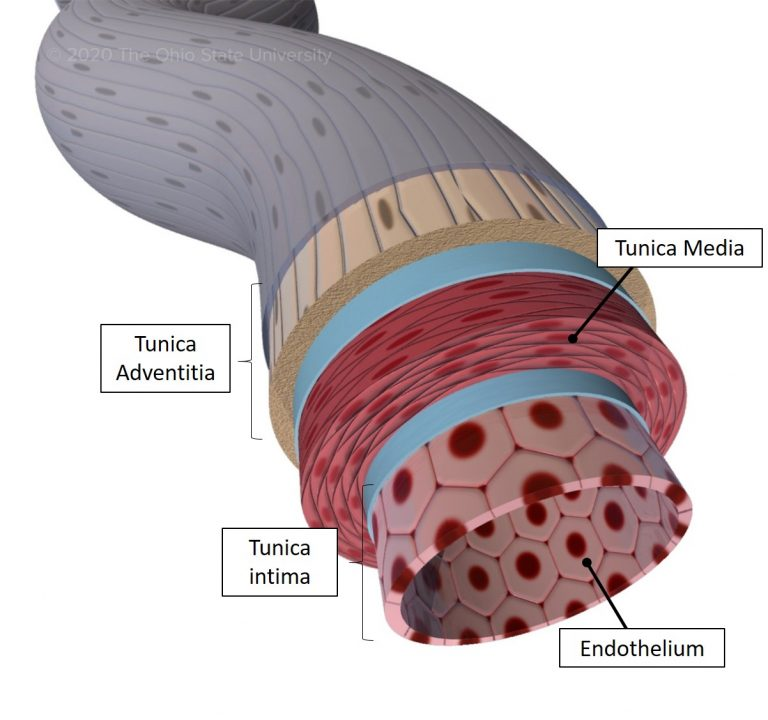
Blood vessels have three layers: the innermost tunica intima, the middle tunica media, and the outer tunica adventitia.
- The tunica intima is composed of a single layer of endothelial cells, supported by a thin layer of connective tissue.
- The tunica media consists of smooth muscle cells and elastic fibers, which allow the blood vessels to constrict or dilate, regulating blood flow and blood pressure.
- The tunica adventitia is a connective tissue layer that provides structural support and contains nerves and blood vessels that supply the blood vessel itself.
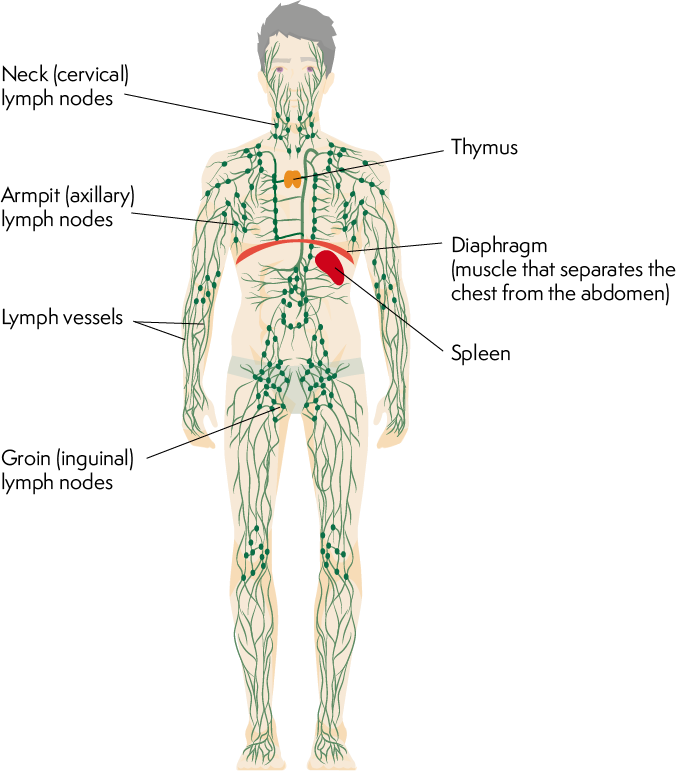
Lymphatics:
Lymphatics are a network of vessels that parallel the blood vessels and form the lymphatic system.
The lymphatic system plays a vital role in maintaining fluid balance, immune function, and the absorption of dietary fats.
Lymphatic vessels collect excess interstitial fluid, called lymph, from tissues and transport it back to the bloodstream.
Lymph nodes are small structures located along the lymphatic vessels that filter lymph, removing pathogens, cellular debris, and foreign substances.
Lymphocytes, a type of white blood cell, reside within the lymphatic system and play a central role in immune responses.
Endothelial Cells:
- Endothelial cells are specialized cells that line the interior surface of blood vessels and lymphatic vessels, forming a thin layer called the endothelium.
- The endothelium serves as a selectively permeable barrier between the blood or lymph and surrounding tissues.
- Endothelial cells have various functions, including regulating the exchange of gases, nutrients, and waste products between the blood/lymph and tissues.
- They also secrete substances involved in blood clotting, immune responses, and the dilation or constriction of blood vessels.
- Endothelial cells produce nitric oxide, a molecule that helps regulate blood vessel tone and promote vasodilation.
A hierarchical stem cell system: Blood cell formation
- Hematopoiesis is the process by which new blood cells are formed in the body.
- It is a highly regulated and hierarchical process that involves various types of stem and progenitor cells.
- Hematopoietic stem cells (HSCs) are at the top of the hierarchy and are responsible for the lifelong production of all blood cell types.
- HSCs are self-renewing, meaning they can divide and generate identical copies of themselves, as well as differentiate into more specialized progenitor cells.
- HSCs reside mainly in the bone marrow, but they can also be found in small numbers in other tissues, such as the umbilical cord blood and peripheral blood.
- HSCs give rise to two main lineages of progenitor cells: the common myeloid progenitors (CMPs) and the common lymphoid progenitors (CLPs).
- CMPs give rise to various myeloid cell types, including red blood cells, platelets, granulocytes (neutrophils, eosinophils, basophils), monocytes, and macrophages.
- CLPs, on the other hand, differentiate into lymphoid cell types, including B cells, T cells, and natural killer (NK) cells.
- The differentiation of HSCs and progenitor cells into specific blood cell types is controlled by a complex network of transcription factors, growth factors, and signaling pathways.
- For example, the transcription factor GATA-1 is crucial for the development of red blood cells, while PU.1 plays a key role in myeloid cell lineage specification.
- As progenitor cells differentiate, they become more restricted in their developmental potential and acquire specific characteristics and functions.
- The differentiation process involves sequential stages of lineage commitment, during which cells undergo morphological and functional changes.
- Precursor cells mature into mature blood cells, which are released into the bloodstream and can circulate throughout the body to perform their respective functions.
- The lifespan of different blood cell types varies. For instance, red blood cells typically circulate for about 120 days, while certain immune cells have longer lifespans.
- In situations of increased demand, such as during infection or blood loss, the hematopoietic system can respond by increasing the production of specific blood cell types.
- This is achieved through a process called hematopoietic lineage expansion, where specific progenitor cells are stimulated to undergo increased proliferation and differentiation.
Regeneration and Repair
- Regeneration and repair are biological processes that aim to restore the structure and function of damaged tissues and organs in the body.
- Regeneration refers to the ability of an organism to replace lost or damaged tissue with newly formed tissue that restores the original structure and function.
- Repair, on the other hand, involves the formation of scar tissue to bridge gaps in tissue that cannot be regenerated fully.
- Regeneration and repair can occur in various tissues and organs throughout the body, including the skin, liver, bone, muscle, and nervous system.
- The capacity for regeneration and repair varies among different tissues and organisms. Some tissues, like the liver and skin, have a high regenerative capacity, while others, like the heart and spinal cord, have limited regenerative abilities.
- The regenerative process typically involves several steps, including inflammation, cell proliferation, differentiation, and remodeling.
- Inflammation is the initial response to tissue injury, characterized by the recruitment of immune cells, release of cytokines, and activation of various signaling pathways.
- Inflammatory cells remove debris, pathogens, and damaged cells from the site of injury, creating an environment conducive to tissue regeneration.
- Cell proliferation occurs as a result of the activation and division of existing cells or the recruitment of stem cells or progenitor cells to the site of injury.
- Differentiation is the process by which newly generated cells become specialized and acquire the characteristics and functions of the damaged tissue.
- Remodeling involves the restructuring and maturation of the newly formed tissue, including the organization of cells, deposition of extracellular matrix, and restoration of tissue architecture.
- Various factors can influence the regenerative capacity of tissues, including the type and extent of injury, the presence of a supportive microenvironment, and the presence of stem cells or progenitor cells.
- Stem cells are undifferentiated cells that have the potential to self-renew and differentiate into various cell types. They play a crucial role in tissue regeneration by replenishing lost or damaged cells.
- In some cases, regenerative capacity can be enhanced through therapeutic interventions, such as the use of growth factors, stem cell transplantation, tissue engineering, or gene therapy.
- However, in situations where the regenerative capacity is limited, repair mechanisms predominate. Repair involves the formation of scar tissue, composed of collagen and other extracellular matrix components, to fill the gap left by the damaged tissue.
- While scar tissue restores structural integrity, it may not fully restore the original tissue's function and can impair organ function in some cases.
- The balance between regeneration and repair varies depending on the tissue and the extent of the injury. In some cases, there may be a trade-off between the two processes, with extensive scarring limiting the regenerative response.
Cell Reprogramming and Pluripotent Stem cells
- Cell reprogramming refers to the process of converting specialized cells into a more flexible state, often resembling embryonic stem cells, which have the ability to give rise to all cell types in the body.
- One of the most well-known methods of cell reprogramming is called induced pluripotent stem cell (iPSC) technology, which was pioneered by Shinya Yamanaka in 2006.
- iPSCs are generated by introducing specific transcription factors, typically Oct4, Sox2, Klf4, and c-Myc, into somatic cells, such as skin fibroblasts, to reprogram them back into a pluripotent state.
- These reprogrammed cells, known as iPSCs, exhibit similar characteristics to embryonic stem cells, including the ability to self-renew and differentiate into cells of all three germ layers: ectoderm, mesoderm, and endoderm.
- The discovery of iPSCs has revolutionized the field of regenerative medicine, as it provides a potentially limitless source of patient-specific pluripotent stem cells for various applications.
- iPSCs have the potential to be differentiated into a wide range of cell types, including neurons, heart cells, liver cells, and pancreatic cells, among others.
- iPSCs hold promise for disease modeling, drug screening, and personalized cell-based therapies, as they can be generated from individuals with specific diseases, allowing researchers to study disease mechanisms and test potential treatments.
- iPSCs also offer a potential alternative to the use of human embryonic stem cells (hESCs) in research and therapy, as they do not raise the same ethical concerns.
- However, challenges remain in the iPSC field, including improving the efficiency of reprogramming techniques, understanding the mechanisms involved in reprogramming, and ensuring the safety and quality of iPSC-derived cells for clinical applications.
- In addition to iPSCs, other methods of cell reprogramming have been developed, such as direct reprogramming or transdifferentiation, where specialized cells can be converted directly into another cell type without going through a pluripotent intermediate.